Abstract
BACKGROUND AND PURPOSE:
It has been shown that patients with a first ischemic stroke are at high risk of developing recurrent stroke due to carotid atherosclerotic plaque rupture. However, no one has defined the difference in plaques between initial and recurrent stroke. This study sought to investigate the characteristics of carotid plaque between patients with first-time and recurrent acute ischemic stroke by using MR imaging.
MATERIALS AND METHODS:
Eighty-nine patients with recent acute ischemic stroke were recruited. All subjects underwent carotid high-resolution black-blood MR imaging. The index carotid arteries, defined as the arteries responsible for the ipsilateral stroke, were analyzed quantitatively and qualitatively. Carotid plaque burden and compositional features between patients with first-time and recurrent ischemic stroke were compared.
RESULTS:
Of 89 recruited patients, 51 had first-time stroke and 38 had recurrent stroke. The mean WA, WT, and PWV were greater in patients with recurrent stroke than in patients with first-time stroke (all, P < .05). Compared with patients with first-time stroke, those with recurrent stroke showed significantly higher prevalence of calcification (44.7% versus 23.5%, P = .035) as well as a larger volume of LRNC (179.14 ± 254.81 mm2 versus 71.65 ± 111.15 mm2, P = .027). IPH or fibrous cap rupture or both were observed in 15.8% of patients with recurrent stroke and 3.9% of patients with first-time stroke.
CONCLUSIONS:
Carotid plaques in patients with recurrent ischemic stroke are significantly aggravated compared with those in patients with first-time stroke, and monitoring carotid plaques in patients with initial stroke by MR imaging may be helpful for secondary stroke prevention.
Previous studies have demonstrated that patients with ischemic cerebrovascular events, such as TIA and stroke, are at high risk of developing recurrent stroke.1–3 Carotid artery atherosclerosis is one of the major causes of acute large-vessel-disease stroke, which is a subtype of ischemic stroke according to the Trial of Org 10172 in Acute Stroke Treatment classification.4 Clinically, measuring luminal stenosis via angiographic techniques has been considered the most effective strategy for evaluation of atherosclerotic disease severity.5 Recent studies, however, have shown that vulnerable plaques may occur in arteries with low-grade stenosis (lumen reduction lower than 50%).6–8 Therefore, it is important to directly visualize atherosclerotic plaques and characterize their vulnerability by using noninvasive imaging techniques.
Studies have demonstrated that high-resolution black-blood MR imaging is an ideal method to study carotid atherosclerosis.6,9–11 MR imaging enables not only quantification of plaque burden but also characterization of the compositional features of carotid atherosclerotic disease, such as calcification, LRNC, IPH, and fibrous cap status.6,10–12 Using this imaging technique, investigators have revealed significant differences in the tissue composition and morphology of symptomatic and asymptomatic plaques13 and have demonstrated that vulnerable plaques defined by MR imaging accelerate recurrent cerebrovascular events.14 However, these authors13,14 did not study the plaque features in patients with recurrent stroke compared with those with initial stroke. Consequently, it is not certain whether the plaque is aggravated from the time of first ischemic attack to recurrent stroke.
We hypothesized that patients with recurrent stroke develop more advanced atherosclerotic lesions than those with first-time stroke, and the purpose of this study was to compare the characteristics of carotid atherosclerotic plaques between patients with first-time and recurrent acute ischemic stroke by using high-resolution black-blood MR imaging techniques.
Materials and Methods
Study Population
The study protocol was approved by the institutional review board, and informed consent was obtained from all patients before initiation of the study. Patients with acute ischemic stroke symptoms in the anterior circulation presenting to the emergency department were recruited in this study. The exclusion criteria were as follows: 1) probable cardiac source of embolism, 2) carotid aneurysm and arteritis, 3) intracranial artery stenosis shown by brain MR angiography, and 4) previous carotid endarterectomy on the index side or previous neck irradiation. All patients underwent neurologic examination, electrocardiography, laboratory analysis, and brain and carotid MR imaging examination. Patient data, including age, sex, stroke risk factors (such as hypertension, diabetes, hyperlipidemia, and history of ischemic heart disease), and stroke status (first-time or recurrent ischemic stroke) were collected from medical records. The presence of acute ischemic stroke in the anterior circulation was identified by 2 experienced neurologists (>10 years' experience) on the basis of the clinical and the imaging findings.15 MR imaging for carotid arteries was performed within 1 week after neurovascular symptom onset.
