Abstract
BACKGROUND AND PURPOSE:
The use of cerebral protection during CAS is an extended practice. Paradoxically it is open to question because it can lead to potential embolic complications. The aim of this study was to evaluate the safety and efficacy of CASWPD in patients with severe symptomatic carotid artery stenosis.
MATERIALS AND METHODS:
A prospective study was performed including 210 consecutive patients (201 symptomatic and 9 asymptomatic) with carotid artery stenosis >70%. All patients were treated by CASWPD. Angiographic results and neurologic complications were recorded during the procedure and within 30 days after it. All patients underwent clinical evaluation and Doppler sonography follow-up at 3, 6, and 12 months after the procedure.
RESULTS:
Two hundred twenty carotid arteries were treated. The average degree of stenosis was 88.9%. The procedure was successfully completed in 212 (96.4%) arteries. After stent placement, 98.6% of arteries showed no residual stenosis or <30%. Balloon angioplasty dilation before stent placement was performed in 16% of cases. During the 30-day periprocedural period, there were 3 major complications (1.4%), including 1 disabling ischemic stroke, 1 acute stent thrombosis, and 1 MI. The last 2 patients died from these complications. At 1-year follow-up 24 (12.8%) restenoses, 2 new ipsilateral strokes, 1 contralateral stroke, and 5 deaths (2.7%) had occurred. None of these deaths were related to the initial stroke.
CONCLUSIONS:
In our study, unprotected stent placement in symptomatic patients with severe carotid artery stenosis has demonstrated a low incidence of complications. We believe that this is due to the reduction of maneuvering and manipulation through the stenosis and to the protective effect of the stent placement before angioplasty balloon dilation.
In the early 1990s, the NASCET and European Carotid Surgery Trials established CEA as the first-choice treatment for symptomatic carotid artery stenosis >70%.1,2 Ten years later, the first randomized trial comparing surgical-versus-endovascular treatment, CAVATAS, was published.3 The results of this study showed no significant difference between procedures, though in the endovascular therapy group, 80% of patients were treated by angioplasty and only 20%, by stent placement. Since then, several randomized trials have unsuccessfully assessed the noninferiority of CAS versus CEA.4–7 Despite this outcome, CAS has been established as an alternative to CEA.
The most feared complication of the stent placement technique is embolism caused by atherosclerotic plaque dislodgment during the procedure. Several cerebral protection devices have been developed to avoid or reduce the risk of periprocedural complications. Nowadays, the use of these systems has widely increased, and it is recommended as a good practice. Many reviews and meta-analyses have shown that the combined rate of stroke and death is lower in patients treated by CAS and protection devices than those treated by CASWPD.8,9 However, in past years, a discussion has emerged regarding the use of distal protection devices during CAS. A subanalysis of data from the SPACE trial has shown other results that did not support the need for protection devices.10 On the other hand, several uncontrolled reports have shown excellent results in patients treated by unprotected stent placement techniques.11–15
We performed a study to assess the safety and efficacy of unprotected CAS. The aim of this study was to establish the cumulative incidence of stroke, death, or myocardial infarction within 30 days after the procedure and at 1-year follow-up.
Materials and Methods
All patients with symptomatic severe carotid artery stenosis treated at our center between January 2002 and January 2011 were prospectively included in our study.
Inclusion criteria were the following: age >18 years, with no upper limit; symptomatic patients with a ≥70% atherosclerotic stenosis demonstrated by angiography, according to NASCET criteria; symptomatic patients with a 50%–70% stenosis despite antiplatelet therapy; and asymptomatic patients with progressive ≥70% stenosis and contralateral carotid occlusion.
Exclusion criteria were the following: intracranial hemorrhage or major surgery within 30 days before the procedure, uncontrolled arterial hypertension, uncontrolled coagulopathy, contraindications to heparin or antiplatelet therapy, lack of percutaneous vascular access, and stenosis secondary to radiation therapy.
A baseline CT scan was performed in all patients. If an acute ischemic infarction was detected on CT, stent placement was delayed at least 21 days after the clinical event to avoid bleeding complications.
Carotid artery stenosis was initially diagnosed by Doppler sonography. MR angiography, including 3D gradient-echo and contrast-enhanced T1 sequences, was performed whenever possible. Doppler criterion for ≥70% stenosis was a peak systolic velocity of >230 cm/s.16,17 Stenosis degree was confirmed by angiography in all cases. Stenosis degree was established by the NASCET criteria in both angiography and MR angiography.
