It is known that risk of cerebrovascular accident is not only associated with degree of carotid artery stenosis but probably more importantly with type of plaque: vulnerable vs stable. Myriad studies have looked at this issue with high-resolution MR imaging but the present one used FDG-PET and CT. Fifty patients with transient ischemic attack/stroke who had ipsilateral stenosis and plaque along with contralateral asymptomatic plaque were imaged. High uptake was seen in ipsilateral plaques when compared with contralateral asymptomatic ones but these differences were not significant. CT also showed larger lipid-rich necrotic cores and thicker arterial walls in symptomatic plaques, but again these differences were not significant. Thus, it remains to be determined if the combination of FDG-PET/CT is valuable.
Abstract
BACKGROUND AND PURPOSE:
There is a need for improved risk stratification of patients with TIA/stroke and carotid atherosclerosis. The purpose of this study was to prospectively investigate the potential of integrated 18F-FDG PET/MDCT in identifying vulnerable carotid plaques.
MATERIALS AND METHODS:
Fifty patients with TIA/stroke with an ipsilateral carotid plaque causing <70% stenosis and a plaque on the contralateral asymptomatic side underwent integrated 18F-FDG PET/MDCT within 36.1 ± 20.0 days (range, 9–95 days) of the last symptoms. Carotid plaque 18F-FDG uptake was measured as both the mean and maximum blood-normalized SUV, known as the TBR. Using MDCT, we assessed volumes of vessel wall and individual plaque components.
RESULTS:
Mean TBR was only significantly larger in the ipsilateral plaques of patients who were imaged within 38 days (1.24 ± 0.04 [SE] versus 1.17 ± 0.05, P = .014). This also accounted for maximum TBR (1.53 ± 0.06 versus 1.42 ± 0.06, P = .015). MDCT-assessed vessel wall and LRNC volumes were larger in ipsilateral plaques of all patients (982.3 ± 121.3 versus 811.3 ± 106.6 mm3, P = .016; 164.7 ± 26.1 versus 134.3 ± 35.2 mm3, P = .026, respectively).
CONCLUSIONS:
In the present study, 18F-FDG PET only detected significant differences between ipsilateral and contralateral asymptomatic plaques in patients with TIA/stroke who were imaged within 38 days, whereas MDCT detected larger vessel wall and LRNC volumes, regardless of time after symptoms. In view of the substantial overlap in measurements of both sides, it remains to be determined whether the differences we found will be clinically meaningful.
Large clinical trials demonstrated that patients with high-grade carotid artery stenosis due to atherosclerosis may benefit from CEA.1 However, for patients with symptomatic 70%–99% stenosis, 6.3 patients needed to undergo surgery to prevent 1 ipsilateral stroke in 5 years, whereas for patients with asymptomatic 60%–99% stenosis, this number was as high as 17.1 These numbers show that there is a considerable difference in stroke risk between both groups and that many patients are undergoing surgery unnecessarily. Histopathologic studies suggest that so-called vulnerable plaques, characterized by attenuated inflammation and large LRNC size, are more prone to rupture.2 Plaque rupture can lead to thrombus formation and subsequent stroke. Difference in stroke risk can be explained by differences in plaque characteristics: Plaques ipsilateral to the symptomatic side may be more vulnerable than asymptomatic plaques.
It has been shown that 18F-FDG PET and MDCT are able to noninvasively assess plaque inflammation and composition, respectively.3,4 Integrated PET/MDCT scanners are now widely available and may be used to differentiate vulnerable from nonvulnerable carotid plaques, which may lead to a better risk stratification of patients. To assess the potential of integrated 18F-FDG PET/MDCT in identifying vulnerable plaques, we prospectively investigated whether these combined imaging modalities can detect differences between carotid plaques ipsilateral to the symptomatic (infarct) side and contralateral to asymptomatic plaques. We performed this study in patients with TIA/stroke with low-grade (<70%) stenosis, because if 18F-FDG PET and/or MDCT can detect differences between ipsilateral and contralateral plaques, it is this group of patients who are expected to benefit most from improved personalized risk stratification.