Carotid MR Imaging Protocol
All patients were imaged with a 3T MR imaging scanner (Achieva; Philips Healthcare, Best, the Netherlands) by using an 8-channel Chenguang carotid coil (Chenguang Medical Technologies, Shanghai, China). A standardized imaging protocol was performed to obtain multicontrast cross-sectional MR images including TOF, T1-weighted, T2-weighted, and MPRAGE imaging16 for bilateral carotid arteries centered on the bifurcation of the index carotid artery. The “index carotid artery” was defined as the artery responsible for the neurologic symptoms. The MR imaging parameters were as follows: 3D TOF: TR/TE, 20/5.1 ms; flip angle, 20°; quadruple inversion recovery17 T1-weighted sequence: TR/TE, 800/10 ms; T2-weighted sequence with multi-double inversion recovery18: TR/TE, 4000/50 ms; 3D MPRAGE sequence: TR/TE, 9.2/5.5 ms; flip angle, 15°. All MR axial images were acquired with a section thickness of 2 mm, FOV of 14 × 14 cm, matrix size of 256 × 256, and an in-plane resolution of 0.54 × 0.55 mm. The longitudinal coverage of black-blood (T1-weighted, T2-weighted, and 3D MPRAGE) and bright-blood (3D TOF) sequences was 32 mm (16 sections) and 44 mm (22 sections), respectively. Fat saturation was applied to the acquisition of black-blood sequences to enhance the tissue contrast between the carotid vessel wall and the surrounding tissues. Maximum-intensity-projection MRA images were reconstructed from the 3D TOF images.
Carotid MR Image Interpretation
All MR images of the index carotid arteries were reviewed by 2 trained reviewers in consensus, blinded to clinical information and stroke status (first-time or recurrent stroke) by using the custom-designed software Cascade (University of Washington, Seattle, Washington).19 Image quality was rated per axial location on a 4-point scale (1, poor; 2 marginal; 3, good; 4, excellent) depending on the overall signal intensity–to-noise ratio and the clarity of the vessel wall boundaries; images with an image quality of 1 were excluded from this study. For all MR images with acceptable image quality, the LA, WA, TVA, and the mean and maximum WT were measured for each axial location. Carotid plaque burden measurements, including mean LA, mean WA, mean WT, mean TVA, and PWV (PWV = Wall Volume/Total Vessel Volume × 100%) for each artery, were also determined. The degree of carotid stenosis was measured by using NASCET criteria (Percentage Stenosis = 100% × [1 − Luminal Diameter at the Point of Maximal Narrowing/Diameter of the Normal Distal Internal Carotid Artery]). The presence or absence of carotid plaque compositional features, such as calcification, LRNC, and IPH and/or fibrous cap rupture, was identified according to previously published criteria.9 The volume of calcification, LRNC, and IPH for each artery was measured.
Statistical Analysis
Patients were divided into 2 groups according to the stroke status, namely a first-time stroke group and a recurrent stroke group. The normality of each continuous variable was tested by using the Kolmogorov-Smirnov Z-test. Normal distribution data were described as the mean value ± SD. The Student t test or Mann-Whitney U test was used to compare the significance of differences of variables between the 2 groups. For adjusting the age-confounding factor, we used the analysis of covariance for age control to compare the significance of differences of variables between the 2 groups. The prevalence of plaque compositional features was presented and compared with the χ2 test or Fisher exact test between the 2 groups. Statistical analyses were performed with the Statistical Package for the Social Sciences, Version 16.0 software (SPSS, Chicago, Illinois). All tests were 2-tailed, and P values <.05 were considered statistically significant.