Preparation
All patients agreed and signed the informed consent according to our protocol. Patients received antiplatelet therapy with clopidogrel (75 mg per day) and aspirin (100 mg per day) at least 4 days before the procedure. When this was not possible, they received a loading dose of clopidogrel (300 mg) and aspirin (300 mg) the day of the procedure.
Procedure
Angiography was performed with the patient under local anesthesia through a femoral or brachial approach by using a 5F diagnosis catheter. Location, length, and degree of stenosis; plaque characteristic; flow compensation through the circle of Willis or pial branches; and the presence of anastomoses between the internal and external carotid arteries were evaluated. After the intravenous heparin bolus administration of 5000 IU, a 90-cm 6F introducer sheath (Super Arrow-Flex percutaneous sheath; Arrow International, Cleveland, Ohio) was advanced into the distal common carotid artery over an exchange guidewire placed in the external carotid artery. A 0.014-in guidewire (Platinum EX Transend; Boston Scientific, Natick, Massachusetts) was used to cross the stenosis until the guidewire reached the carotid cavernous artery; then, the stent-delivery system (carotid Wallstent; Schneider Boston Scientific, Galway, Ireland, as first choice) was smoothly advanced through the stenosis over the guidewire. Finally, the stent was deployed, and the residual stenosis degree was evaluated. When it was not possible to get through the stenosis with the stent-delivery system, a predilation was performed by using a 2- to 4-mm angioplasty balloon (Gateway PTA balloon catheter; Boston Scientific, Fremont, California) at 6 atm. When residual stenosis was >30%, a postprocedural dilation was performed by using a 4-mm balloon (Viatrac; Guidant, St. Paul, Minnesota). A low-pressure dilation at 8 atm was performed to achieve a residual stenosis <30%.
All procedures were performed by 2 neuroradiologists with experience in endovascular techniques (F.D. and R.O. with 20 and 6 years of experience, respectively). Patients were monitored by a critical care physician. Atropine was administered only if asystolia (absence of complex waves in 3 sweeps of the electrocardiogram display) or extreme bradycardia (<30 systoles per minute) occurred during the procedure.
After the procedure, all patients remained for 24–48 hours at the neurology unit. A neurologic evaluation was performed by a neurologist within 24 hours. Dual antiplatelet therapy was prescribed during the first month poststenting; then, a single antiplatelet agent was continued indefinitely. Follow-up was performed by neurologic evaluation and Doppler sonography examination at 3, 6, and 12 months after the procedure. All data were included on an Access data base (Microsoft, Bothell, Washington) created for this purpose.
We performed a descriptive observational study. Continuous values were expressed as mean and nominal variables as counts and percentages. The primary end point was to determine the cumulative incidence of death, stroke, or myocardial infarction within 30 days after intervention. The secondary end point was to establish the incidence of ipsilateral stroke and/or death between 31 days and 1 year after the procedure.
Results
Two hundred ten patients, 178 men and 32 women, with a mean age of 66.2 years (range, 20–84 years) were included in the study (Table 1). All patients were symptomatic except 9; 4 presented with >70% stenosis and 5 had a stenosis between 90% and 99%. In these 9 patients, stent placement was considered because of repeated syncope in 5, bilateral progressive stenosis (>70%) in 2, and contralateral carotid occlusion in 2.
Table 1:
Baseline characteristics of patients and stenoses
| Characteristics | No. | % |
|---|---|---|
| Patients | 210 | 100 |
| Men | 178 | 85 |
| Women | 32 | 15 |
| Mean age (yr) | 66.2 (±9.6) | |
| Vascular risk factors | ||
| Hypertension | 145 | 69.4 |
| Diabetes mellitus | 86 | 41.1 |
| Dyslipidemia | 84 | 40.2 |
| Coronary artery disease | 38 | 18.2 |
| Peripheral artery disease | 36 | 17.2 |
| Smoker | 41 | 19.6 |
| Carotid arteries | ||
| Stenosis | 220 | 100 |
| <70 % | 6 | 2.7 |
| 70%–79% | 51 | 23.2 |
| 80%–89% | 23 | 10.5 |
| 90%–99% | 121 | 55.0 |
| Pseudo-occlusion | 18 | 8.2 |
| Occlusion | 1 | 0.5 |
A Doppler sonography examination was performed in all cases, and MR angiography, in 114 patients (51.8%). The degree of stenosis was always confirmed by angiography. From the 220 carotid arteries treated, 163 (74.1%) had >80% stenosis, 140 (63.6%) had >90% stenosis, 18 (8.2%) showed pseudo-occlusions, and 1 was totally occluded (0.5%). There were also 6 patients with <70% stenosis: Three presented with progressive stenosis and contralateral carotid artery occlusion, and 3 presented with TIAs, despite dual antiplatelet therapy. The average degree of stenosis was 88.9%. On the basis of angiography, complex stenoses were categorized into unfavorable geometry, excessive angulation, kinking or extreme carotid artery tortuosity (41.8%), ulceration (15.9%), and hypertrophic calcification (15.9%).
