Abstract
BACKGROUND AND PURPOSE:
Injection therapies play a major role in the treatment of lower back pain and are to date performed mainly under CT or fluoroscopic guidance. The benefits of US-guided instillation procedures have been shown in many studies. We conducted this study to simplify an US-guided approach to the lumbar spinal nerves and to assess the feasibility and preliminary accuracy by means of CT and anatomic dissection.
MATERIALS AND METHODS:
Ten US-guided injections at 5 different levels (L1-L5) were performed on 1 embalmed cadaver. Images in 3 sagittal/parasagittal scanning planes were obtained at each lumbar level: 1) the plane of the spinous processes, 2) the plane of the lumbar arches/zygapophyseal-joints, and 3) the plane of the transverse processes. The PAP was then defined by positioning the transducer perpendicularly over the medial part of the respective transverse processes, depicting the hyperechoic intertransverse ligament. In the “in-plane technique,” spinal needles were advanced through the respective segmental intertransverse ligament. A solution consisting of a contrast agent and a pigmented dispersion was subsequently injected into the pararadicular compartment. An anatomic dissection of the specimen and CT scans were performed to verify the exact placement of the needle tips and to evaluate fluid dispersion in the punctured compartment.
RESULTS:
CT examination confirmed that each needle tip was correctly placed within the intended compartment with sufficient contrast accumulation around the respective proximal segment of the spinal nerve. On each anatomic section, dye was identified in the correct compartment and directly around each targeted spinal nerve with needles shown in the correct position.
CONCLUSIONS:
This modified US approach for therapeutic root injections in the lumbar spine by using the intertransverse ligament as a new anatomic landmark allows an easy and correct needle placement within the pararadicular compartment.
Lower back pain and radiculopathy are common conditions and are, at least in part, due to our modern lifestyle. In fact, most individuals will experience neck and/or low back pain at least once in their lives, and with increasing age, a greater number of patients with such symptoms is seen by family physicians and in outpatient clinics.1–4 Aside from physical therapy and other rehabilitative methods, injection therapy targeted to the facet joints or the nerve roots is well established in the treatment of lumbar radiculopathy and has been performed without image guidance for many years. Today minimally invasive imaging-guided techniques have entered the tool box of the pain physician and, because of their ease of use and better success rates, are becoming an integral part of multidisciplinary pain management.5,6
Imaging guidance has increased the precision of spinal injection, and CT or fluoroscopy is, to date, preferentially used.7–10 US guidance for spinal injection is a recent development but, according to the literature, seems promising.11–20 US has proved sufficiently reliable and accurate in the demonstration of lumbar paravertebral anatomy,11–14,21 and the feasibility of US-guided injection therapy at the spine has also been demonstrated in several studies.11–20 Although US is considered reliable for different injection procedures of the lumbar spine,11–14 some disadvantages of the currently proposed methods—particularly in the upper lumbar spine—have been discussed. Aside from bony structures, no reliable landmark has been described for pararadicular injection. This makes targeting with US no better than fluoroscopy—except for a lack of ionizing radiation—which also relies on indirect access to the nerve root by definition of only the bony structures.
We propose the intertransverse ligament as a new anatomic landmark for a more simplified US-guided approach for pararadicular injections in the lumbar spine in this CT-controlled cadaver study.
Materials and Methods
Approval of the institutional review board for the use of a human cadaver in this study was obtained. To achieve a reasonable sonographic tissue similarity to live patients, we ethanol-glycerol–embalmed the cadaver. One radiologic specialist, experienced in interventional musculoskeletal sonography, performed all US-guided interventions according to the protocol specified below. All US examinations and interventions were performed on a standard US device under default settings (iU22; Philips Ultrasound, Bothell, Washington) with a broadband 9- to 4-MHz curved array transducer. CT (Syntec Synergy; GE Healthcare, Vienna, Austria) was subsequently performed to confirm the respective needle positions and fluid spreading. Finally the specimen was dissected to definitely validate fluid spreading and position of the needles.
