Abstract
BACKGROUND AND PURPOSE:
The microcatheter protective technique positions an additional microcatheter in the parent or side-branching artery to protect it during coil embolization. The purpose of this study was to describe this method and to evaluate its efficacy and safety as an alternative to a multiple-microcatheter or balloon- or stent-assisted technique for wide-neck aneurysms.
MATERIALS AND METHODS:
A retrospective review of 74 patients (43 women; mean age, 59.6 years) with 75 wide-neck aneurysms treated with the microcatheter protective technique between January 2003 and April 2010 was performed. Immediate postembolization angiograms were evaluated by using a conventional angiographic scale, and clinical evaluation was performed by using the GOS. Clinical and imaging follow-up were available in 57 (76%) patients, with a mean of 14.7 months.
RESULTS:
Postembolization angiograms demonstrated total occlusion in 45 of 75 (60%) aneurysms, a neck remnant in 17 (22.7%), and body filling in 13 (17.3%). The technique-related complication rate was 17.4% (13/75), and the procedural-related morbidity rate was 1.3% (1/74). All patients, except 3 complicated cases with a GOS of <4, had a GOS of 5 at the end of the study period. Of the 57 aneurysms with follow-up, recanalization developed in 5 (8.8%) aneurysms, and 3 (5.3%) cases of major recanalization were re-treated endovascularly.
CONCLUSIONS:
The microcatheter protective technique is feasible and safe for coil embolization of wide-neck aneurysms, especially in cases that are not suitable for multiple catheter or balloon- or stent-assisted techniques.
Despite advances in devices and techniques, coil embolization of wide-neck aneurysms remains a technical challenge. Neck-remodeling techniques with balloon microcatheters or stents were previously used to treat wide-neck aneurysms.1–3 Although these techniques may be effective for aneurysmal neck protection during endovascular treatment, there are some technical difficulties in performing them, as well as disadvantages and limitations.4,5
For several years, we have used the microcatheter protective technique, which means positioning an additional microcatheter in the parent or side-branching artery to protect it, for coil embolization of wide-neck aneurysms. The key concept is making a stable coil frame under the protection of a microcatheter. In the present study, we describe this method and evaluate its efficacy and safety as an alternative to a multiple-catheter and balloon- or stent-assisted technique for wide-neck aneurysm.
Materials and Methods
From January 2003 to April 2010, 74 patients with 75 aneurysms were treated with the microcatheter protective technique at Seoul National University Hospital. Of the 74 patients, 43 were women and 31 were men. The mean age of the patients was 59.6 years (range, 23–79 years). Ten (13.5%) of 74 patients with 10 aneurysms presented with SAH.
Biplane Integris Allura angiography units (Phillips Healthcare, Best, the Netherlands) were used. The size of each aneurysm and neck was measured in 3D angiographic images by using the angiography unit software. The locations of aneurysms were as follows: middle cerebral artery (24/75, 32%), anterior communicating/anterior cerebral arteries (18, 24%), basilar artery bifurcation (13, 17.3%), posterior communicating artery (9, 12%), anterior choroidal artery (5, 6.7%), internal carotid artery bifurcation (3, 4%), vertebral/posterior inferior cerebellar arteries (2, 2.6%), and posterior cerebral artery (1, 1.3%). Sixty-five (86.7%) of 75 aneurysms were unruptured. The mean aneurysm neck size was 3.9 mm (range, 1.9–13.8 mm), and the mean dome-to-neck ratio was 1:0 (range, 0.7–2.8). Forty-two (56%) of 75 aneurysms had a neck of <4 mm; however, all of these aneurysms had a dome-to-neck ratio of <2.
In all patients, coil embolization was performed as the primary treatment. The microcatheter protective technique was chosen after the failure of a conventional single-catheter or multiple-microcatheter technique in 29 (38.7%) aneurysms. In the remaining 46 (61.3%) aneurysms, the microcatheter protective technique was adopted from the beginning. Immediate postembolization angiography was performed in all patients and was evaluated by using a conventional angiographic scale: total occlusion, neck remnant, or aneurysmal body filling.6 Simultaneously, vPD was calculated with a previously reported equation.7 The vPD of >24% was considered a compact coil mesh.
Clinical outcome evaluations were performed by using a GOS8: 5, good recovery, able to return to work or school; 4, moderate disability, able to live independently, however unable to work or go to school; 3, severe disability, able to follow commands, unable to live independently; 2, vegetative status, unable to interact with the environment; 1, dead.