Other Findings
Eighty-two patients presented with severe aortoiliac atherosclerosis, 32 showed contralateral carotid artery stenosis ≥70%, 20 showed vertebral artery stenosis, 18 had stenosis at the origin of the great vessels from the aortic arch, and 8 had stenosis in the intracranial internal carotid arteries. Thirty-nine patients had flow compensation through anterior and posterior communicating arteries, and 87, only through 1 of these. Ninety-four patients showed no flow compensation through the circle of Willis. Two patients showed compensation through pial collateral vessels, and 3, through the ophthalmic artery, which had reversed flow. Two vertebral artery steal syndromes due to subclavian artery occlusion were detected. Three other patients had abdominal aorta aneurysms.
Immediate Results
Technical success was achieved in 212 of the 220 arteries (96.4%) treated. In 8 patients, it was not possible to pass the wire through the stenosis because of irregularity and high-grade stenosis >90% (n = 4) or the presence of pseudo-occlusion (n = 4). Of the 212 arteries successfully treated, 169 (79.7%) showed no residual stenosis, 40 (18.9%) had residual stenosis <30%, 2 (0.94%) had 30%–40% residual stenosis, and 1 (0.47%) had 50% residual stenosis. A single stent was placed in 198 arteries (Fig 1), 2 stents were placed in 12 arteries (Fig 2), and 3 stents were placed in 2 cases. We treated bilateral stenoses (10 patients) in 2 different sessions, waiting at least 1 month between.
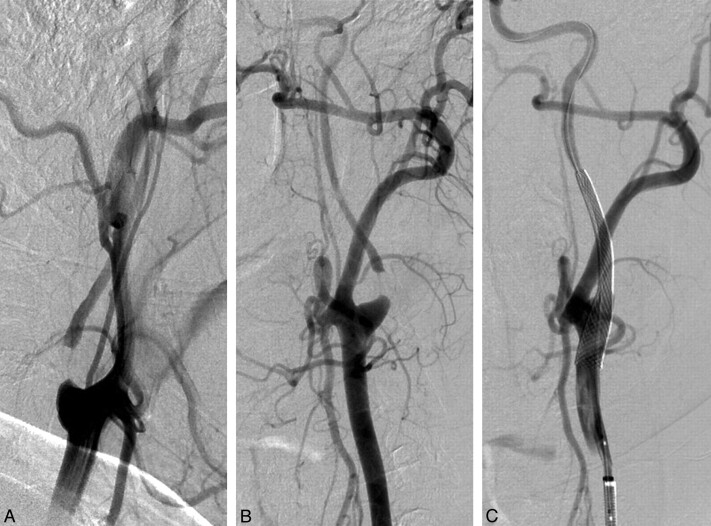
Fig 1.
Left common carotid artery angiography showing pseudo-occlusion of internal carotid artery. Oblique view (A) and lateral view (B). The internal carotid artery filling was delayed regarding to the external carotid artery. C, Immediate control after stent deployment (7 × 40 mm Carotid Wallstent) and postdilatation with a 4 × 20 mm balloon. No predilation was performed.
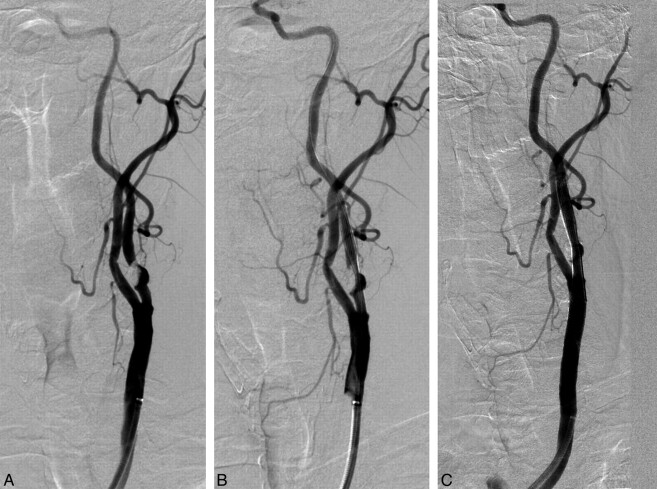
Fig 2.