Materials and Methods
Patients
Fifty consecutive patients (34 men; mean age, 67.8 ± 8.9 years) who were diagnosed by a neurologist as having recent TIA or nondisabling ischemic stroke in the carotid artery territory and ipsilateral carotid plaque causing <70% stenosis at duplex sonography5 underwent integrated 18F-FDG PET/MDCT of both carotid arteries. Forty-five patients were on statin therapy. The mean time interval between the date of last symptoms and imaging was 36.1 ± 20.0 days (range, 9–95 days). The mean blood glucose level, measured before each 18F-FDG PET/MDCT study was 102 ± 14 mg/dL. Exclusion criteria were the following: atrial fibrillation, history of clinical cerebral ischemia at the contralateral side, no visible plaque or occlusion at the contralateral side at duplex ultrasonography,5 glomerular filtration rate < 60 mL/min/1.73 m2, and blood glucose level > 150 mg/dL. This study was approved by the institutional review board of our hospital, and all patients gave written informed consent.
Imaging Protocol
Scanning was performed on an integrated PET/MDCT scanner (Gemini TF-64; Philips Healthcare, Cleveland, Ohio) with 4.8-mm PET resolution (full width at half maximum). After overnight fasting and 1 hour after 18F-FDG injection (2.75 MBq/kg body weight), 3D PET images were acquired with 576 × 576 mm FOV; 144 × 144 matrix; and 4-mm thickness. In all cases, low-dose CT scanning (30 mAs) was performed. Using the standard Philips time-of-flight ordered-subsets expectation maximization reconstruction (33 subsets, 3 iterations), we obtained images corrected for random events, scattered radiation, and attenuation. After PET scanning, contrast-enhanced CT images were obtained by using 90 mL of iobitridol (Xenetix 350; Guerbet, Aulnay-sous-Bois, France), collimation of 64 × 0.625 mm, 120 kVp, 175 mAs, 0.5-second tube rotation, and pitch of 0.671. With an intermediate kernel, image reconstructions were made with 250 × 250 FOV, 512 × 512 matrix, and 0.7-mm thickness.
Image Review
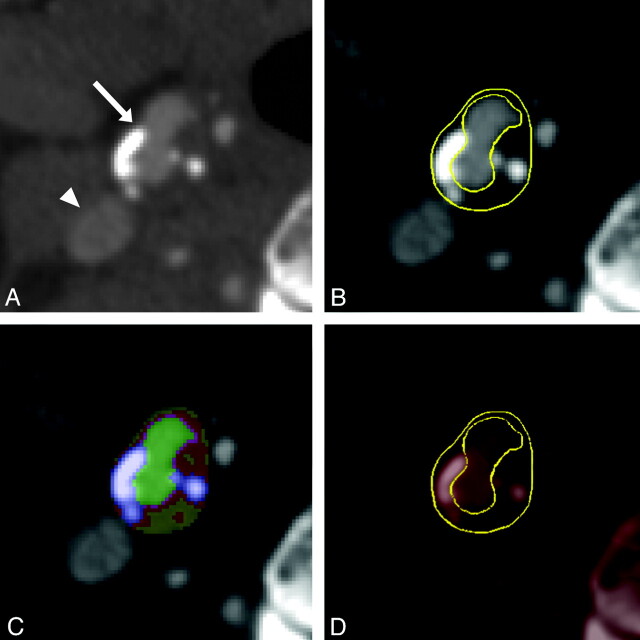
One investigator with 2 years of experience in plaque analysis (R.M.K.) evaluated 18F-FDG PET/MDCT images, blinded to all clinical information. Stenosis grade was assessed on the MDCT images by using the NASCET method.6 Plaque was identified as the presence of calcification and/or thickening of the vessel wall on the MDCT images. Regions of interest were manually drawn, encompassing the plaque (Fig 1), and were placed to avoid plaque borders to reduce partial volume effects. Regions of interest were drawn on all sections around the carotid bifurcation where plaque was identified for both arteries, and each one was transferred onto the corresponding coregistered PET images (Fig 1). Dedicated fusion software (Syntegra, Philips Healthcare) was used to analyze the PET images. Mean and maximum 18F-FDG SUVs of the plaque (excluding the lumen) were normalized for blood 18F-FDG activity by dividing them by the mean SUV of blood as measured in the internal jugular vein. This resulted in mean and maximum TBRs. A second trained observer (M.T.B.T.) also analyzed 18F-FDG PET/MDCT images of 20 consecutive patients independently. Overall, interobserver agreement for the assessment of mean and maximum TBRs was moderate, with ICCs of 0.61 and 0.65, and CVs of 12.1% and 10.8%, respectively.