Imaging follow-up by using plain radiography, MR angiography, or DSA and clinical follow-up was available in 57(80.3%) of 74 patients, with a mean of 14.7 months (range, 3–36 months). “Minor aneurysm recanalization” was defined as minimal coil compaction at the aneurysm neck, and “major recanalization” was defined as contrast filling within the aneurysm dome or significant coil loosening or compaction.
Microcatheter Protective Technique
All procedures were performed with the patient under general anesthesia. Systemic heparinization was given during the procedure as follows: A bolus of 3000 U of heparin was administered after femoral sheath placement in the case of an unruptured aneurysm and 2000 U after protection of the ruptured part in the case of a ruptured aneurysm. Then, intermittent boluses of 1000 U/h of heparin were administered during the entire procedure. Since March 2006, clopidogrel has been administered to patients with unruptured aneurysms before the procedure in our institution, as described previously.9 In the present study, 61 (82.4%) of 74 patients received clopidogrel before the procedure. In most cases, the antiplatelet agent was discontinued immediately after the procedure. On the other hand, no antiplatelet premedication was administered to 2 (2.7%) patients with unruptured aneurysms at the earlier period of March 2006 and in 11 (14.9%) patients with ruptured aneurysms. In all cases, the microcatheters were inserted through a single 6F guiding catheter. All microcatheters inserted through a guiding catheter were flushed continuously with heparinized saline by using a pressurized bag.
The key concept of the microcatheter protective technique is to obtain a stable coil frame with the microcatheter under the protection of a parent or side-branching artery. Under the fluoroscopic and roadmap guidance, a coil-delivering microcatheter is introduced to a selected aneurysm. Simultaneously, an additional protective microcatheter is positioned over the aneurysm neck (Fig 1) or is introduced into the side-branching artery (Figs 2 and 3). When there are some difficulties in selecting a side-branching artery, the tip of the protective microcatheter is positioned at the orifice of the side-branching artery.10 Thereafter, detachable platinum coils of appropriate size, length, and shape are introduced and deployed through ≥1 coil-delivering microcatheter placed in the aneurysmal sac. During frame-coil deployment, appropriate tension of a protective microcatheter is needed to avoid coil protrusion into the parent or side-branching artery. If necessary, a microwire can be positioned within a microcatheter to achieve higher tension of a coil mesh (Fig 1).
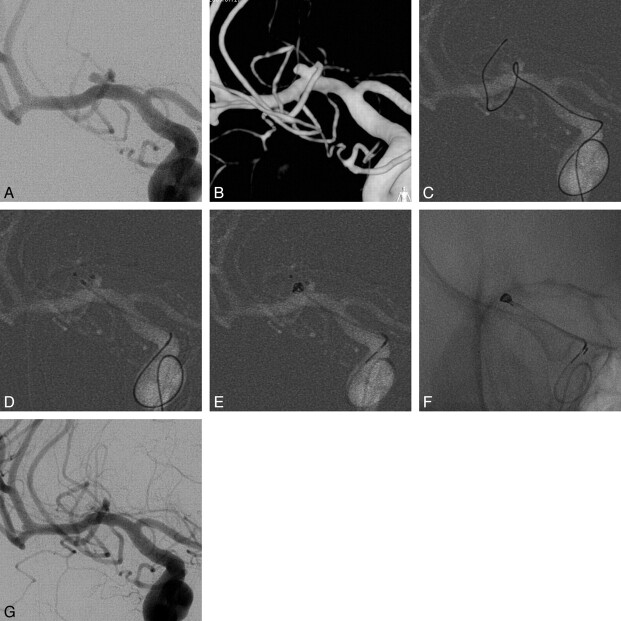
Fig 1.
A 51-year-old female patient with an unruptured aneurysm of the AcomA. A, Right internal carotid angiogram shows a wide-neck aneurysm of the AcomA. B, Clear aneurysmal configurations are seen in the 3D image (1.9 × 3.0 × 1.6 mm). C, A coil-delivering catheter is positioned within the aneurysm. Simultaneously, a protective microcatheter is introduced into the proximal segment of the ipsilateral A2. D, The first coil (Trufill Orbit Mini Complex, Cordis, 2 × 4 cm) is deployed via a coil-delivering microcatheter. A microwire remains in a protective microcatheter to apply higher tension to the frame coil. E, Angiogram obtained immediately after embolization shows total occlusion of the aneurysmal sac.