A, selective angiogram that shows a high-grade stenosis of >90% in the left internal carotid artery due to a great atheromatous plaque with calcification. B, After sent deployment (7 × 40 mm Carotid Wallstent), a significant residual stenosis is revealed despite performing a postdilation with a 4 × 20 mm balloon. C, Final result after a second overlapped stent placement with no residual stenosis.
Predilation was necessary in 34/212 arteries (16%), postdilation, in 152 cases (71.6%), and both, in 17 cases (8%). We deployed 228 stents: 198 Carotid Wallstents (Boston Scientific), 21 Precise (Cordis, Miami Lakes, Florida), 3 Express (Boston Scientific), 2 Wingspan (Boston Scientific), 1 Enterprise (Cordis), 1 Herculink (Abbott Vascular, Abbott Park, Illinois), 1 Multi-Link (Guidant), and 1 Prokinetic. (Biotronic, Ann Arbor, Michigan).
Thirty-Day Results
There were 3 major complications (1.36%) within 30 days after the procedure: 1 ischemic disabling stroke, 1 death related to acute stent thrombosis, and another death due to myocardial infarction. In addition, there were 2 nondisabling strokes and 4 TIAs. The combined end point including any stroke, death, or MI was reached in 7 patients (3.18%). Other reported events are presented in Table 2.
Table 2:
Peri-interventional and 30-day complications
| Peri-Interventional | 24 hours–30 days | Total | % | |
|---|---|---|---|---|
| Medical complications | ||||
| Hypertension | 7 | 7 | 3.18 | |
| Asystolia (atropine) | 8 | 8 | 3.64 | |
| Bradycardia <30 bpm (atropine) | 18 | 18 | 8.18 | |
| Bradycardia 30–40 bpm | 7 | 7 | 3.18 | |
| Groin or brachial hematoma | 3 | 1 | 4 | 1.82 |
| Neurologic complications | ||||
| TIA | 2 | 2 | 4 | 1.82 |
| Ischemic nondisabling stroke | 2 | 2 | 0.91 | |
| Ischemic disabling stroke | 1 | 1 | 0.45 | |
| Intracranial hemorrhage | 1 | 1 | 2 | 0.91 |
| Deaths | ||||
| Stroke | 1 | 1 | 0.45 | |
| MI | 1 | 1 | 0.45 |
One-Year Follow-Up
At the end of the study, 33 of 210 patients were lost to follow-up (censored cases), so patients with 187 of 212 arteries treated completed 1-year follow-up (88.2%). There were 2 new strokes and 1 TIA from the ipsilateral carotid artery, 1 contralateral stroke, and 5 deaths. One patient died of a vertebrobasilar stroke 33 days after the procedure. The other deaths were related to different causes (1 MI, 1 metastatic disease, 1 heart failure, and 1 urologic septic shock). All deaths occurred between 8 and 11 months after the intervention. Twenty-four patients (12.8%) had in-stent restenosis on Doppler sonography follow-up, 6 at the 3-month control, 9 at the 6-month control, and 9 at the 1-year control. In 4 of the restenoses, the immediate results after stent placement were a <30% residual stenoses, 1 patient had a residual stenosis of 50%, and the 19 remaining patients had no residual stenosis in the immediate control. All restenoses were asymptomatic except for 3.
Discussion
The results of this study show a relatively low incidence of complications within 30 days after the procedure: 1.36% with disabling stroke, death, or MI; and 3.18% with any stroke, death, or MI. In randomized trials, complications ranged from 5.2% to 9.6% in patients treated with CAS and 3.4%–5.7% in patients treated with CEA.4–7 However, these trials are heterogeneous regarding patient selection criteria, degree of stenosis, type of stents deployed, and the use of cerebral protection devices. A large meta-analysis including 54 713 patients treated with CAS estimated that the 30-day combined rate of stroke or death was 4.7%.18 Most CAS trials used cerebral protection devices. On the other hand, the subanalysis of the SPACE trial showed a lower complication rate in the CASWPD group (6.5%) than the CAS with the protection device group (8.3%).10
Nonrandomized reports by using CASWPD have shown that 30-day complication rates ranged from 1.2% to 4.2%. These reports included symptomatic and asymptomatic patients, with an average degree of stenosis ranging from 80% to 82%.12–15 In our series, all cases were symptomatic except 9, and the mean degree of stenosis was 89%. In addition, our report included the largest number of symptomatic patients and the highest degree of stenosis.