Results
Of the total of 94 consecutive subjects recruited from August 2009 to November 2010, 5 were excluded from this study due to poor MR image quality of the carotid artery. Of the remaining 89 subjects, the mean age was 63.00 ± 10.73 years (range, 31–82 years) and 67 (75.2%) were male. First-time stroke and recurrent stroke were found in 51 and 38 subjects, respectively. The mean age of patients with recurrent stroke was significantly greater than that of patients with first-time stroke (67.05 ± 9.69 years versus 59.98 ± 10.55 years, P = .002). Patients with recurrent stroke showed a significantly higher level of total cholesterol compared with those with first-time stroke (4.96 ± 0.91 mmol/L versus 4.50 ± 1.05 mmol/L, P = .031). The demographics and clinical information of this study population are summarized in Table 1.
Table 1:
Patients demographic and clinical informationa
| First-Time Stroke (n = 51) | Recurrent Stroke (n = 38) | P Value | |
|---|---|---|---|
| Age (yr) | 59.98 ± 10.55 | 67.05 ± 9.69 | .002b |
| Sex, male | 72.5% | 78.9% | .489 |
| Body mass index | 24.06 ± 2.76 | 23.69 ± 2.93 | .543 |
| Total cholesterol (mmol/L) | 4.50 ± 1.05 | 4.96 ± 0.91 | .031b |
| High-density lipoprotein (mmol/L) | 1.08 ± 0.27 | 1.14 ± 0.24 | .346 |
| Low-density lipoprotein (mmol/L) | 2.74 ± 0.94 | 2.95 ± 0.69 | .223 |
| Hypertension | 70.6% | 86.8% | .069 |
| Diabetes | 37.3% | 39.5% | .831 |
| Smoking | 58.8% | 50.0% | .408 |
| Coronary heart disease | 17.6% | 22.5% | .732 |
Mean ± SD or %.
P < .05.
Comparison of Carotid Plaque Burden
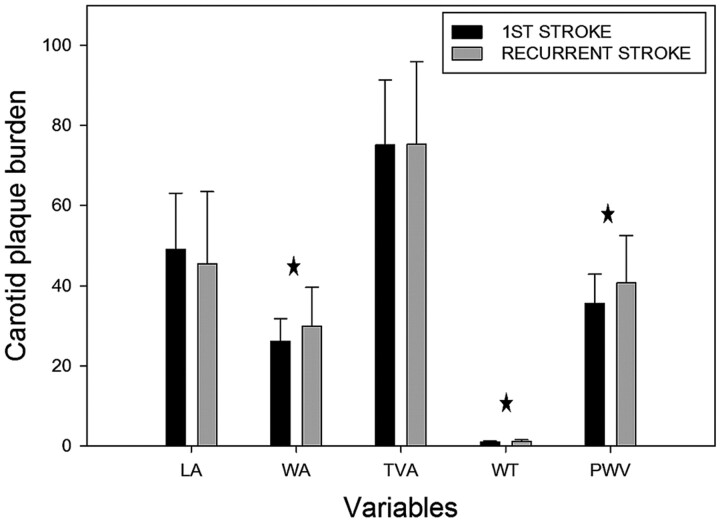
Most of carotid atherosclerotic burden measurements, including mean WA, mean WT, and PWV, of patients with recurrent stroke were significantly greater than those of patients with first-time stroke after adjusting the age (all P values <.05) (Table 2 and Fig. 1). No significant differences were found in mean LA (45.46 ± 17.98 mm2 versus 49.06 ± 14. 06 mm2, P = .292) and mean TVA (75.28 ± 20.62 mm2 versus 75.22 ± 16.09 mm2, P = .988) between patients with recurrent stroke and those with first-time stroke (Table 2 and Fig. 1). Additionally, the prevalence of lumen stenosis of >50% of patients with recurrent stroke was higher, but insignificant statistically, than that of patients with first-time stroke (15.8% versus 7.8%, P = .315).