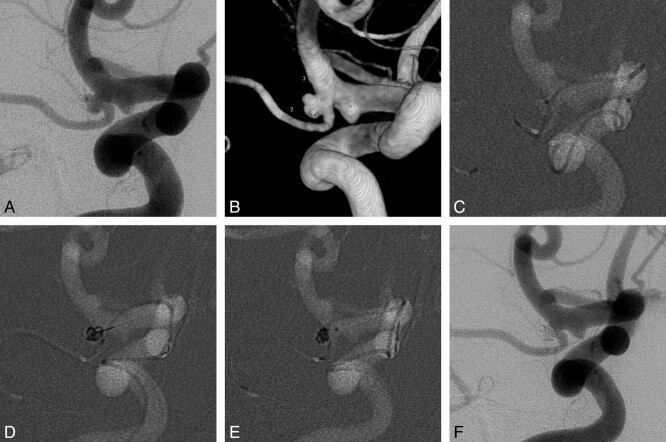
Fig 2.
A 52-year-old female patient with a middle cerebral artery (M1) aneurysm. A, Right internal carotid angiogram shows a wide-neck aneurysm of the M1 segment. B, The aneurysmal configurations are visible in a 3D image (2.1 × 2.3 × 3.9 mm). C, A protective microcatheter is introduced into the anterior temporal branch along with the microwire. D, A coil-delivering microcatheter is positioned within the aneurysm. E, The first frame coil (Trufill Orbit Mini Complex 2 × 3 cm) is deployed via a coil-delivering microcatheter, and the anterior temporal branch is preserved. F, Although the first coil was fully deployed without coil protrusion into the parent artery, the first coil frame is still unstable. Therefore, the first coil remains undetached. To achieve coil stability with the second coil, an additional coil-delivering microcatheter is required. Accordingly, the protective microcatheter is retrieved, and it is repositioned into the aneurysmal sac to use as a coil-delivering microcatheter for the second coil. After that, a final coil (Axium Helical, ev3, Irvine, California; 1.5 × 2 cm) is deployed through the repositioned protective microcatheter by using a double-microcatheter technique. G, Completion angiogram obtained immediately after embolization shows total occlusion of the aneurysmal sac preserving the anterior temporal branch.
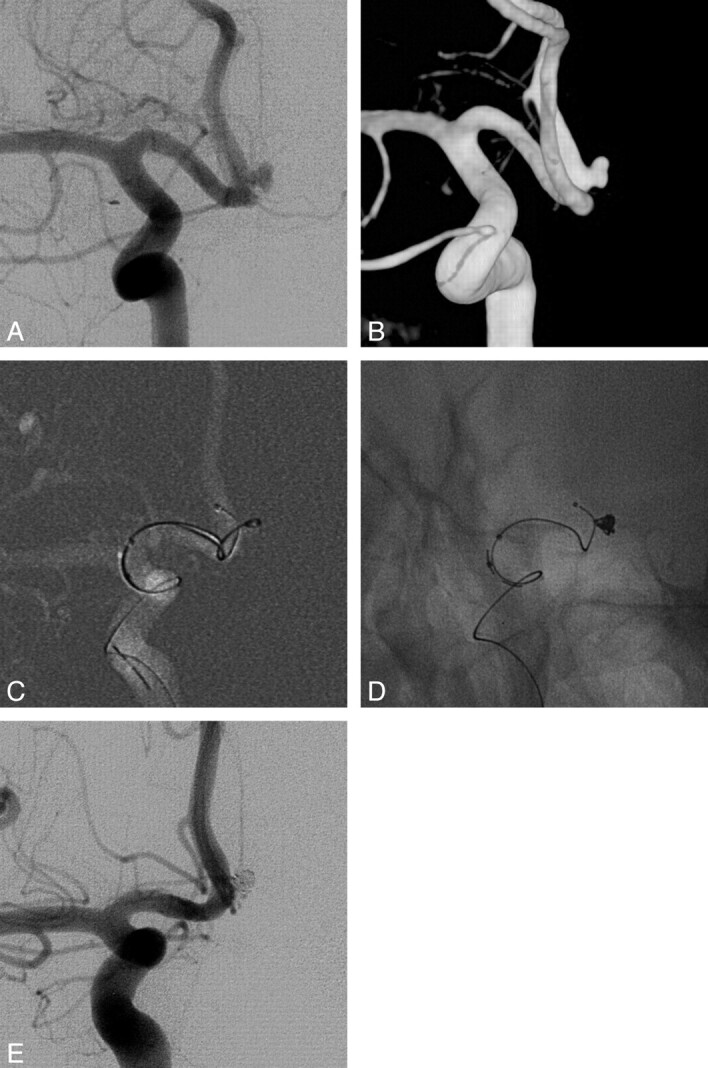
Fig 3.