Two hemorrhagic complications were reported. One patient had a pseudo-occlusion, contralateral carotid artery stenosis (>70%), and vertebral artery stenosis. The patient presented with headache, vomiting, decreased level of consciousness, and hemiparesis 5 days after stent placement. CT revealed a basal ganglia hematoma extending into the ventricular system and subarachnoid space. The patient was admitted to the intensive care unit. His symptoms regressed, and he was discharged with residual hemiparesis. The clinical findings and favorable evolution suggest that the hemorrhage was probably related to hyperperfusion syndrome, associated with loss of vascular autoregulation mechanisms in a chronically hypoperfused area.19
The other patient presented with a >90% stenosis. Immediately after the procedure, in the angiography room, he developed progressive aphasia. An urgent CT scan was performed, revealing a putaminal hematoma on a previously infarcted area. Protamine was administered, and he was transferred to the intensive care unit. The patient showed progressive favorable evolution in the following days. We proposed 2 possible mechanisms for early bleeding: embolism during the procedure, and secondary hemorrhagic transformation due to reperfusion on a cerebral infarct area. However, in our case the exact cause of hemorrhage could not be clarified.20,21
Two patients developed stent thrombosis. One occurred 9 days after the procedure, causing a complete middle cerebral artery infarct. This patient had a previous stenosis of >90%. The other thrombosis occurred 6 days after the procedure. The patient was readmitted after discharge, with acute hemiplegia and aphasia. An urgent CT scan demonstrated a cerebral infarction, and a carotid Doppler sonography showed stent thrombosis. A rescue revascularization was unsuccessfully attempted, but the patient died of a malignant infarction 24 hours later. Stent thrombosis can be a fatal complication.22 It has been associated with insufficient antiplatelet therapy and antiplatelet agent resistance.23 The first patient received previous treatment with aspirin (100 mg per day) and clopidogrel (75 mg per day) for 5 days before the procedure, but the antiplatelet response in this patient was unknown because there was no aggregometry analysis method at that time. More recently, in the second patient, an aggregometry test showed clopidogrel resistance, so the aspirin dose was increased empirically to 300 mg per day. However, stent thrombosis occurred.
One patient presented with hemicranial and neck pain, ptosis, and enophthalmos during angioplasty before stent placement. Despite this event, the stent was deployed and the procedure was concluded. An urgent CT scan was performed showing gyral enhancement. A second scan 24 hours later showed no abnormalities. This episode was probably due to arterial dissection provoked during the predilation with the angioplasty balloon.
Two TIAs occurred in the first 24 hours after the procedure and the other 2 cases, within 30 days. Except for these 2 peri-interventional TIAs, we think complications would not have avoided with the use of cerebral protection devices.
The main mechanism of stroke in patients with atherosclerosis of the large arteries is thromboembolism from unstable plaques. The plaque rupture causes platelet aggregation, local thrombosis, or plaque material embolism.24 Although other mechanisms such as low-flow hemodynamic stroke are uncommon, they can have a synergic effect added to embolism.25 The goal of treatment should be the protection of the embolic source, whereas the correction of the hemodynamic problem has less relevance. Although a residual stenosis <30% after stent placemen has usually been regarded as adequate, the degree of stenosis correction necessary to reduce the risk of further embolic events is still unclear.26
Embolism can occur both in CEA and in CAS.27,28 Embolism related to the procedure can be assessed by transcranial Doppler sonography and cerebral MR imaging with DWI. However, there is no consensus regarding the true incidence and relevance of these events. Correlation between the number of microembolism signals registered by transcranial Doppler and the appearance of new ischemic lesions in DWI is unclear.29 In addition, most of these ischemic foci are clinically silent. Some authors have found a higher rate of microembolism by transcranial Doppler in CAS with protective devices than in CASWPD.30 A higher rate of cerebral ischemic lesions on DWI associated with the use of protection devices has also been found.31,32 Finally, it has been estimated that cerebral protection devices prevent only 25% of clinical embolisms.33 Therefore, it is reasonable to question their usefulness.
Embolism is related to the instrumentation and manipulation of the plaque and may occur during all of the procedural phases of CAS, including cerebral protection device introduction, placement, and removal.34 Therefore, it is very important to reduce the maneuvers as much as possible. Less manipulation with the guidewire or the catheter and the minimizing of balloon dilation reduces the number of emboli detected on transcranial Doppler.35 The passing of the protection device through the stenosis is really an unprotected maneuver and sometimes requires predilation, which may increase the risk of embolism or may cause other complications such as vasospasm or dissection. In addition, device withdrawal involves more manipulation, which increases the embolism risk.