Fig 1.
MDCT (A−C) and fused 18F-FDG PET/MDCT (D) images of a transverse section of plaque at the carotid bifurcation. The arrow indicates the bifurcation with plaque, while the arrowhead indicates the internal jugular vein (A). Regions of interest encompassing the plaque and arterial lumen have been drawn on the MDCT image (B), from which a pixel map based on differences in Hounsfield units was obtained (green indicates arterial lumen; yellow, lipids; red, fibrous tissue; blue/white, calcifications) (C). Regions of interest were transferred onto the fused 18F-FDG PET/MDCT image to assess plaque 18F-FDG uptake, which was normalized for blood 18F-FDG uptake, resulting in a TBR (D).
A polymeasure plug-in for the software package ImageJ (Wayne Rasband; National Institutes of Mental Health, Bethesda, Maryland) was used to semiautomatically quantify volumes of the vessel wall, LRNC (including hemorrhage), fibrous tissue, and calcifications, as described before.4,7 MDCT segmentation thresholds were LRNC < 60 HU; fibrous tissue = 60–130 HU; and calcification > 130 HU. Previously, it was shown that overall interobserver agreement for the assessment of carotid plaque components by MDCT was moderate, with ICCs ranging from 0.76 to 0.97 and CVs ranging from 13% to 47%.7
Statistical Analysis
Statistical analyses were performed by using the Statistical Package for the Social Sciences, Version 11.5 (SPSS, Chicago, Illinois). Because the degree of plaque macrophage content and/or activity may be time-dependent,2,8 we assessed correlations between the time after symptoms and TBRs by Pearson rank correlation tests. Very weak, weak, moderate, strong, and very strong correlations were defined as Pearson ρs of 0–0.19, 0.20–0.39, 0.40–0.59, 0.60–0.79, and 0.80–1.00, respectively.9 Wilcoxon signed rank tests were used to compare 18F-FDG PET and MDCT findings between ipsilateral and contralateral carotid plaques. Because of the time dependency of plaque macrophage content and/or activity, we also performed a sensitivity analysis to assess whether patients who were imaged sooner after symptoms had significantly larger TBRs in the ipsilateral plaques. When a certain plaque parameter was found to be significantly higher at 1 side, we assessed whether this difference was not related to stenosis grade. This was done by performing linear regression analysis for ipsilateral and contralateral plaques with overlapping stenosis grade, by using ipsilateral/contralateral plaque status and stenosis grade as independent variables. Logarithmic transformation was performed to normalize distribution of data. Correlations between TBRs of ipsilateral and contralateral asymptomatic plaques were assessed by Pearson rank correlation tests. All levels of statistical significance were set at .05.