A 54-year-old male patient with a right anterior choroidal artery aneurysm. A, Right internal carotid angiogram shows a wide-neck aneurysm of the anterior choroidal artery. B, The aneurysmal configurations are visible in the 3D image (2.4 × 3.7 × 2.3 mm). C, A coil-delivering microcatheter is positioned within the aneurysm. A protective microcatheter is introduced into the hyperplastic anterior choroidal artery. D, The first frame coil (Trufill Orbit Mini Complex, 2.5 × 3.5 cm) is deployed via a coil-delivering microcatheter. The coil protrudes and partially compromises the anterior choroidal artery. E, A protective microcatheter applies tension to the frame coil. After deployment of 1 more coil (Axium Helical, 1.5 × 2 cm), a stable coil mass is achieved. F, Angiogram obtained immediately after embolization shows total occlusion of the aneurysmal sac with a preserved anterior choroidal artery.
The tension of the protective microcatheter is released before its removal to confirm the stability of the frame coil. After achieving a stable coil frame, the protective microcatheter is retrieved and additional coils are deployed through the coil-delivering microcatheter. If the coil frame is not stable, the protective microcatheter could remain until a stable coil mesh is made. If necessary, to maintain the stability of the coil mass or an anticipated coil protrusion due to coils deployed subsequently, an additional coil-delivering microcatheter can be used to select and fill an aneurysm with coils under the maintenance of a protective microcatheter. In the present study, a protective microcatheter technique was applied to protect a parent artery in 56 (74.7%) of 75 aneurysms, and it was performed to protect side-branching arteries in 19 (25.3%) aneurysms.
Results
All aneurysms were successfully embolized. Immediate postembolization angiograms demonstrated total occlusion in 45 (60%) of 75 aneurysms, neck remnants in 17 (22.7%), and body filling in 13 (17.3%). The mean vPD was 34.4%, ranging from 17% to 56%. Compact coil mesh was achieved in 69 (92%) of 75 aneurysms.
Procedure-related complications developed in 13 (17.4%) of 75 aneurysms: 11 (14.6%) thromboembolic events and 2 (2.7%) coil protrusions without a subsequent thromboembolic complication. Two (2.7%) of 11 thromboembolic complications were symptomatic, and a permanent neurologic deficit developed in 1 (1.3%) of 75 aneurysms. One symptomatic thromboembolic complication was a ruptured AcomA aneurysm. The immediate postembolization angiogram of this patient showed occlusion of the inferior division of the middle cerebral artery. Even though mechanical and chemical thrombolysis was intensively performed by using 10-sized microcatheter, 10-sized microware, and intra-arterial infusion of tirofiban, we did not achieve recanalization of the occluded major branch. As a result, permanent sensory dysphagia developed. In the other case, a weakness of a lower extremity developed following coil embolization of a ruptured AcomA aneurysm. The completion angiogram showed thrombus formation around the deployed coil without occlusion of the arterial branch. To resolve the clot, we performed an intra-arterial infusion of tirofiban. Thereafter, the clot was completely resolved.
After the procedure, however, the patient had a weakness of the right lower extremity. One day after the procedure, follow-up angiography was performed to verify thrombus formation around the deployed coil or occlusion of arterial branches. It revealed a vascular blush and early venous opacification in the region of the left posterior internal frontal area without thrombus formation or arterial occlusion. The weakness was fully recovered 6 weeks after the procedure. In 8 of 9 cases of asymptomatic thromboembolic complications, thrombi were found around the deployed coil at the end of the procedure, but they resolved with an intra-arterial infusion of tirofiban. The other case was complicated by a silent superior cerebellar infarction following coil embolization of a ruptured basilar tip aneurysm. Two of these 9 cases presented with SAH. There was no significant difference in the rate of thromboembolic events between parent artery protecting and side-branching artery protecting groups (14.3% versus 15.8%, P = .57, Fisher exact test).