CASWPD is a simple procedure that minimizes the manipulation over the plaque. We believe that the use of low-profile stent-device systems allows advancing through the stenosis with minimal risk of embolism. The placement of a closed stent cell represents a protection system against periprocedural embolism, especially during the dilation with a balloon after stent deployment.
When stent placement in 1 step is not possible from the beginning, a predilation with an angioplasty balloon represents the most critical point of the technique. The need for predilation is variable according to reports (2%–46%),11–13 suggesting that it depends heavily on the experience and methodology of the operator. According to our experience, it is possible to navigate with the stent device system without predilation in most cases. Predilation was only performed in 26/140 (18.7%) stenoses of >90%. In contrast to other authors,14 we have observed that the degree of residual stenosis after stent placement requires further dilation in most cases. This is probably because of a higher initial average stenosis degree in our series. However, postdilation was not associated with complication increase. We believe that closed-cell stents have a protective effect during postdilation.
In all cases, dilation maneuvers were minimized, not only to avoid embolic complications but also to prevent vagal reactions.36 These events are usually treated with atropine even in a prophylactic mode.37 We do not support the indiscriminate use of atropine, to avoid its adverse effects (tachycardia, gastrointestinal disturbances, bronchoconstriction, blurred vision, dizziness, and photophobia). In an empirical way, we controlled the arterial pressure and cardiac rate during the procedure and treated with urapidil and beta blockers only when it was necessary. In our series, we administered atropine in only 2.7% of the procedures.
According to the literature, in long-term follow-up, there are no significant differences between CAS and CEA.38 The results should probably be independent of the use of cerebral protective devices. In our series, the 1-year mortality rate was 2.7%. Only 1 death was related to a new stroke but involved a different vascular territory from the treated carotid artery. The other deaths were due to comorbidity and showed no temporal relation with the procedure.
Restenosis was observed in 12.8% of cases; most were asymptomatic. This finding is not comparable with that in other groups because the diagnosis depends on the criteria used in Doppler sonography. In this report, because the study was long, data were reanalyzed, establishing a peak systolic velocity of >300 cm/s or an end-diastolic velocity >90 cm/s, as in-stent restenosis >70%.39 Restenosis is related to intimal hyperplasia, the progression of atherosclerosis, the control of cardiovascular risk factors, and the appropriate treatment with antiplatelet therapy. The antiplatelet therapy effect and its resistance could not be evaluated from the beginning of the study because the impedance aggregometry (Verify Now system; Accumetrics, San Diego, California) was not available until September 2009. Since then, all candidates for carotid stent placement are subjected to aggregometry by using this device. Sixty-eight patients were evaluated. Clopidogrel resistance was observed in 33%, and aspirin resistance, in 13%. Only 1 patient (1.5%) was resistant to both drugs. Because antiplatelet resistance was not evaluated from the beginning, a new study is being performed to assess the influence of the aggregometry test and the appropriate long-term treatment regimen in these patients.
Limitations
Results obtained in this study have been compared with the ones in the literature. The study period was long due to such restrictive inclusion criteria. Despite being so long, the stent placement technique has always been the same.
Inclusion of patients with symptomatic stenosis ≥50% would have increased the number of patients and would have shortened the study period. However, we believed that it was necessary to achieve the results proposed in the reference guides.40 The maximum complications rate in this report was <6% recommended for symptomatic patients.
Asymptomatic patients were also excluded, because the benefit of CAS in this group is still unclear.41,42 Concerning stent placement in these patients, one should consider other risk criteria and not only the stenosis degree.43
Conclusions
In this series, unprotected CAS in patients with symptomatic high-grade stenosis was a safe technique with low risk of periprocedural complications. Adequate clinical indications, the use of closed-cell stents, and minimizing of maneuvers through the stenosis are needed to obtain optimal results.