Results
Correlations between the time after symptoms and TBRs are shown in Table 1. There were moderate negative correlations between time after symptoms and mean and maximum TBRs at the ipsilateral side. Comparisons and correlations of 18F-FDG PET and MDCT findings between ipsilateral and contralateral asymptomatic plaques are shown in Table 2 . Plaques ipsilateral to the symptomatic side caused a significantly higher degree of stenosis. For all patients, there was no significant difference between mean and maximum 18F-FDG TBRs of ipsilateral and contralateral asymptomatic plaques. When we limited the analysis to patients who were imaged within 38 days after symptoms, the mean TBR was significantly larger in ipsilateral plaques (1.24 versus 1.17, P = .014). This also accounted for maximum TBR (1.53 versus 1.42, P = .015).
Table 1:
Correlations between time after symptoms and mean and maximum TBRs of ipsilateral and contralateral asymptomatic plaques
| Plaques Ipsilateral to the Symptomatic Side |
Contralateral Asymptomatic Plaques |
|||
|---|---|---|---|---|
| Pearson ρ | P value | Pearson ρ | P value | |
| Mean TBR | −0.396 | .005 | −0.099 | .498 |
| Maximum TBR | −0.365 | .010 | −0.100 | .494 |
Table 2:
Comparison between ipsilateral and contralateral asymptomatic plaques: results of Wilcoxon signed rank tests
| Plaques Ipsilateral to the Symptomatic Side (Mean Value ± SE) | Contralateral Asymptomatic Plaques (Mean Value ± SE) | P Value | |
|---|---|---|---|
| Degree of stenosis according to NASCET method6 (%) | 37.1 ± 2.1 | 16.1 ± 2.9 | <.001 |
| Mean TBR | |||
| All patients (N = 50) | 1.19 ± 0.03 | 1.17 ± 0.04 | .318 |
| Only patients in whom 18F-FDG PET/MDCT was performed within 38 days after symptoms (n = 38) | 1.24 ± 0.04 | 1.17 ± 0.05 | .014 |
| Maximum TBR | |||
| All patients (N = 50) | 1.46 ± 0.05 | 1.44 ± 0.06 | .160 |
| Only patients in whom 18F-FDG PET/MDCT was performed within 38 days after symptoms (n = 38) | 1.53 ± 0.06 | 1.42 ± 0.06 | .015 |
| Vessel wall volume (mm3) | 982.3 ± 121.3 | 811.3 ± 106.6 | .016 |
| LRNC volume (mm3) | 164.7 ± 26.1 | 134.3 ± 35.2 | .026 |
| Volume of fibrous tissue (mm3) | 491.2 ± 56.2 | 430.5 ± 49.9 | .081 |
| Volume of calcifications (mm3) | 312.4 ± 61.6 | 246.9 ± 45.8 | .067 |
MDCT-assessed vessel wall and LRNC volumes were larger in ipsilateral plaques of all patients (982.3 versus 811.3 mm3, P = .016; 164.7 versus 134.3 mm3, P = .026, respectively). Linear regression analyses (after log-transformation of data) showed no association between stenosis grade and mean and maximum TBRs (B = 0.009 ± 0.063 [SE], P = .885; and B = 0.012 ± 0.071, P = .864, respectively). Linear regression analyses (after log-transformation of data) showed no association between stenosis grade and vessel wall and LRNC volume (B = 0.191 ± 0.220 [SE], P = .387; B = 0.141 ± 0.393, P = .721, respectively). Correlations between TBRs of ipsilateral and contralateral asymptomatic plaques were strong (for mean TBR: ρ = 0.723, P < .001; and for maximum TBR: ρ = 0.785, P < .001) (Fig 2 A, -B).
Fig 2.
