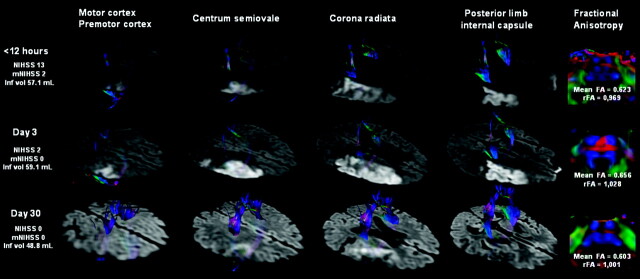
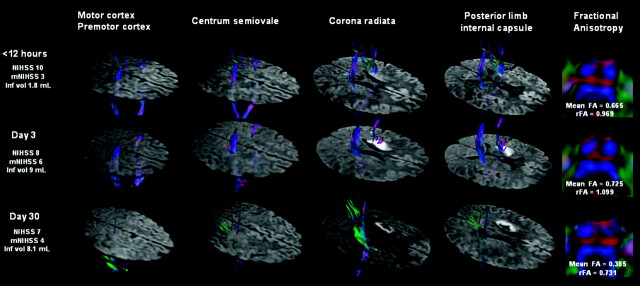
Practical applications of diffusion tensor imaging are few, but this seems to be an interesting and a potentially important one: can it be used to predict motor outcome after stroke? Sixty patients within 12 hours of stroke were assessed with tractography at 5 different locations in the corticospinal tracts at admission, and at days 3 and 30. Patients with acute damage to the posterior limb of the internal capsule had the worst outcome and clinical severity at presentation. Conclusions: In the acute setting, tractography is promising for stroke mapping to predict motor outcome. Acute corticospinal tract damage at the level of the posterior limb of the internal capsule is a significant predictor of unfavorable motor outcome.
Abstract
BACKGROUND AND PURPOSE:
Early prediction of motor outcome is of interest in stroke management. We aimed to determine whether lesion location at DTT is predictive of motor outcome after acute stroke and whether this information improves the predictive accuracy of the clinical scores.
MATERIALS AND METHODS:
We evaluated 60 consecutive patients within 12 hours of middle cerebral artery stroke onset. We used DTT to evaluate CST involvement in the motor cortex and premotor cortex, centrum semiovale, corona radiata, and PLIC and in combinations of these regions at admission, at day 3, and at day 30. Severity of limb weakness was assessed by using the motor subindex scores of the National Institutes of Health Stroke Scale (5a, 5b, 6a, 6b). We calculated volumes of infarct and fractional anisotropy values in the CST of the pons.
RESULTS:
Acute damage to the PLIC was the best predictor associated with poor motor outcome, axonal damage, and clinical severity at admission (P < .001). There was no significant correlation between acute infarct volume and motor outcome at day 90 (P = .176, r = 0.485). The sensitivity, specificity, and positive and negative predictive values of acute CST involvement at the level of the PLIC for motor outcome at day 90 were 73.7%, 100%, 100%, and 89.1%, respectively. In the acute stage, DTT predicted motor outcome at day 90 better than the clinical scores (R2 = 75.50, F = 80.09, P < .001).
CONCLUSIONS:
In the acute setting, DTT is promising for stroke mapping to predict motor outcome. Acute CST damage at the level of the PLIC is a significant predictor of unfavorable motor outcome.