ABBREVIATIONS:
- CAS
carotid artery stent placement
- CEA
carotid endarterectomy
- CASWPD
carotid artery stent placement without distal protection device
- CAVATAS
Carotid and Vertebral Artery Transluminal Angioplasty Study
- MI
myocardial infarction
- SPACE
Stent Protected Angioplasty versus Carotid Endarterectomy study
References
- 1. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–53 [DOI] [PubMed] [Google Scholar]
- 2. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99) or with mild (0–29%) carotid stenosis: European Carotid Surgery Trialists' Collaborative Group. Lancet 1991;337:1235–43 [PubMed] [Google Scholar]
- 3. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet 2001;357:1729–37 [PubMed] [Google Scholar]
- 4. Mas JL, Chatellier G, Beyssen B, for the EVA-3S Investigators . Carotid angioplasty and stenting with or without cerebral protection: Clinical alert from endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (EVAS-3S) trial. Stroke 2004;35:e18–20. Epub 2003 Dec 4 [DOI] [PubMed] [Google Scholar]
- 5. Ringleb PA, Allenberg J, Brückmann H, et al. for the SPACE Collaborative Group. 30-day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomized non-inferiority trial. Lancet 2006;368:1239–47. Erratum in: Lancet. 2006 Oct 7 [DOI] [PubMed] [Google Scholar]
- 6. Silver B. Stenting versus endarterectomy treatment of carotid artery stenosis. N Engl J Med 2010;363:11–23. Author reply 1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ederle J, Dobson J, Featherstone RL, et al. for the International Carotid Stenting Study investigators. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Artery Stenting Study): an interim analysis of randomised controlled trial. Lancet 2010;375:985–97. Epub 2010 Feb 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kastrup A, Groschel K, Krapf H, et al. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke 2003;34:813–19 [DOI] [PubMed] [Google Scholar]
- 9. Garg N, Karagiorgos N, Pisimisis T, et al. Cerebral protection devices reduce periprocedural strokes during carotid angioplasty and stenting: a systematic review of the current literature. J Endovas Ther 2009;16:412–27 [DOI] [PubMed] [Google Scholar]
- 10. Jansen O, Fiehler J, Hartman M, et al. Protection or non-protection in carotid stent angioplasty: the influence of interventional techniques on outcome data from the SPACE trial. Stroke 2009;40:841–46 [DOI] [PubMed] [Google Scholar]
- 11. Maynard M, Baldi S, Rostagno R, et al. Carotid stenting without use of balloon angioplasty and distal protection devices: preliminary experience in 100 cases. AJNR Am J Neuroradiol 2007;28:1378–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tietke MW, Kerby T, Alfke K, et al. Complication rate in unprotected carotid artery stenting with closed-cell stents. Neuroradiology 2010;52:611–18 [DOI] [PubMed] [Google Scholar]
- 13. Mohammadian R, Sohrabi B, Mansourizadeh R, et al. Unprotected carotid artery stenting: complications in 6 months follow-up. Neuroradiology 2012;54:225–30 [DOI] [PubMed] [Google Scholar]
- 14. Baldi S, Zander T, Rabellino M, et al. Carotid artery stenting without angioplasty and cerebral protection: a single-center experience with up to 7 years' follow-up. AJNR Am J Neuroradiol 2011;32:759–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mansour OY, Weber J, Niesen W, et al. Carotid angioplasty and stenting without protection devices: safety and efficacy concerns—single center experience. Clin Neuroradiol 2011;2:65–73 [DOI] [PubMed] [Google Scholar]
- 16. Oates CP, Naylor AR, Hartshorne T, et al. Joint recommendations for reporting carotid ultrasound investigations in the United Kingdom. Eur J Vasc Endovasc Surg 2009;37:251–61 [DOI] [PubMed] [Google Scholar]
- 17. Tahmasebpour HR, Buckley AR, Cooperberg PL, et al. Sonographic examination of the carotid arteries. Radiographics 2005;25:1561–75 [DOI] [PubMed] [Google Scholar]
- 18. Touzé M, Trinquart M, Chatellier G, et al. Systematic review of the perioperative risks of stroke or death after carotid angioplasty and stenting. Stroke 2009;40:e683–93. Epub 2009 Nov 5 [DOI] [PubMed] [Google Scholar]
- 19. Ogasawara K, Sakai N, Kuroiwa T, et al. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 2007;107:1130–36 [DOI] [PubMed] [Google Scholar]
- 20. Timaran CH, Veith FJ, Rosero EB, et al. Intracranial hemorrhage after carotid endarterectomy and carotid stenting in the United States in 2005. J Vasc Surg 2009;49:623–29 [DOI] [PubMed] [Google Scholar]