Sonographic Localization
With the cadaver in a prone position, 3 sagittal/parasagittal scans were obtained to define the necessary anatomic landmarks for the injection as follows:
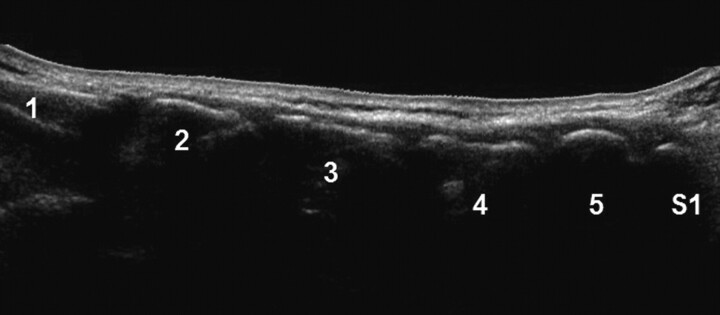
In a midline scan along the spinous processes, the typical transition from the first sacral to the fifth lumbar spinous process was defined (Fig 1). After definition of the fifth lumbar spinous process, the respective spinal segment for the injection was localized by cephalad counting of the spinous processes.
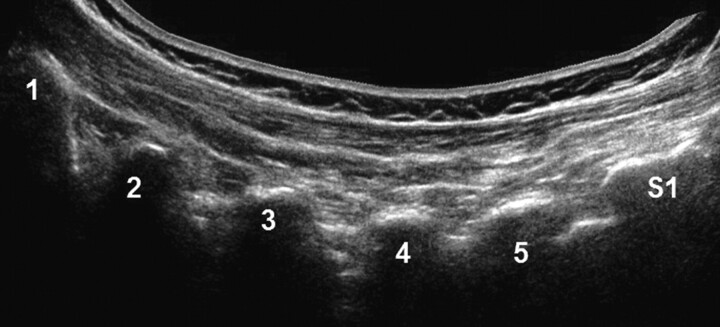
From a midline position over the segment of interest, the transducer was offset laterally in a paravertebral parasagittal orientation toward the transition from the vertebral arch to the zygapophyseal joint (Fig 2).
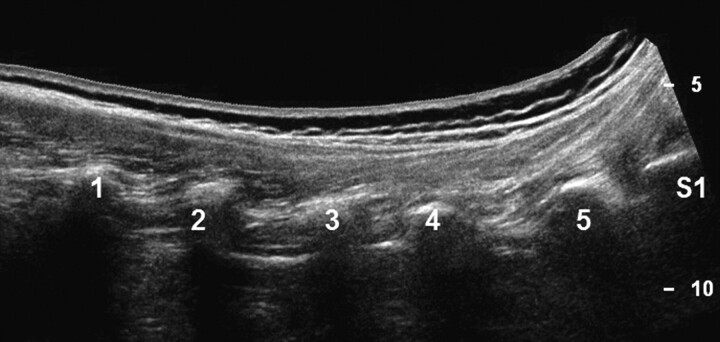
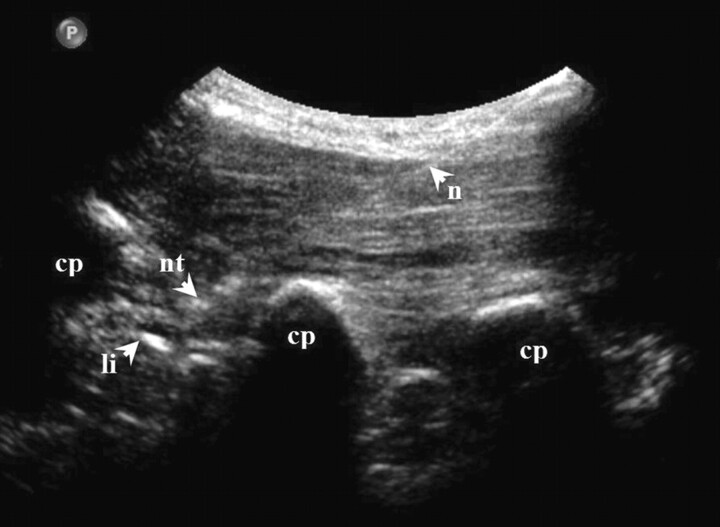
The transducer was advanced further until the transverse process was shown (Fig 3) and then moved back toward the midline until the edge of the zygapophyseal joint was viewed again. In this final scanning plane (called the PAP), the intertransverse ligament was seen as a thin hyperechoic band between 2 adjacent transverse processes (Fig 4). The spinal nerve —if identified at all—was present in the PAP ventral to the intertransverse ligament as a slightly hypoechoic roundish structure surrounded by hyperechoic fat.