Table 2:
Comparison of carotid plaque burden after adjusting the age
| Carotid Plaque Burden | First-Time Stroke (n = 51) | Recurrent Stroke (n = 38) | P Value |
|---|---|---|---|
| Mean LA (mm2) | 49.06 ± 14.06 | 45.46 ± 17.98 | .292 |
| Mean WA (mm2) | 26.16 ± 5.58 | 29.82 ± 9.78 | .043a |
| Mean TVA (mm2) | 75.22 ± 16.09 | 75.28 ± 20.62 | .988 |
| Mean WT (mm2) | 0.96 ± 0.22 | 1.13 ± 0.42 | .043a |
| PWV (%) | 35.49 ± 7.36 | 40.66 ± 11.86 | .021a |
P < .05.
Fig 1.
Comparison of carotid plaque burden in first-time and recurrent stroke after adjusting the age. The star indicates a significant difference (P < .05) between adjacent data.
Comparison of Carotid Plaque Compositional Features
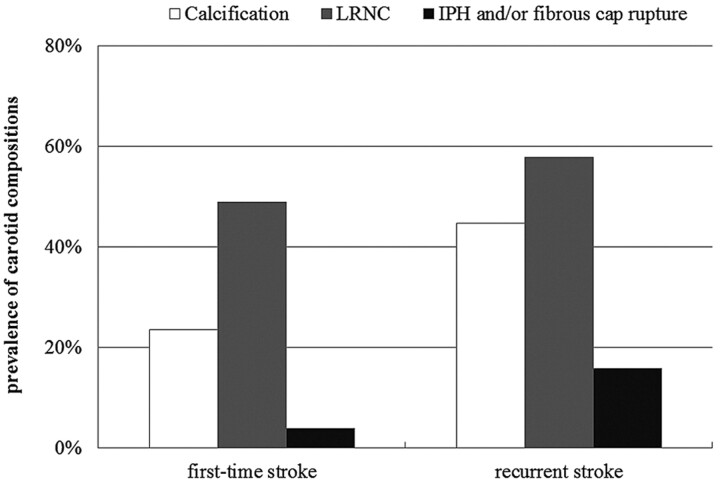
Patients with recurrent stroke had a significantly higher prevalence of calcification compared with those with first-time stroke (44.7% versus 23.5%, P = .035) (Fig 2). The prevalence of LRNC (57.9% versus 49.0%, P = .407) and IPH and/or fibrous cap rupture (15.8% versus 3.9%, P = .069) in patients with recurrent stroke was greater, but statistically insignificant, than that of patients with first-time stroke. In the carotid arteries with LRNC, patients with recurrent stroke showed a significantly larger volume of LRNC compared with patients with first-time stroke (179.14 ± 254.81 mm3 versus 71.65 ± 111.15 mm3, P = .027). For carotid arteries with calcification, patients with recurrent stroke had a slightly larger volume of calcification compared with those with first-time stroke (32.60 ± 32.13 mm3 versus 25.20 ± 27.98 mm3, P = .525) (Figs 3 and 4).
Fig 2.
The prevalence of MR imaging−defined carotid plaque compositions (ie, calcification, LRNC, and IPH and/or fibrous cap rupture) in patients with first-time stroke compared with those with recurrent stroke. A high prevalence of calcification, LRNC, and IPH and/or fibrous cap rupture was found in the patients with recurrent stroke.
Fig 3.