- 21. van Mook WN, Rennenberg RJ, Schurink GW, et al. Cerebral hyperperfusion syndrome. Lancet Neurol 2005;4:877–88 [DOI] [PubMed] [Google Scholar]
- 22. Chaturvedi S, Shorab S, Teslis A. Carotid stent thrombosis: report of 2 fatal cases. Stroke 2001;32:2700–02 [PubMed] [Google Scholar]
- 23. Nicosia A, Nikas D, Castriota F. et al. Classification for carotid artery stenting complications: manifestation, management and prevention. J Endovasc Ther 2010;17:275–94 [DOI] [PubMed] [Google Scholar]
- 24. Beal MF, Williams RS, Richardson EP, et al. Cholesterol embolism as a cause of transient ischemic attacks and cerebral infarction. Neurology 1981;31:860–65 [DOI] [PubMed] [Google Scholar]
- 25. Derdeyn CP. Mechanisms of ischemic stroke secondary to large artery atherosclerotic disease. Neuroimaging Clin N Am 2007;17:303–11 [DOI] [PubMed] [Google Scholar]
- 26. Yadav JS, Wholey MH, Kuntz RE, et al. , for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–501 [DOI] [PubMed] [Google Scholar]
- 27. Malik RK, Landis GS, Sundick S, et al. Predicting embolic potential during carotid angioplasty and stenting: analysis of captured particulate debris, ultrasound characteristics, and prior carotid endarterectomy. J Vasc Surg 2010;51:317–22 [DOI] [PubMed] [Google Scholar]
- 28. Green DW, Sanchez LA, Parodi JC, et al. Acute thromboembolic events during carotid artery angioplasty and stenting: etiology and a technique of neurorescue. J Endovasc Ther 2005;12:360–65 [DOI] [PubMed] [Google Scholar]
- 29. Piñero P, González A, Mayol A, et al. Silent ischemia after neuroprotected percutaneous carotid stenting: a diffusion-weighted MRI study. AJNR Am J Neuroradiol 2006;27:1338–45 [PMC free article] [PubMed] [Google Scholar]
- 30. Vos JA, van den Berg JC, Ernst SM, et al. Carotid angioplasty and stent placement: comparison of transcranial Doppler US data and clinical outcome with and without filtering cerebral protection devices in 509 patients. Radiology 2005;234:493–99 [DOI] [PubMed] [Google Scholar]
- 31. Macdonald S, Evans DH, Griffiths PD, et al. Filter-protected versus unprotected carotid artery stenting: a randomized trial. Cerebrovasc Dis 2010;29:282–89. Epub 2010 Jan 15 [DOI] [PubMed] [Google Scholar]
- 32. Bonati LH, Jongen LM, Haller S, et al. , for the ICSS-MRI study group. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol 2010;9:353–62 [DOI] [PubMed] [Google Scholar]
- 33. Cloft HJ. Distal protection: maybe less than you think. AJNR Am J Neuroradiol 2008;29:407–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al-Mubarak N, Roubin GS, Vitek JJ, et al. Effect of the distal-balloon protection system on microembolization during carotid stenting. Circulation 2001;104:1999–2002 [DOI] [PubMed] [Google Scholar]
- 35. Men S, Lownie SP, Pelz DM. Carotid stenting without angioplasty. Can J Neurol Sci 2002;29:175–79 [PubMed] [Google Scholar]
- 36. Nano G, Dalainas I, Bianchi P, et al. Ballooning-induced bradycardia during carotid stenting in primary stenosis and restenosis. Neuroradiology 2006;48:533–36 [DOI] [PubMed] [Google Scholar]
- 37. Cayne NS, Faries PL, Trocciola SM, et al. Carotid angioplasty and stent-induced bradycardia and hypotension: impact of prophylactic atropine administration and prior carotid endarterectomy. J Vasc Surg 2005;41:956–61 [DOI] [PubMed] [Google Scholar]
- 38. Zarins CK, White RA, Diethrich EB, et al. , for the CaRESS Steering Committee and CaRESS Investigators. Carotid revascularization using endarterectomy or stenting systems (CaRESS): 4-year outcomes. J Endovasc Ther 2009;16:397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou W, Felkai DD, Evans M, et al. Ultrasound criteria for severe in-stent restenosis following carotid artery stenting. J Vasc Surg 2008;47:74–80 [DOI] [PubMed] [Google Scholar]
- 40. Moore WS, Barnett HJ, Beebe HG, et al. Guidelines for carotid endarterectomy: a multidisciplinary consensus statement from the ad hoc committee, American Heart Association. Stroke 1995;26:188–201 [DOI] [PubMed] [Google Scholar]
- 41. Halliday A, Mansfield A, Marro J, et al. , for the MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004;363:1491–502 [DOI] [PubMed] [Google Scholar]
- 42. Lanzino G, Rabinstein AA, Brown RD. Treatment of carotid artery stenosis: medical therapy, surgery, or stenting. Mayo Clin Proc 2009;84:362–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nicolaides AN, Kakkos SK, Griffin M, et al. , for the Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events; results from the ACSRS study. Eur J Vasc Endovasc Surg 2005;30:275–84 [DOI] [PubMed] [Google Scholar]