Fig 1.
Panoramic (extended FOV) US image of the spinous processes (L1-S1) in a posterior paravertebral parasagittal plane.
Fig 2.
Panoramic (extended FOV) US image of the zygapophyseal joints (L1-S1) in a posterior paravertebral parasagittal plane.
Fig 3.
Panoramic (extended FOV) US image of the costal processes (L1-S1) in a posterior paravertebral parasagittal plane.
Fig 4.
US image of pararadicular injection (PAP) at levels L2-L3 showing delineation of the needle, needle tip, intertransverse ligament, and costal processes.
Sonographically Guided Pararadicular Injection
After localization of the first-to-fifth lumbar spinal levels, a spinal needle (21 ga, 80 mm; Sterican, Braun, Melsungen, Germany) was advanced into the pararadicular space of each segment (both sides) under real-time US guidance to simulate lumbar nerve root infiltration. The needle was inserted in an “in-plane technique” (needle advanced strictly parallel to the long axis of the transducer at an angle of approximately 45°, which enables real-time visualization of the entire needle path). The tip of each needle was advanced until it reached the respective intertransverse ligament. Afterward the needle was advanced a little deeper until the ligament was barely penetrated by the needle tip and the tip thus reached the pararadicular compartment. After all needles had been placed (5 segments, both sides), 1 mL of a solution consisting of 1 part water-soluble iodinated contrast agent (iopamidol, Jopamiro 300 mg J/mL; Bracco, Milan, Italy) and 2 parts of a highly pigmented aqueous dispersion (Unisperse Blue B-E; Ciba Specialty Clinicals, London, United Kingdom) was injected into each pararadicular compartment.
The placement of the needle tips and the distribution of the injected fluid in the cadaver were checked by CT (section thickness, 1.0 mm; pitch, 0.5). After imaging, the cadaver was split longitudinally: The left half was dissected anatomically, so that the position of the needles could be controlled. The right half of the specimen was frozen, and transverse cryosections were acquired according to the orientation of CT sections, to evaluate the spread of the injected solution.
Results
CT confirmed that all 10 needle tips were correctly placed inside the pararadicular compartment, with sufficient contrast accumulation around the respective spinal nerve root (Fig 5). Also in the anatomic dissection and the transverse anatomic cryosections, the needle tips and the blue dye distribution were equally found in the correct pararadicular compartment of each lumbar spinal level. A sufficient pararadicular dye coating of the respective lumbar root was confirmed in all cases without direct needle contact to any nerve root (Figs 6–8).
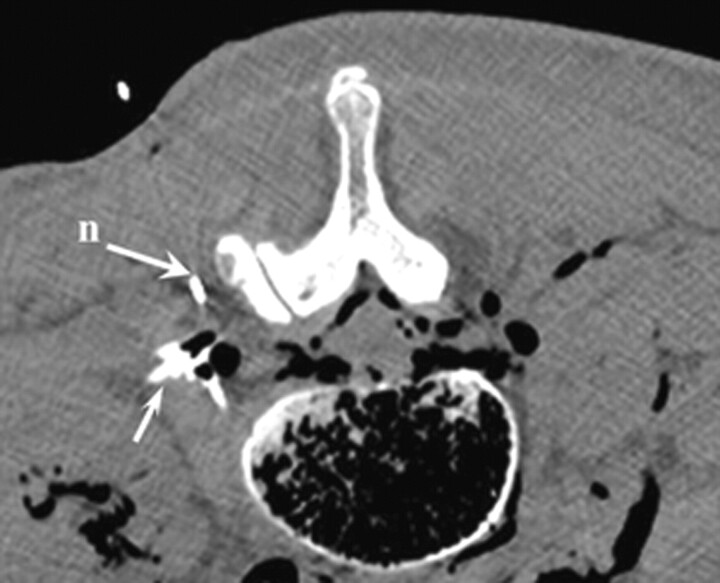
Fig 5.