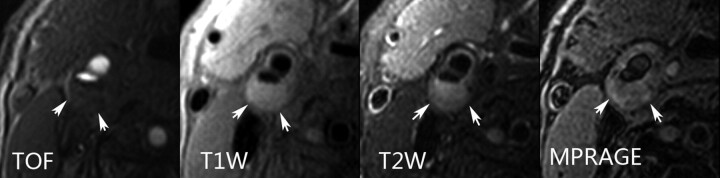
An atherosclerotic plaque with a large LRNC was found in the right internal carotid artery of a 65-year-old male patient who had ipsilateral first-time acute ischemic stroke. The plaque exhibits an intact surface and typical signal-intensity patterns of LRNC (arrows) on TOF, T1-weighted, and T2-weighted images. No high signal intensity was present on the MPRAGE image representing IPH.
Fig 4.
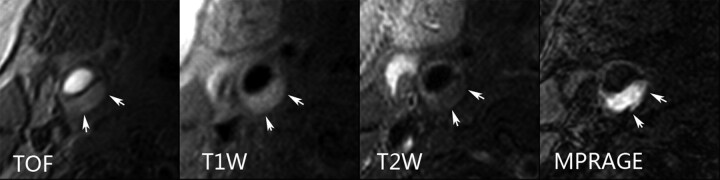
A large complicated plaque can be seen in the left common carotid artery of a 77-year-old male patient who experienced ipsilateral recurrent acute ischemic stroke. Inhomogeneous signal intensity (arrows) on TOF, T1-weighted, and T2-weighted images indicates LRNC with IPH and fibrous cap rupture. The hemorrhage was depicted more clearly by MPRAGE images.
Discussion
This study is one of the first to compare the characteristics of carotid atherosclerotic plaques determined by high-resolution black-blood MR imaging between patients with first-time and recurrent acute ischemic stroke. We found that patients with recurrent stroke had significantly greater carotid atherosclerotic plaque burden. A higher prevalence of calcification and IPH and/or fibrous cap rupture in the carotid arteries was observed in patients with recurrent stroke compared with those with first-time stroke. In addition, the volume of LRNC was markedly larger in patients with recurrent stroke than in patients with first-time stroke.
In this study, patients with recurrent stroke were found to have similar traditional risk factors to those in patients with first-time stroke except for age and total cholesterol level. It has been shown that ischemic stroke is related to modifiable risk factors that have multiplied and compounded for many years. A number of studies have documented that the risk factors for recurrent stroke are the same as those for the first stroke.2,20 The findings of our study are consistent with previous findings that no significant differences can be found between these 2 groups of patients in hypertension, diabetes, smoking, and history of coronary heart disease. Moreover, a very recent study investigated the carotid atherosclerotic plaques of 1385 subjects undergoing carotid endarterectomy and demonstrated that plaque vulnerability and risk of stroke increase gradually with age.21 In our study, the total cholesterol level was significantly higher, and there was a trend in low-density lipoprotein toward hyperlipidemia in patients with recurrent stroke. Given the recent results of the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis trial22 demonstrating that aggressive medical management is very important in prevention of a secondary event, the prevention of hyperlipidemia may also protect patients from a recurrent stroke.
For carotid atherosclerosis in this study population, we found that patients with recurrent stroke exhibit larger plaque burdens compared with those with first-time stroke, even after adjusting the age factor. In this study, the PWV was used to measure the plaque burden because it can fully exploit the 3D data acquisition inherent in carotid MR imaging and is the most reproducible parameter to evaluate plaque burden among all the morphologic measurements by MR imaging.23,24 Similarly, a previous study has shown that carotid plaque burden as measured by sonographic intima-media thickness is associated with recurrence of cardiovascular events including stroke.25 Additionally, we found that there was no significant difference in the prevalence of carotid lumen stenosis of >50% between the 2 patient groups, indicating that the degree of arterial stenosis alone is a relatively poor predictor of recurrent neurologic events. This finding can be explained by the positive remodeling effect of the arterial wall.26 Recent studies have shown that plaque burden measurements, such as PWV, are better indicators than luminal stenosis for evaluating the severity of atherosclerotic disease in the carotid artery.7 The findings of this study imply that measures of carotid plaque burden by using vessel wall imaging techniques might be more effective indicators than the degree of luminal stenosis for assessing the risk of developing recurrent stroke for those symptomatic patients.