Accurate early prediction of motor functional outcome in the early stage of stroke is important for clinicians and researchers in management and rehabilitation.1 Motor deficit after stroke is common and has a considerable influence on quality of life.2 Several observational studies have demonstrated that the grade of initial motor deficit is the most important determinant of motor recovery.1–5 Other valid predictors in regression models have included infarct site, volume of stroke, age, demographics, comorbidities, infarct side, and stroke subtype.1–3,6
The CST is the main pathway that mediates voluntary movements, and neurophysiological and structural imaging studies have evidenced that motor outcome is heavily dependent on the integrity of the motor fibers.6–13 Thus, the involvement of motor-related cortical regions, CR, and internal capsule progressively decrease the probability of upper limb functional recovery.6,14 Recently, these findings were complemented by DTI studies that have demonstrated the usefulness of DTT for predicting poor motor outcome when infarct involves the CST.15–20 DTI enables in vivo visualization and quantification of microstructural damage to white matter tracts.21 DTT uses data acquired through DTI to reconstruct a 3D macroscopic orientation of the white matter fibers that enables the specific topographic relation between lesion location and CST fibers to be evaluated.22 Decreases in FA, a DTI-derived structural measure, have been interpreted as WD and proposed as an index of axonal damage.23 Decreased FA in the CST correlates with motor impairment 1 month after stroke.24 In contrast, although patients with large infarcts tend to have a poor outcome, functional deficits due to moderate-sized infarcts are more difficult to predict.7,25,26 One of the major reasons functional outcome does not correlate strongly with infarct volume is that the specific site of the lesion is not taken into account.4,9
In the current study, we aimed to 1) evaluate whether the specific site of a lesion in the CST (primary MC, PMC, CS, CR, PLIC, and combinations among these) at DTT predicts axonal damage to the motor pathway and functional motor outcome after acute stroke, and 2) assess whether a model incorporating DTT information on the specific location of the stroke and clinical scores is more accurate in predicting motor outcome than clinical scores alone.
Materials and Methods
Patients
The data reported here were obtained from the same cohort of patients included in our previous study relating WD and motor outcome.24 Patients included had a nonlacunar, first-ever MCA infarction and were admitted to our stroke unit within 12 hours of symptom onset during a 19-month period. Patients with other lesions, cerebral hemorrhage, significant pre-existing nonischemic neurologic deficit (including dementia or extrapyramidal disease), or a history of prior stroke that would hinder the interpretation of clinical and imaging data were excluded. Our institutional ethics committee approved the study, and written informed consent was obtained from all patients or from close relatives.
Clinical Examination
A senior certified staff neurologist used the National Institutes of Health Stroke Scale to assess clinical deficit at admission, at day 3, at day 30, and at day 90 from stroke onset. The m-NIHSS subindex (5a, 5b, 6a, 6b) was used to categorize the severity of limb weakness as grade I (total m-NIHSS score of 0), grade II (m-NIHSS, score of 1–4), or grade III (m-NIHSS, score of 5–8). The mRS and BI were used to measure disability and dependence in activities of daily living at day 90. Poor overall outcome was defined as mRS >3, BI <60, or both.27 All clinical assessments were performed without knowledge of the MR imaging findings. Patients were treated according to published guidelines.28
MR Imaging Protocol
All scans were performed with a whole-body 1.5T MR system (Gyroscan Intera; Philips Medical Systems, Best, the Netherlands) with a SENSE head coil. The routine protocol included axial trace DWI, fluid-attenuated inversion recovery, T2-weighted gradient-echo, perfusion-weighted imaging, time-of-flight angiography, and DTI sequences. DTI was performed by using a single-shot echo-planar imaging sequence with the sensitivity encoding parallel-imaging scheme (acceleration factor, 2) after contrast agent administration. Diffusion-sensitized gradients were applied along 15 noncollinear directions with a b-value of 1000 s/mm2. In addition, diffusion-weighted B0 images were obtained. Other acquisition parameters were TR/TE, 6795 ms/72 ms; 23 × 23-cm FOV; and 112 × 112 matrix size. DTI voxel size was 2.05 × 2.05 × 3 mm. Forty sections covering the entire brain were obtained parallel to the bicommissural line without intersection gaps. DTI acquisition took 3 minutes and 10 seconds.
Data Processing and DTI
Diffusion-sensitized image sets were transferred to an off-line workstation for data analysis. We used DTIWeb version 2.0 (http://trueta.udg.edu/DTI/index.html) to calculate tensor values for tractography.29 Anisotropy maps were obtained by using orientation-independent FA, and color FA maps were generated following the standard convention (red, left-right; green, anteroposterior; and blue, superior-inferior).