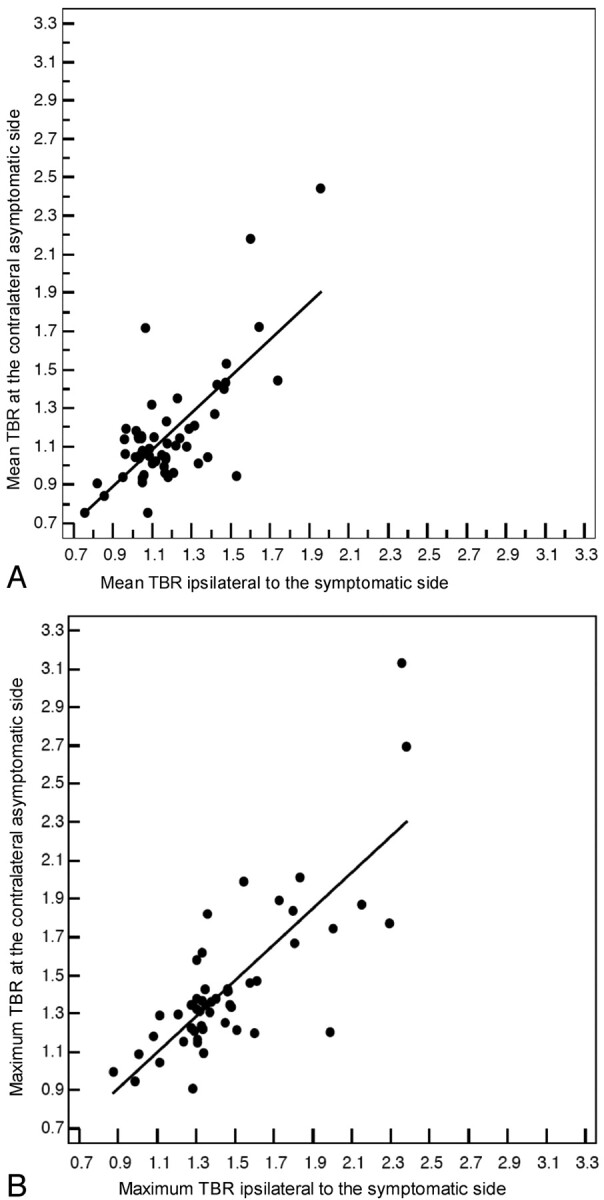
Scatterplots with regression lines. Correlations between mean (A) and maximum (B) TBRs of ipsilateral and contralateral asymptomatic plaques.
Discussion
Integrated PET/MDCT scanners are now widely available and can provide information on plaque inflammation and composition in a single examination. This technique may be used to differentiate vulnerable from nonvulnerable carotid plaques, which may lead to a better risk stratification of patients with TIA/stroke with carotid atherosclerosis. This study compared 18F-FDG PET and MDCT findings between plaques ipsilateral to the side of symptoms and contralateral asymptomatic plaques causing <70% stenosis. Overall 5-year risk of (recurrent) ipsilateral stroke for the former is approximately 20%,10 while for the latter, it is approximately 2%.11 In the present study, 18F-FDG PET only detected significant differences in patients who were imaged within 38 days. However, in this subgroup of patients, there was still substantial overlap in 18F-FDG uptake measurements.
In light of the error in TBR measurement (CV of 10.8%–12.1%), our findings suggest that selection of vulnerable versus nonvulnerable plaques on the basis of a TBR threshold may have a low sensitivity and/or specificity. In addition, we found strong positive correlations between 18F-FDG uptake in ipsilateral and contralateral asymptomatic plaques, which support the concept that atherosclerosis is a systemic inflammatory disease and may also explain why differences between both sides are small. MDCT detected larger vessel wall and LRNC volume in ipsilateral plaques, regardless of the time after symptoms. However, there was also substantial overlap between both sides. Moreover, interobserver variability of semiautomatic assessment of vessel wall and LRNC volume by MDCT, as previously reported by de Weert et al,6 is still considerable (CV of 23%–34% and 42%–58%, respectively), which may limit its clinical usefulness.