In 2 cases of coil protrusion, part of the loop of the previously deployed coil protruded into the parent artery during the deployment of the subsequent coil, but the coil loop was small and did not interfere with blood flow or cause any thromboembolic complication. Neither aneurysmal rupture nor occlusion of the parent or side-branching artery occurred.
One (1.3%) of 74 patients died (GOS = 1). This 70-year-old female patient presented with cardiac arrest, and she was successfully resuscitated. After that, a brain CT established the diagnosis of SAH with intraventricular hemorrhage (HH scale grade, V; Fisher grade, IV). After an EVD insertion, DSA was performed and showed a ruptured basilar tip aneurysm. Coil embolization was performed promptly. Twelve days after treatment, however, she died.
One other patient (1.3%) was severely disabled (GOS = 3). On arrival, this 53-year-old male patient was stuporous. A CT revealed SAH with hydrocephalus (HH scale grade, IV). After an EVD insertion, DSA was performed, and an immediate coil embolization of a ruptured basilar tip aneurysm was performed. Sixteen days after treatment, due to vasospasm, a grade II motor weakness of the left lower extremity developed. His neurologic status has not improved at 14 weeks after treatment. Procedure-related complications did not develop in these 2 cases.
Ultimately, procedure-related morbidity occurred in 1 of 74 (1.3%) cases, resulting in permanent sensory dysphagia (GOS = 4). The poor clinical outcomes of 2 cases (GOS = 1 and 3) mentioned above were not associated with procedure-related complication, but SAH itself. All patients, except these 3, showed excellent clinical outcomes (GOS = 5).
Of the 57 aneurysms with follow-up, recanalization developed in 5 (8.8%) aneurysms, and 3 (5.3%) cases of major recanalization were retreated by endovascular procedures.
Discussion
The microcatheter protective technique has several advantages over the multiple-microcatheter or balloon- or stent-assisted techniques. First, this method is technically simple. No additional femoral puncture is required, and use of one 6F guiding catheter is conventional. The only difference is the use of an additional protective microcatheter, and this is not technically demanding. Because the microcatheter was constructed with stainless steel−braided, nitinol-braided, or platinum-coiled segments, depending on the type and section of microcatheters, it would have sufficient force to resist protrusion of the coil loops. If necessary, a microwire can be inserted into a microcatheter to apply stronger tension to a protruded coil loop or coil mesh.
Another advantage of the microcatheter protective technique is that a microcatheter is readily available for navigating in small or narrowed distal tortuous arteries. The profiles of compliant balloon microcatheters are 2.3F to 3.5F, depending on the type of device; and the delivery catheter Neuroform stent (Boston Scientific, Natick, Massachusetts) is 3F. These large-profile devices have risks in navigating difficult arteries. Recently, the Enterprise self-expending stent (Cordis, Miami Lakes, Florida) has become available for stent-assisted coil embolization. It needs a 0.021-inch delivery microcatheter. Although the profile of the Enterprise delivery catheter is much smaller than other stent-delivery devices, it is more dangerous when navigating difficult arteries than the 10- or 14-sized series microcatheters.
Sometimes, microcatheter exchange is needed to position a 0.021-inch microcatheter at the proper site for deployment of the Enterprise stent (Cordis). These large-profile devices have more risk of developing procedural complications such as rupture of the parent artery, intimal injury, and procedure-related thromboembolic complications.1,4,5 In particular, there is minimal space for the deployment of the large-profile protective device at an acute angle between the aneurysm and the parent artery.11 Such cases are frequently observed at the ophthalmic artery, posterior communicating artery, anterior choroidal artery, and anterior temporal artery arising at the M1 segment. This technique would be a very effective alternative treatment for aneurysms of these arteries. In addition, small and wide-neck aneurysms (maximal diameter, <4 mm; dome-to-neck ratio, <1.5), especially located at the distal segment beyond the circle of Willis, are difficult to embolize by using a multiple-microcatheter technique.12,13 A large part of the aneurysm could be occupied by tips of microcatheters, and these would disturb deployment of coils. In this case, the microcatheter protective technique would be a good substitution.