Corresponding axial CT scan at levels L2-L3, demonstrating the needle and contrast enhancement in the pararadicular area (arrow).
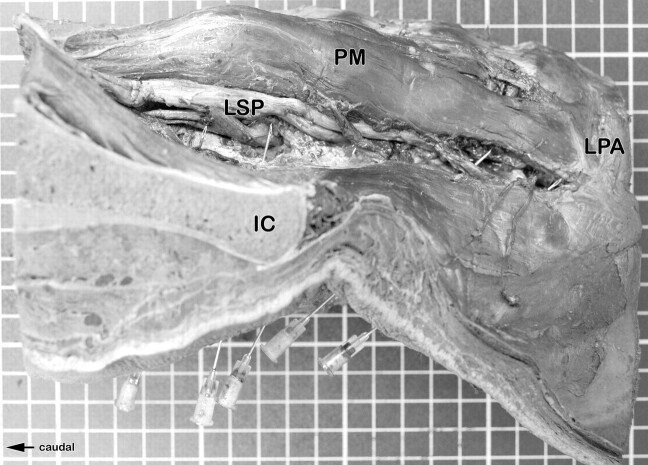
Fig 6.
Anatomic dissection of the left side of the injected specimen. The position of the needles can be seen.
Fig 7.
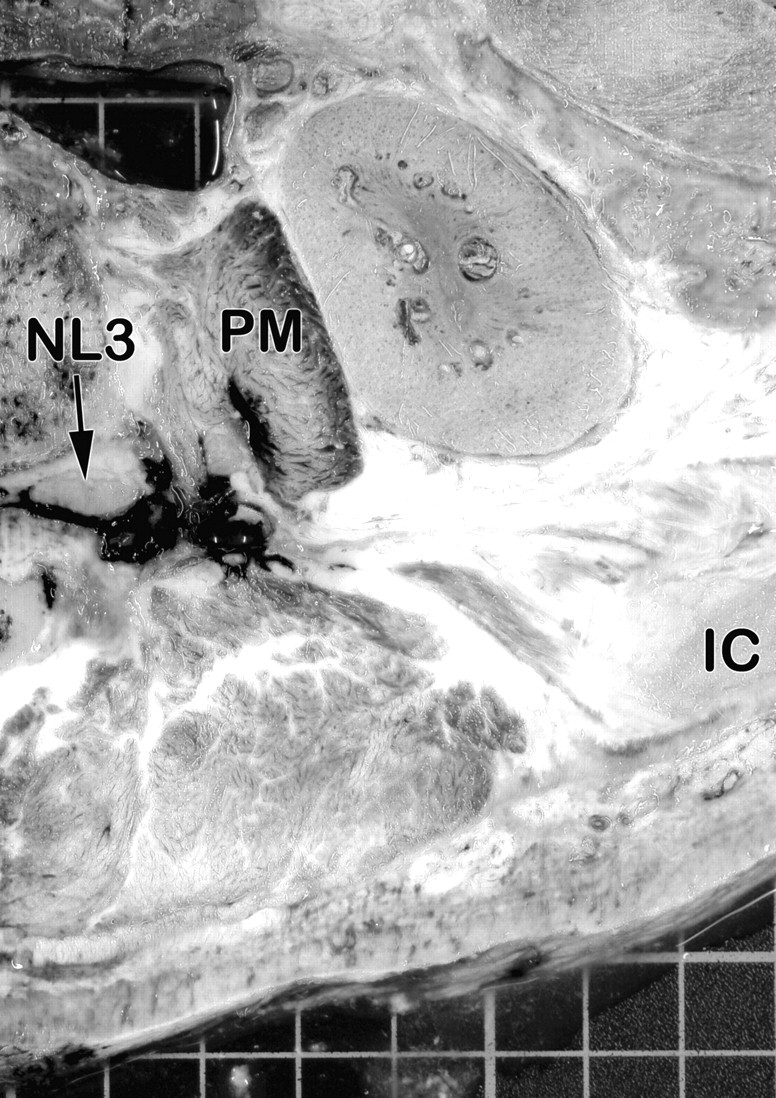
Transversal section of the right side of the injected specimen at the level of the third lumbar intervertebral foramen (superior aspect). The deposit of the injected dye can be seen around the third lumbar spinal nerve. The dye also extends to the intramuscular parts of the lumbar plexus (within the psoas muscle).