To date, only a few studies have compared 18F-FDG uptake between ipsilateral and contralateral asymptomatic plaques in relatively small sample sizes.12,13 Using a stand-alone PET scanner, Rudd et al12 performed a study in 8 patients with TIA with an ipsilateral carotid stenosis of at least 70%. The median time between symptoms and 18F-FDG PET imaging was 3.5 months.12 Their analysis was limited to 6 of the 8 included patients, showing 34% higher 18F-FDG uptake on the symptomatic side.12 However, this series of patients12 may be too small to make any assumptions. Moreover, statins attenuate plaque inflammation on 18F-FDG PET,13 whereas the study of Rudd et al12 was performed in an era when statin therapy was not as intensive or prevalent as it is at present. Font et al14 studied 15 patients with >70% stenosis, of whom 10 were defined as being symptomatic. In these 10 symptomatic patients who underwent an 18F-FDG PET scanning between 7 and 114 days (median, 33 days) after symptoms, there was no significant difference in 18F-FDG uptake between ipsilateral and contralateral asymptomatic plaques (1.19 versus 1.15, P = .185). However, due to the relatively limited sample size,14 no analysis with regard to timing of 18F-FDG PET could be performed.
In the present study, in which all patients were defined as symptomatic according to NASCET and European Carotid Surgery Trial criteria,6,15 we did find a negative correlation between TBRs of ipsilateral plaques and the time after symptoms. This finding is in accordance with the results of a large histopathologic study8 showing that symptomatic carotid lesions remodel into more stable plaques with time after stroke. Sensitivity analysis indicated that 18F-FDG PET only detected significant differences if performed within 38 days after symptoms. Future independent studies should confirm our findings.
We found that MDCT identified significantly larger vessel wall and LRNC volume in ipsilateral plaques. Wintermark et al,16 who performed an MDCT study in 40 patients with stroke, also found larger vessel wall volume in ipsilateral plaques (1404.1 versus 1281.9 mm3, P = .020). They also found larger LRNC volumes, though this finding was not significant (24.9 versus 16.4 mm3, P = .196). In accordance, an MR imaging study by Saam et al17 demonstrated that plaques at the symptomatic side had larger mean LRNC areas (8.1 versus 6.3 mm2, P = .2). A disadvantage of MDCT is that LRNC can only be adequately quantified in mildly calcified plaques.4 Other disadvantages of MDCT are that it cannot reliably distinguish intraplaque hemorrhage from LRNC, and fibrous cap status cannot be assessed. These parameters, which are also potential markers of plaque vulnerability, can be assessed by MR imaging.17 Whether MDCT or MR imaging may be more helpful in identifying vulnerable plaques can only be determined by prospective longitudinal studies.
Our study has several limitations. First, 90% of the included patients were on statin therapy, which may have attenuated plaque inflammation on 18F-FDG PET.13 However, the fact that most of the included patients with TIA/stroke used statins reflects current clinical practice. Second, the time between 18F-FDG injection and PET imaging was 1 hour. By increasing this interval, higher lesion-to-background contrast could have been achieved.12 However, this was not possible due to too much patient discomfort. Third, 18F-FDG PET measurements may have been affected by partial volume errors. Future studies may use methods to reduce these errors.18 Fourth, we used a manual outlining method for 18F-FDG PET measurements, whereas a semiautomated method was used for MDCT measurements. The development and use of fully automated software may enhance reproducibility. Last, our study had a cross-sectional design. The true value of integrated 18F-FDG PET/MDCT in identifying vulnerable plaques can only be determined by performing a large prospective clinical trial.
Conclusions
In the present study, 18F-FDG PET only detected significant differences between ipsilateral and contralateral asymptomatic plaques in patients with TIA/stroke who were imaged within 38 days, whereas MDCT detected larger vessel wall and LRNC volume regardless of time after symptoms. However, there was substantial overlap in measurements of both sides. Whether integrated 18F-FDG PET/MDCT is clinically meaningful can only be assessed by a large prospective clinical trial.
Acknowledgments
We thank Dr. E. Meijering and Dr. T.T. de Weert (Department of Radiology and Biomedical Imaging Group, Erasmus Medical Center, Rotterdam, the Netherlands) for providing us the software to analyze the MDCT images.