Tractography was based on a diffusion tensor deflection algorithm.30 The threshold for stopping fiber propagation was FA <0.2 and angle <70°. The seeding method put 1 starting seed randomly inside each voxel with an FA >0.4. To reconstruct the CST, the ROIs were placed at the level of the cerebral peduncle and around the CR in the direction-coded color axial sections. Unrelated fibers, such as those going to the contralateral hemisphere, cerebellum, or thalamus, were removed by using specific ROIs. All ROIs were placed by 2 of the authors (A.P.-G., G.B.); the CST depicted and the evaluation of the PMC were validated by using landmarks from neuroanatomy atlases.31
Assessment of Damage to Specific CST Regions
To decide which structures were affected by infarct, the tractograms of CSTs were superimposed on DWI, and the following specific regions were evaluated: MC, PMC, CS, CR, PLIC, and combinations of these regions (On-line Fig. 1). These regions were scored separately on each section on 2 separate occasions 6 weeks apart by 1 rater (J.P.) and once by 2 raters (J.P., S.R.); all raters were blinded to the clinical ratings. Discordant ratings were resolved by consensus.
Measurement of the FA Values of CSTs
First, FA values for each region of interest on axial sections of the affected and unaffected CST at the rostral pons were obtained by averaging all voxels of 3 contiguous sections. Second, the ipsilateral-to-contralateral CST FA ratios were calculated (rFA = FAaffected side/FAunaffected side). Two readers (J.P., G.B.) blinded to the clinical scores quantified FA.24
Calculation of Infarct Volume
Infarct volumes were determined off-line. Two readers (J.P., G.B.) manually outlined the areas of abnormal hyperintensity on each axial trace DWI. Surface areas of abnormal hyperintensity were summed and multiplied by section thickness (6 mm) and intersection gap (1 mm) to calculate infarct volumes. The results of the 2 readers were averaged.24
Statistical Analysis
To determine whether acute-stage involvement of specific CST regions and combinations of CST regions were associated with stroke severity, clinical and motor outcome at day 30 and at day 90, axonal damage, and/or acute-phase infarct volume, we used the chi-squared test to compare categoric variables and Student t test to compare quantitative variables. We used the Cohen κ coefficient to assess intraobserver and interobserver reliability. Intra- and interobserver agreement were classified as slight (κ = 0.0–0.20), fair (κ = 0.21–0.40), moderate (κ = 0.41–0.60), substantial (κ = 0.61–0.80), or almost perfect (κ = 0.81–1.00) according to the scale proposed by Landis and Koch.32
Motor outcome was first analyzed by using bivariate statistics. We calculated the correlation coefficients for lesion site and for clinical scores with motor deficit at day 3, day 30, and day 90. Each specific CST region was coded as 0 (unaffected by infarct) or 1 (affected by infarct). The predictive dataset contained both dichotomous (involvement of specific CST region) and numeric and ratio variables (infarct volume, rFA, and m-NIHSS) for which an rpb or correlation coefficient was used, respectively. Coefficients with a P value <.05 were considered significant.
Multiple regression analysis was used to predict motor outcome at day 90 after stroke by using a combination of motor deficit, specific CST region involved, and imaging data. We also evaluated the additional predictive value conferred by adding the effect of region involved to that of the motor deficit. The dependent variable was the m-NIHSS score at day 90 after stroke, predicted from the following combinations of independent variables: 1) the specific CST region, m-NIHSS, and infarct volume in the first 12 hours after stroke; 2) the specific CST region, m-NIHSS, and infarct volume at day 3; and 3) the specific CST region, m-NIHSS, infarct volume, and rFA at day 30. To determine which combination of independent variables yielded the best predictive model, variables were deleted one by one from the model on the basis of the significance of their regression coefficients and the R2 selection method. The models with the highest R2 and all predictor variables that were significant (P < .05) were retained for each prediction. Only the model selected for the dataset obtained at day 3 fulfilled the assumption of normality. All statistical analyses were performed by using MINITAB version 15.1.0.0 (Minitab, State College, Pennsylvania).