Fig 8.
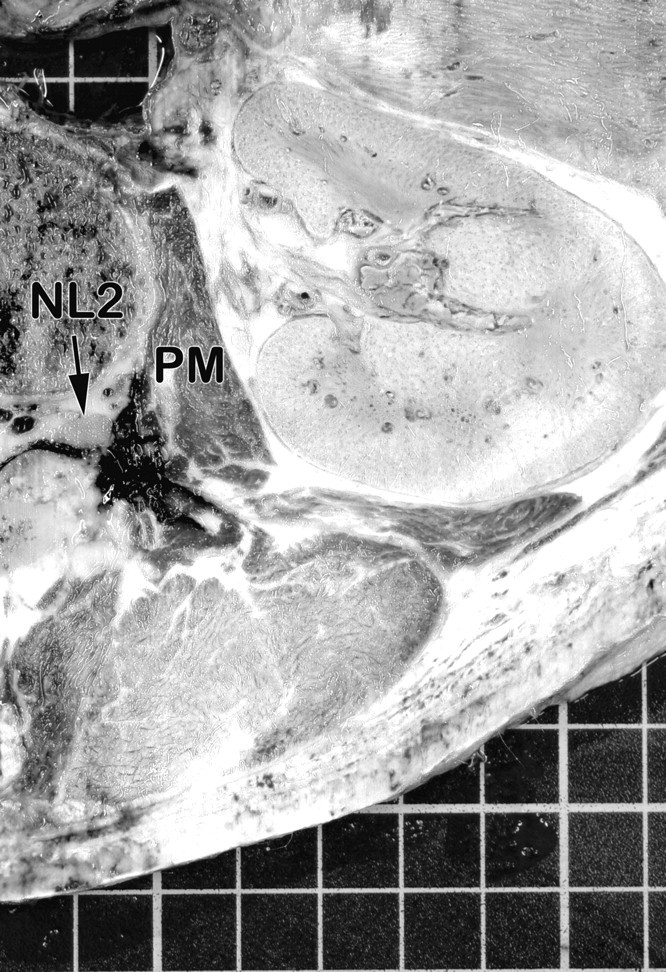
Transversal section of the right side of the injected specimen at the level of the second lumbar intervertebral foramen (superior aspect). The deposit of the injected dye can be seen around the second lumbar spinal nerve.
Discussion
Today injection therapy plays a major role in the treatment of various pain conditions and so has also become an integral part in the treatment of lumbar radiculopathy.5,6 Imaging-guided pararadicular injections are, to date, mainly performed under CT or fluoroscopic guidance. The latter, however, is time-consuming and may be performed only on-site in appropriately equipped clinical institutions with considerable expenditure of resources.7,8 US is already used successfully to guide a variety of instillation procedures in different anatomic regions, showing many benefits: direct visualization of the target of interest, real-time needle guidance, visualization of the spread of local anesthetics and thus minimal risk of complications, potential for dose reduction of local therapeutics, and shortening of procedure time, just to mention a few.
The currently established technique for sonography-guided lumbar nerve root instillation as proposed by Galiano et al14 consists of an axial approach toward the respective spinal level. While this technique is optimally suited for lumbar zygapophyseal joint injections because of the ideal visualization of the joint space in a transverse scanning plane, the authors of this article reported difficulties with nerve root injection in the upper lumbar segments. In the upper lumbar vertebrae, the isthmus is straighter and the laminae of the vertebral arch are narrower. Therefore, the space between the transverse processes is small, and the vertebral isthmus can appear like a straight fissure.22,23 With the transverse approach, a reliable definition of the lamina, however, is the key for the localization of the zygapophyseal joint space and the adjacent neuroforamen. In contrast to this approach, our technique does not rely on depiction of the vertebral lamina but on the intertransverse ligament. The latter is an easy-to-find anatomic landmark even in the upper lumbar spine: In the PAP scans, the respective intertransverse ligament is seen as a thin hyperechoic well-defined band between 2 adjacent transverse processes. Compared with the transverse approach, our technique proved more convenient, especially for localization of the correct level and for the advancement of the needle into the pararadicular space.