Abbreviations
- CEA
carotid endarterectomy
- CV
coefficient of variation
- 18F-FDG
[ 18F] fluorodeoxyglucose
- ICC
intraclass correlation coefficient
- LRNC
lipid-rich necrotic core
- MDCT
multidetector row spiral CT
- NASCET
North American Symptomatic Carotid Endarterectomy Trial
- PET
positron-emission tomography
- SE
standard error
- SUV
standardized uptake value
- TBR
target-to-background ratio
- TIA
transient ischemic attack
Footnotes
This study was supported by the Dutch Heart Foundation grant 2006B061.
References
- 1. Chaturvedi S, Bruno A, Feasby T, et al. , for the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Carotid endarterectomy: an evidence-based review—report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005;65:794–801 [DOI] [PubMed] [Google Scholar]
- 2. Redgrave JN, Lovett JK, Gallagher PJ, et al. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford Plaque Study. Circulation 2006;113:2320–28 [DOI] [PubMed] [Google Scholar]
- 3. Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818–24 [DOI] [PubMed] [Google Scholar]
- 4. de Weert TT, Ouhlous M, Meijering E, et al. In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vasc Biol 2006;26:2366–72 [DOI] [PubMed] [Google Scholar]
- 5. Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003;229:340–46 [DOI] [PubMed] [Google Scholar]
- 6. North American Symptomatic Carotid Endarterectomy Trial: methods, patient characteristics, and progress. Stroke 1991;22:711–20 [DOI] [PubMed] [Google Scholar]
- 7. de Weert TT, de Monyé C, Meijering E, et al. Assessment of atherosclerotic carotid plaque volume with multidetector computed tomography angiography. Int J Cardiovasc Imaging 2008;24:751–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peeters W, Hellings WE, de Kleijn DP, et al. Carotid atherosclerotic plaques stabilize after stroke: insights into the natural process of atherosclerotic plaque stabilization. Arterioscler Thromb Vasc Biol 2009;29:128–33 [DOI] [PubMed] [Google Scholar]
- 9. BMJ. Statistics at Square One. Correlation and regression. http://www.bmj.com/collections/statsbk/11.dtl. Accessed May 16, 2010 [Google Scholar]
- 10. Rothwell PM, Eliasziw M, Gutnikov SA, et al. , for the Carotid Endarterectomy Trialists' Collaboration. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107–16 [DOI] [PubMed] [Google Scholar]
- 11. Longstreth WT, Jr, Shemanski L, Lefkowitz D, et al. Asymptomatic internal carotid artery stenosis defined by ultrasound and the risk of subsequent stroke in the elderly: the Cardiovascular Health Study. Stroke 1998;29:2371–76 [DOI] [PubMed] [Google Scholar]
- 12. Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with (18F)-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708–11 [DOI] [PubMed] [Google Scholar]
- 13. Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006;48:1825–31. Epub 2006 Oct 17 [DOI] [PubMed] [Google Scholar]
- 14. Font MA, Fernandez A, Carvajal A, et al. Imaging of early inflammation in low-to-moderate carotid stenosis by 18-FDG-PET. Front Biosci 2009;14:3352–60 [DOI] [PubMed] [Google Scholar]
- 15. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis—European Carotid Surgery Trialists' Collaborative Group. Lancet 1991;337:1235–43 [PubMed] [Google Scholar]
- 16. Wintermark M, Arora S, Tong E, et al. Carotid plaque computed tomography imaging in stroke and nonstroke patients. Ann Neurol 2008;64:149–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saam T, Cai J, Ma L, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology 2006;240:464–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Izquierdo-Garcia D, Davies JR, Graves MJ, et al. Comparison of methods for magnetic resonance-guided [18-F]fluorodeoxyglucose positron emission tomography in human carotid arteries: reproducibility, partial volume correction, and correlation between methods. Stroke 2009;40:86–93 [DOI] [PubMed] [Google Scholar]