The other advantage is that a pre- or postprocedural antiplatelet aggregation therapy is not necessarily required. In case of a ruptured aneurysm, the risk of rebleeding would be increased by preprocedural antiplatelet preparation.14 Moreover, further surgical procedures, such as external ventricular drainage or decompressive craniectomy, could be required after endovascular treatment. In this case, postprocedural antiplatelet aggregation therapy is unfavorable. However, the stent-assisted technique requires pre- or postprocedural antiplatelet aggregation therapy.15,16
With the use of ≥2 microcatheters in the artery, an increased risk of thromboembolic complications is expected in addition to all the usual risks associated with other protective device−assisted endovascular treatments of wide-neck aneurysms. The current study presents 11 (14.6%) cases of procedure-related thromboembolic complications. Among these, a permanent neurologic deficit due to a thromboembolic complication developed in 1 (1.3%) patient who presented with SAH. Usually, the overall incidence of thromboembolic complications with balloon- and stent-assisted techniques ranges from 4% to 14%4,17,18 and from 0% to 21%, respectively.15,16,19–21 In the present study, 2 symptomatic thromboembolic complications developed in patients presenting with SAH, which is a hypercoagulable state in which the likelihood of thrombosis is high.22 This may be reflected in the development of symptomatic thromboembolic complications. In addition, aneurysms in the current study are wide-neck aneurysms and have more risk of thromboembolic complications than other aneurysms.18 Ultimately, the overall risk of thromboembolic complications with the microcatheter protective technique would not be higher than that with the other protective device−assisted techniques. Ultimately, this technique would provide direct protection of the aneurysmal neck, such as in the balloon- or stent-assisted technique, by using a low-profile device with minimal procedure-related complications.
On the other hand, the microcatheter protective technique has a limitation compared with the balloon- or stent-assisted techniques. The present study shows a relatively high rate of incomplete occlusion of aneurysms (17.3%) compared with the balloon-remodeling technique.1 Therefore, a microcatheter could not provide reliable and robust parent artery protection compared with a balloon or stent. For this reason, this technique could not be applied in every case of wide-neck aneurysms. When the aneurysm neck is too large to protect with a microcatheter, this technique could not be used. In addition, in case of an aneurysm arising from a large-diameter parent artery, such as a paraclinoid aneurysm, a protective microcatheter could not be steadily positioned across the aneurysmal neck. This problem leads to coil instability within the aneurysm or protrusion of coil loops on the parent artery. However, this technique would be a useful alternative when there are problems using the balloon- or stent-assisted techniques due to difficult anatomies, such as tortuous parent arteries, which are risky to navigate with these protective devices, or small side-branching arteries that could not be preserved by a balloon or stent.
The protective microcatheter is used for the parent artery or side-branching artery. Because a side-branching artery is usually narrower, more tortuous, and more susceptible to being injured by a microcatheter or microwire than a parent artery, it would be inferred that the risk of thromboembolic complications would increase in the group with side-branching artery protection. However, there was no significant difference in risk between the parent artery and side-branching artery protection groups. This is likely related to technical considerations—that is, a protective microcathter is not wedged into the artery to preserve antegrade blood flow of a side-branching artery and is always flushed with heparinized saline during the entire procedure.
Conclusions
Our experience with 74 patients with 75 wide-neck aneurysms treated by using the microcatheter protective technique shows that it is feasible and safe for coil embolization of wide-neck aneurysms. No specific additional device requiring special education is used. It simply involves introduction of ≥1 microcatheter into the parent or side-branching artery. Especially in cases of wide-neck aneurysms, which are not suitable for other protective techniques, it is a reliable alternative method for endovascular treatment of these aneurysms with an acceptable procedural-related complication rate.