The prevalence of carotid calcification was found to be associated with recurrent stroke in this study. Our finding is consistent with that in a previous study by Wattanakit et al.25 However, the role of calcification in carotid plaque vulnerability and its relation to the risk of developing ischemic stroke remains controversial. A previous systematic review suggested that clinically symptomatic carotid plaques have a lower degree of calcification than asymptomatic plaques.27 In contrast, studies have demonstrated that the presence of carotid calcified plaque is an effective predictor for initial and recurrent vascular events.25,28 In this study, the greater prevalence of calcification in patients with recurrent stroke may be attributable to the older age of subjects and the increasing presence of calcification from intermediate-to-advanced atherosclerotic lesions (American Heart Association types IV-VIII).9 Investigators have observed that calcifications at superficial regions of the plaque29 or in the fibrous caps30 increase the risk of developing IPH or fibrous cap rupture. This phenomenon may be due to the increases in the mechanical stress at the interface between noncalcified and calcified compositions.31,32 As such, for evaluation of the role of calcification of atherosclerosis in plaque stability and vascular events in symptomatic subjects, location may be key.33
Compared with patients with first-time stroke, a larger volume of carotid LRNC was seen in patients with recurrent stroke in this study. In a prospective study, Takaya et al34 demonstrated that the size of the LRNC is associated with subsequent ischemic cerebrovascular events. These findings can be explained by the hypothesis in a prospective investigation that LRNC size may govern the risk of future surface disruption.35 Previous studies have shown that the LRNC of carotid atherosclerosis regresses after treatment with lipid-lowering drugs.36 For symptomatic patients, therefore, early detection of LRNC size by using accurate MR imaging techniques and receiving systemic lipid-lowering therapy may be helpful for prevention of recurrent stroke.
The prevalence of IPH and/or fibrous cap rupture tended to be higher in patients with recurrent than in those with first-time stroke in this study. This finding is in accordance with those of a number of previous studies that have demonstrated that carotid IPH37–39 and fibrous cap rupture40 are correlated with recurrent cerebrovascular events. Accordingly, early identification of high-risk plaque features may be necessary for prevention of secondary ischemic stroke.
Because a longer scanning time can lower the overall image quality due to poor cooperation of patients with severe diseases, most of our patients had mild-to-moderate ischemic stroke with few IPHs. Moreover, only MR images of at least average quality were considered for review, and patients with poor image quality were excluded, including 5 patients with image quality scores of 1. Development of new MR imaging protocols with shorter scanning times for the analysis of carotid atherosclerotic disease may broaden the use of carotid MR imaging in research and clinical work. Although the sample is small, this study represents a promising future regarding the potential value of MR imaging of plaque for secondary stroke prevention. However, larger and prospective studies are needed to confirm the findings of this study.
Conclusions
Our study reveals significant differences in carotid atherosclerotic plaque features between patients with first-time and recurrent acute ischemic stroke. Patients with recurrent stroke tend to develop more advanced carotid atherosclerotic lesions and more high-risk plaque features compared with those with first-time stroke. Monitoring of carotid atherosclerosis may be needed, and MR imaging seems to represent a promising tool for secondary stroke prevention.
ABBREVIATIONS:
- IPH
intraplaque hemorrhage
- LA
lumen area
- LRNC
lipid-rich necrotic core
- MPRAGE
magnetization-prepared rapid acquisition of gradient echo
- PWV
percentage wall volume
- TOF
time-of-flight
- TVA
total vessel area
- WA
wall area
- WT
wall thickness
Footnotes
This work was supported in part by Philips Healthcare as well as the Leading Academic Discipline Project of Shanghai, China (No. S30203).