In contrast to the method proposed by Galiano et al, 14 the technique described here has a theoretic disadvantage: Even with optimized scanning conditions, the lumbar nerve root itself is only seen by chance. Our needle path, however, is not directed toward the lumbar root but toward the respective intertransverse ligament, which is punctured to install the therapeutic agent into the pararadicular compartment. In this context, exact adherence to the proposed PAP (ie, an injection plane closer to the neural foramen) is of utmost importance because the lumbar roots are closer to the inner edge of the intertransverse ligament. Although the spinal nerve roots were not directly visualized, the CT examination and the anatomic dissection confirmed correct needle placement within the pararadicular compartment without any contact to the spinal nerve. This result is remarkable because the needles were placed in the correct pararadicular compartment, similar to placement with CT or fluoroscopic guidance.
We always used a freehand in-plane puncture technique originally developed and described for the psoas compartment block.24 In this technique, the needle is advanced strictly parallel to the long axis of the transducer to keep it entirely under real-time control within the sonographic scanning plane. Nevertheless, precise and careful handling of the transducer in interaction with the puncture needle is mandatory to achieve sufficient control over the procedure.
Conclusions
Our new US-based approach proved to be reliable and accurate in placing a therapeutic needle for lumbar pararadicular instillations. The presented technique is rather simple to perform because it uses the intertransverse ligament as an anatomic landmark, which is easy to localize. The difficulties encountered in the study of Galiano et al14 in depicting and puncturing the pararadicular compartment in the upper lumbar spine are avoided because the intertransverse ligament could be clearly delineated in all lumbar segments. Additionally, the lack of exposure to ionizing radiation, in contrast to CT and fluoroscopy-guided procedures, favors this US-guided instillation technique. The clinical impact of the proposed technique on patients with lumbar pain in contrast to existing procedures must be validated in a larger clinical trial.
Abbreviations
- cp
costal processes
- IC
iliac crest
- li
intertransverse ligament
- LPA
lateral arcade of the psoas muscle
- LSP
lumbosacral plexus
- n
needle
- NL2
second lumbar spinal nerve root
- NL3
third lumbar spinal nerve root
- nt
needle tip
- PAP
pararadicular aditus plane
- PM
psoas muscle
- US
ultra-sonography
References
- 1. Bogduk N. On the definitions and physiology of back pain, referred pain, and radicular pain. Pain 2009; 147: 17–19 [DOI] [PubMed] [Google Scholar]
- 2. Moore RA, Straube S, Derry S, et al. Chronic low back pain analgesic studies: a methodological minefield. Pain 2010; 149: 431–34 [DOI] [PubMed] [Google Scholar]
- 3. O'Neill S, Graven-Nielsen T, Manniche C, et al. Ultrasound guided, painful electrical stimulation of lumbar facet joint structures: an experimental model of acute low back pain. Pain 2009; 144: 76–83 [DOI] [PubMed] [Google Scholar]
- 4. Schiltenwolf M, Schneider S. Activity and low back pain: a dubious correlation. Pain 200;143:1–2 [DOI] [PubMed] [Google Scholar]
- 5. Carrino JA, Morrison WB, Parker L, et al. Spinal injection procedures: volume, provider distribution, and reimbursement in the US Medicare population from 1993 to 1999. Radiology 2002; 225: 723–29 [DOI] [PubMed] [Google Scholar]
- 6. Kim PS. Role of injection therapy: review of indications for trigger point injections, regional blocks, facet joint injections, and intra-articular injections. Curr Opin Rheumatol 2002; 14: 52–57 [DOI] [PubMed] [Google Scholar]