Abbreviations
- AcomA
anterior communicating artery
- DSA
digital subtraction angiography
- EVD
external ventricular drain
- GOS
Glasgow Outcome Scale
- Hunt and Hess
HH
- SAH
subarachnoid hemorrhage
- vPD
volumetric packing density
References
- 1. Shapiro M, Babb J, Becske T, et al. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: a literature review. AJNR Am J Neuroradiol 2008; 29: 1777–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biondi A, Janardhan V, Katz JM, et al. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery 2007; 61: 460–68, discussion 468–69 [DOI] [PubMed] [Google Scholar]
- 3. Higashida RT, Halbach VV, Dowd CF, et al. Initial clinical experience with a new self-expanding nitinol stent for the treatment of intracranial cerebral aneurysms: the Cordis Enterprise stent. AJNR Am J Neuroradiol 2005; 26: 1751–56 [PMC free article] [PubMed] [Google Scholar]
- 4. Sluzewski M, van Rooij WJ, Beute GN, et al. Balloon-assisted coil embolization of intracranial aneurysms: incidence, complications, and angiography results. J Neurosurg 2006; 105: 396–99 [DOI] [PubMed] [Google Scholar]
- 5. Akpek S, Arat A, Morsi H, et al. Self-expandable stent-assisted coiling of wide-neck intracranial aneurysms: a single-center experience. AJNR Am J Neuroradiol 2005; 26: 1223–31 [PMC free article] [PubMed] [Google Scholar]
- 6. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001; 32: 1998–2004 [DOI] [PubMed] [Google Scholar]
- 7. Piotin M, Daghman B, Mounayer C, et al. Ellipsoid approximation versus 3D rotational angiography in the volumetric assessment of intracranial aneurysms. AJNR Am J Neuroradiol 2006; 27: 839–42 [PMC free article] [PubMed] [Google Scholar]
- 8. Jennett B, Snoek J, Bond MR, et al. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry 1981; 44: 285–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang HS, Kwon BJ, Kim JE, et al. Preinterventional clopidogrel response variability for coil embolization of intracranial aneurysms: clinical implications. AJNR Am J Neuroradiol 2010; 31: 1206–10. Epub 2010 Mar 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kang HS, Kwon BJ, Kwon OK, et al. Endovascular coil embolization of anterior choroidal artery aneurysms: clinical article. J Neurosurg 2009; 111: 963–69 [DOI] [PubMed] [Google Scholar]
- 11. Ihn YK, Kim DI, Kim BS, et al. Utility of catheter-assisted Guglielmi detachable coiling in the treatment of wide-necked aneurysms. Acta Neurochir (Wien) 2006; 148: 1045–52, discussion 1052 [DOI] [PubMed] [Google Scholar]
- 12. Im SH, Han MH, Kwon OK, et al. Endovascular coil embolization of 435 small asymptomatic unruptured intracranial aneurysms: procedural morbidity and patient outcome. AJNR Am J Neuroradiol 2009; 30: 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwon OK, Kim SH, Oh CW, et al. Embolization of wide-necked aneurysms with using three or more microcatheters. Acta Neurochir (Wien) 2006; 148: 1139–45, discussion 1145. Epub 2006 Sep 29 [DOI] [PubMed] [Google Scholar]
- 14. Tumialan LM, Zhang YJ, Cawley CM, et al. Intracranial hemorrhage associated with stent-assisted coil embolization of cerebral aneurysms: a cautionary report. J Neurosurg 2008; 108: 1122–29 [DOI] [PubMed] [Google Scholar]
- 15. Benitez RP, Silva MT, Klem J, et al. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery 2004; 54: 1359–67, discussion 1368 [DOI] [PubMed] [Google Scholar]
- 16. Fiorella D, Albuquerque FC, Han P, et al. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery 2004; 54: 6–16 [DOI] [PubMed] [Google Scholar]
- 17. Cottier JP, Pasco A, Gallas S, et al. Utility of balloon-assisted Guglielmi detachable coiling in the treatment of 49 cerebral aneurysms: a retrospective, multicenter study. AJNR Am J Neuroradiol 2001; 22: 345–51 [PMC free article] [PubMed] [Google Scholar]
- 18. Layton KF, Cloft HJ, Gray LA, et al. Balloon-assisted coiling of intracranial aneurysms: evaluation of local thrombus formation and symptomatic thromboembolic complications. AJNR Am J Neuroradiol 2007; 28: 1172–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lubicz B, Francois O, Levivier M, et al. Preliminary experience with the Enterprise stent for endovascular treatment of complex intracranial aneurysms: potential advantages and limiting characteristics. Neurosurgery 2008; 62: 1063–69, discussion 1069–1070 [DOI] [PubMed] [Google Scholar]
- 20. Yahia AM, Gordon V, Whapham J, et al. Complications of Neuroform stent in endovascular treatment of intracranial aneurysms. Neurocrit Care 2008; 8: 19–30 [DOI] [PubMed] [Google Scholar]
- 21. dos Santos Souza MP, Agid R, Willinsky RA, et al. Microstent-assisted coiling for wide-necked intracranial aneurysms. Can J Neurol Sci 2005; 32: 71–81 [DOI] [PubMed] [Google Scholar]
- 22. Fujii Y, Takeuchi S, Sasaki O, et al. Serial changes of hemostasis in aneurysmal subarachnoid hemorrhage with special reference to delayed ischemic neurological deficits. J Neurosurg 1997; 86: 594–602 [DOI] [PubMed] [Google Scholar]