References
- 1. Kelly BM, Pangilinan PH, Jr, Rodriguez GM. The stroke rehabilitation paradigm. Phys Med Rehabil Clin N Am 2007;18:631–50, v [DOI] [PubMed] [Google Scholar]
- 2. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics: 2011 update—a report from the American Heart Association. Circulation 2011;123:e18–e209. Epub 2010 Dec 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alberts MJ, Atkinson R. Risk reduction strategies in ischaemic stroke: the role of antiplatelet therapy. Clin Drug Investig 2004;24:245–54 [DOI] [PubMed] [Google Scholar]
- 4. Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial—TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 [DOI] [PubMed] [Google Scholar]
- 5. Moore WS, Barnett HJ, Beebe HG, et al. Guidelines for carotid endarterectomy: a multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Circulation 1995;91:566–79 [DOI] [PubMed] [Google Scholar]
- 6. Wasserman BA, Wityk RJ, Trout HH, 3rd, et al. Low-grade carotid stenosis: looking beyond the lumen with MRI. Stroke 2005;36:2504–13 [DOI] [PubMed] [Google Scholar]
- 7. Zhao X, Underhill HR, Zhao Q, et al. Discriminating carotid atherosclerotic lesion severity by luminal stenosis and plaque burden: a comparison utilizing high-resolution magnetic resonance imaging at 3.0 Tesla. Stroke 2011;42:347–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saam T, Underhill HR, Chu B, et al. Prevalence of American Heart Association type VI carotid atherosclerotic lesions identified by magnetic resonance imaging for different levels of stenosis as measured by duplex ultrasound. J Am Coll Cardiol 2008;51:1014–21 [DOI] [PubMed] [Google Scholar]
- 9. Cai JM, Hatsukami TS, Ferguson MS, et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106:1368–73 [DOI] [PubMed] [Google Scholar]
- 10. Clarke SE, Hammond RR, Mitchell JR, et al. Quantitative assessment of carotid plaque composition using multicontrast MRI and registered histology. Magn Reson Med 2003;50:1199–208 [DOI] [PubMed] [Google Scholar]
- 11. Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol 2005;25:234–39 [DOI] [PubMed] [Google Scholar]
- 12. Hatsukami TS, Ross R, Polissar NL, et al. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000;102:959–64 [DOI] [PubMed] [Google Scholar]
- 13. Saam T, Cai J, Ma L, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology 2006;240:464–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin K, Zhang ZQ, Detrano R, et al. Carotid vulnerable lesions are related to accelerated recurrence for cerebral infarction magnetic resonance imaging study. Acad Radiol 2006;13:1180–86 [DOI] [PubMed] [Google Scholar]
- 15. Kidwell CS, Warach S. Acute ischemic cerebrovascular syndrome: diagnostic criteria. Stroke 2003;34:2995–98 [DOI] [PubMed] [Google Scholar]
- 16. Ota H, Yarnykh VL, Ferguson MS, et al. Carotid intraplaque hemorrhage imaging at 3.0-T MR imaging: comparison of the diagnostic performance of three T1-weighted sequences. Radiology 2010;254:551–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yarnykh VL, Yuan C. T1-insensitive flow suppression using quadruple inversion-recovery. Magn Reson Med 2002;48:899–905 [DOI] [PubMed] [Google Scholar]
- 18. Yarnykh VL, Yuan C. Multislice double inversion-recovery black-blood imaging with simultaneous slice reinversion. J Magn Reson Imaging 2003;17:478–83 [DOI] [PubMed] [Google Scholar]
- 19. Kerwin W, Xu D, Liu F, et al. Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson Imaging 2007;18:371–78 [DOI] [PubMed] [Google Scholar]
- 20. Leoo T, Lindgren A, Petersson J, et al. Risk factors and treatment at recurrent stroke onset: results from the Recurrent Stroke Quality and Epidemiology (RESQUE) study. Cerebrovasc Dis 2008;25:254–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Lammeren GW, Reichmann BL, Moll FL, et al. Atherosclerotic plaque vulnerability as an explanation for the increased risk of stroke in elderly undergoing carotid artery stenting. Stroke 2011;42:2550–55. Epub 2011 Jul 7 [DOI] [PubMed] [Google Scholar]