- 7. Derby R, Kine G, Saal JA, et al. Response to steroid and duration of radicular pain as predictors of surgical outcome. Spine (Phila Pa 1976) 1992; 17 (6 suppl): S176–83 [DOI] [PubMed] [Google Scholar]
- 8. Gangi A, Dietemann JL, Mortazavi R, et al. CT-guided interventional procedures for pain management in the lumbosacral spine. Radiographics 1998; 18: 621–33 [DOI] [PubMed] [Google Scholar]
- 9. Fritz J, Niemeyer T, Clasen S, et al. Management of chronic low back pain: rationales, principles, and targets of imaging-guided spinal injections. Radiographics 2007; 27: 1751–71 [DOI] [PubMed] [Google Scholar]
- 10. Silbergleit R, Mehta BA, Sanders WP, et al. Imaging-guided injection techniques with fluoroscopy and CT for spinal pain management. Radiographics 2001; 21: 927–39 [DOI] [PubMed] [Google Scholar]
- 11. Galiano K, Obwegeser AA, Walch C, et al. Ultrasound-guided versus computed tomography-controlled facet joint injections in the lumbar spine: a prospective randomized clinical trial. Reg Anesth Pain Med 2007; 32: 317–22 [DOI] [PubMed] [Google Scholar]
- 12. Galiano K, Obwegeser AA, Bale R, et al. Ultrasound-guided and CT-navigation-assisted periradicular and facet joint injections in the lumbar and cervical spine: a new teaching tool to recognize the sonoanatomic pattern. Reg Anesth Pain Med 2007; 32: 254–57 [DOI] [PubMed] [Google Scholar]
- 13. Galiano K, Obwegeser AA, Bodner G, et al. Ultrasound guidance for facet joint injections in the lumbar spine: a computed tomography-controlled feasibility study. Anesth Analg 2005; 101: 579–83 [DOI] [PubMed] [Google Scholar]
- 14. Galiano K, Obwegeser AA, Bodner G, et al. Real-time sonographic imaging for periradicular injections in the lumbar spine: a sonographic anatomic study of a new technique. J Ultrasound Med 2005; 24: 33–38 [DOI] [PubMed] [Google Scholar]
- 15. Galiano K, Obwegeser AA, Bodner G, et al. Ultrasound-guided facet joint injections in the middle to lower cervical spine: a CT-controlled sonoanatomic study. Clin J Pain 2006; 22: 538–43 [DOI] [PubMed] [Google Scholar]
- 16. Galiano K, Obwegeser AA, Bodner G, et al. Ultrasound-guided periradicular injections in the middle to lower cervical spine: an imaging study of a new approach. Reg Anesth Pain Med 2005; 30: 391–96 [DOI] [PubMed] [Google Scholar]
- 17. Greher M, Scharbert G, Kamolz LP, et al. Ultrasound-guided lumbar facet nerve block: a sonoanatomic study of a new methodologic approach. Anesthesiology 2004; 100: 1242–48 [DOI] [PubMed] [Google Scholar]
- 18. Kapral S, Krafft P, Eibenberger K, et al. Ultrasound-guided supraclavicular approach for regional anesthesia of the brachial plexus. Anesth Analg 1994; 78: 507–13 [DOI] [PubMed] [Google Scholar]
- 19. Marhofer P, Schrogendorfer K, Koinig H, et al. Ultrasonographic guidance improves sensory block and onset time of three-in-one blocks. Anesth Analg 1997; 85: 854–57 [DOI] [PubMed] [Google Scholar]
- 20. Marhofer P, Schrogendorfer K, Wallner T, et al. Ultrasonographic guidance reduces the amount of local anesthetic for 3-in-1 blocks. Reg Anesth Pain Med 1998; 23: 584–88 [DOI] [PubMed] [Google Scholar]
- 21. Kirchmair L, Entner T, Wissel J, et al. A study of the paravertebral anatomy for ultrasound-guided posterior lumbar plexus block. Anesth Analg 2001; 93: 477–81 [DOI] [PubMed] [Google Scholar]
- 22. Rickenbacher J, Landolt AM, Rücken TK. Das Skelett des Rückens. In: Lang J, Wachsmuth W. eds. Praktische Anatomie. Heidelberg, Germany: Springer-Verlag;1982:17–22 [Google Scholar]
- 23. Parke WW. Applied anatomy of the spine. In: Rothman RH, Simeone FA. eds. The Spine. Philadelphia: WB Saunders;1992:35–88 [Google Scholar]
- 24. Kirchmair L, Entner T, Kapral S, et al. Ultrasound guidance for the psoas compartment block: an imaging study. Anesth Analg 2002; 94: 706–10 [DOI] [PubMed] [Google Scholar]