Results
Subjects
Sixty-five consecutive patients with ischemic MCA stroke were scanned on admission, but data from 5 patients were incomplete at day 90 due to recurrence of stroke, death, and the presence of motion artifacts. Analyses were therefore based on 60 subjects (37 men, 23 women; aged 68 ± 13 years). One patient missed the MR imaging study at admission but completed studies on day 3 and day 30. All patients underwent MR imaging and clinical assessment at day 30.
Clinical Characteristics and DTT Analysis
On-line Table 1 presents detailed clinical and MR data for all the patients. The m-NIHSS score at admission was 11 (interquartile range 7–17), indicating that most patients had moderate to severe neurologic deficits. All patients had started physiotherapy within 2 weeks after the stroke. At admission, 47 (78.3%) of 60 patients presented some motor deficits, and 28 (59.6%) of these patients had moderate-severe motor deficit (m-NIHSS III). At day 3, 28 patients (46.7%) in total presented some motor deficits, and 13 (46.4%) of these patients were classified at m-NIHSS III. Improvements with respect to baseline scores were observed in 67.8% of patients at day 30 and in 85.7% at day 90, and 42.8% and 39.2% of motor deficits were categorized as m-NIHSS III at day 30 and day 90, respectively. The MCA territories most frequently involved were the peripheral territory, striatocapsular territory, or both. Thirty-one patients (51.67%) received intravenous rtPA (alteplase). There were no significant differences in rtPA treatment at admission among the m-NIHSS groups at day 90. BI and mRS scores before the stroke were 100 and 0 in all patients, respectively.
The mean time for reconstructing and assessing the DTT to evaluate the damage to CST regions was 3 minutes and 30 seconds. At admission, the CST did not seem disrupted or displaced in any patient. Intrarater and interrater agreement about the region of the CST affected was almost perfect (κ = 0.88 and κ = 0.84, respectively). No CST involvement by infarct on admission was observed in 14 (23.34%) patients; however, 5 of these had motor deficits (On-line Table 1). In contrast, CST involvement was observed on admission in 5 patients without motor deficits; the areas affected were the PMC (n = 2), PMC and CR (n = 1), CR (n = 1), and PLIC (n = 1). At day 30, involvement of at least one specific CST region was observed in all patients with motor deficit. Finally, PLIC involvement in the first 12 hours was associated with unfavorable overall outcome (mRS >3, BI <60, or both) (P < .001).
Motor Outcome Prediction and the Involvement of the Specific CST Regions
Damage to the PLIC in the first 12 hours and at day 3 after stroke correlated with clinical severity, axonal damage expressed as decreased FA and rFA values, and motor outcome at day 30 and day 90 (P < .001) better than damage to any other CST region (Table 1). There was no significant correlation between acute infarct volume and motor outcome at day 90 (P = 0.176, r = 0.485) (Figs 1 and 2).
Table 1:
Sensitivity, specificity, and positive and negative predictive values for motor outcome according specific CST regions in acute stroke
| Motor Outcome | m-NIHSS | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| PLIC <12 hr | Day 30 | I vs. II/III | 66.67 | 100.00 | 100.00 | 84.78 |
| II vs. III | 100.00 | 70.00 | 78.57 | 100.00 | ||
| Day 90a | I vs. II/IIIa | 73.68a | 100.00a | 100.00a | 89.13a | |
| II vs. IIIa | 100.00a | 71.43a | 85.71a | 100.00a | ||
| PLIC at day 3 | Day 30 | I vs. II/III | 71.43 | 100.00 | 100.00 | 86.67 |
| II vs. III | 100.00 | 60.00 | 73.33 | 100.00 | ||
| Day 90 | I vs. II/III | 78.95 | 100.00 | 100.00 | 91.11 | |
| II vs. III | 100.00 | 57.14 | 80.00 | 100.00 | ||
| CS at day 3 | Day 30 | I vs. II/III | 68.75 | 87.18 | 68.75 | 77.27 |
| II vs. III | 54.55 | 50.00 | 54.55 | 50.00 | ||
| Day 90 | I vs. II/III | 47.37 | 82.93 | 56.25 | 77.27 | |
| II vs. III | 50.00 | 57.14 | 66.67 | 40.00 | ||
| CR at day 3 | Day 30 | I vs. II/III | 71.43 | 56.41 | 46.88 | 78.57 |
| II vs. III | 63.64 | 20.00 | 46.67 | 33.33 | ||
| Day 90 | I vs. II/III | 73.68 | 56.10 | 43.75 | 82.14 | |
| II vs. III | 58.33 | 0.00 | 50.00 | 0.00 |
Indicates the highest overall values for all determinations.