- 22. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saam T, Kerwin WS, Chu B, et al. Sample size calculation for clinical trials using magnetic resonance imaging for the quantitative assessment of carotid atherosclerosis. J Cardiovasc Magn Reson 2005;7:799–808 [DOI] [PubMed] [Google Scholar]
- 24. Zhao X, Zhao Q, Chu B, et al. Prevalence of compositional features in subclinical carotid atherosclerosis determined by high-resolution magnetic resonance imaging in Chinese patients with coronary artery disease. Stroke 2010;41:1157–62 [DOI] [PubMed] [Google Scholar]
- 25. Wattanakit K, Folsom AR, Chambless LE, et al. Risk factors for cardiovascular event recurrence in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2005;149:606–12 [DOI] [PubMed] [Google Scholar]
- 26. Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. New Engl J Med 1987;316:1371–75 [DOI] [PubMed] [Google Scholar]
- 27. Kwee RM. Systematic review on the association between calcification in carotid plaques and clinical ischemic symptoms. J Vasc Surg 2010;51:1015–25 [DOI] [PubMed] [Google Scholar]
- 28. Prabhakaran S, Singh R, Zhou X, et al. Presence of calcified carotid plaque predicts vascular events: the Northern Manhattan Study. Atherosclerosis 2007;195:e197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu X, Ju H, Cai J, et al. High-resolution MR study of the relationship between superficial calcification and the stability of carotid atherosclerotic plaque. Int J Cardiovasc Imaging 2010;26 (suppl 1): 143–50 [DOI] [PubMed] [Google Scholar]
- 30. Bobryshev YV, Killingsworth MC, Lord RS, et al. Matrix vesicles in the fibrous cap of atherosclerotic plaque: possible contribution to plaque rupture. J Cell Mol Med 2008;12:2073–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee RT, Grodzinsky AJ, Frank EH, et al. Structure-dependent dynamic mechanical behavior of fibrous caps from human atherosclerotic plaques. Circulation 1991;83:1764–70 [DOI] [PubMed] [Google Scholar]
- 32. Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 2004;24:1161–70. Epub 2004 May 20 [DOI] [PubMed] [Google Scholar]
- 33. Li ZY, Howarth S, Tang T, et al. Does calcium deposition play a role in the stability of atheroma? Location may be the key. Cerebrovasc Dis 2007;24:452–59 [DOI] [PubMed] [Google Scholar]
- 34. Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI: initial results. Stroke 2006;37:818–23 [DOI] [PubMed] [Google Scholar]
- 35. Underhill HR, Yuan C, Yarnykh VL, et al. Predictors of surface disruption with MR imaging in asymptomatic carotid artery stenosis. AJNR Am J Neuroradiol 2010;31:487–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Underhill HR, Yuan C, Zhao XQ, et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial. Am Heart J 2008;155: 584 e581–88 [DOI] [PubMed] [Google Scholar]
- 37. Altaf N, MacSweeney ST, Gladman J, et al. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke 2007;38:1633–35 [DOI] [PubMed] [Google Scholar]
- 38. Altaf N, Daniels L, Morgan PS, et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg 2008;47:337–42 [DOI] [PubMed] [Google Scholar]
- 39. Kurosaki Y, Yoshida K, Endo H, et al. Association between carotid atherosclerosis plaque with high signal intensity on T1-weighted imaging and subsequent ipsilateral ischemic events. Neurosurgery 2011;68:62–67, discussion 67 [DOI] [PubMed] [Google Scholar]
- 40. Kume S, Hama S, Yamane K, et al. Vulnerable carotid arterial plaque causing repeated ischemic stroke can be detected with B-mode ultrasonography as a mobile component: jellyfish sign. Neurosurg Rev 2010;33:419–30 [DOI] [PubMed] [Google Scholar]