Fig 1.
A 55-year-old man (patient 40) presented with moderate right peripheral MCA territory infarction. DTT images show infarction near the right CST at the level of the CR and PLIC, although there is no direct involvement. At admission, ischemic penumbra (not shown) involved part of the CST at the level of the CR and could explain the motor deficit at this time. FA indices reveal CST axonal integrity at the anterior part of the pons.
Fig 2.
A 47-year-old man (patient 15) presented a mild right-sided hemiparesis lasting 45 minutes. DTT of motor tracts superimposed on DWI are shown tridimensionally. A small infarct in the striatocapsular MCA territory involves the left CST. Note the slight hyperintensity of the CST involved by the infarct due to the short time elapsed since the onset of symptoms. The markedly reduced brightness on the left side and decreased FA value in the left descending CST at day 30 are regarded as WD.
CS involvement, CR involvement, or both at day 3 was associated with motor deficit at day 30 and day 90 (P < .004) and axonal damage (P < .003) (On-line Table 2). It is noteworthy that although other significant associations can be observed, combined PLIC and CS or CR involvement at day 3 was not significant for motor outcome at day 30 or at day 90 (P = .157 and P = .218 for the interaction between PLIC and CS at day 30 and at day 90, respectively; P = .521 and P = .457 for the interaction between PLIC and CR at day 30 and at day 90, respectively). Therefore, the motor outcome at day 30 and at day 90 is secondary to PLIC damage.
Damage to the PLIC in the first 12 hours yielded the highest sensitivity, specificity, and predictive values for the prediction of motor outcome at day 90. Interestingly, PLIC damage by acute stroke clearly distinguishes subjects without motor deficit (m-NIHSS I) from those with motor deficit (NIHSS II and III) and even differentiates m-NIHSS II from m-NIHSS III at day 90 (Table 1).
Correlations analysis revealed significant coefficients only between PLIC involvement in the first 12 hours and motor outcome at day 90 (On-line Table 3). Damage to the CR, CS, or both; m-NIHSS; and acute-stage infarct volume were not related to motor outcome at 90 days. At day 3, PLIC damage and m-NHISS showed the most significant correlations with motor outcome at 90 days. At day 30, PLIC damage, m-NHISS, and axonal damage showed the most significant correlations with motor outcome at day 90. The only relation between infarct volume and motor outcome at day 90 was a modest correlation observed at day 3.
Table 2 summarizes the best predictive models achieved at each time point. The simplest model to predict m-NIHSS at day 90 based on the data available in the first 12 hours consisted only of PLIC damage; PLIC damage alone accounted for 75.5% of the variance in outcome. At day 3, regression analyses indicated that m-NIHSS accounted for 79% of the variance in motor outcome at day 90, and PLIC damage had a significant contribution of only 6.62%. Regression coefficients for these assessments were positive, indicating that an infarct affecting CST and m-NIHSS is predictive of greater motor deficit from day 3 to 90 days after stroke. The best model for predicting motor outcome at day 90 based on the assessments at day 30 included only the m-NIHSS, which accounted for 90.10% of the variance in the measurement.
Table 2:
Models selected from multiple regression analyses for predicting m-NIHSS 90 days after stroke from motor scores and specific CST regions
| Predictor | Regression Coefficient | tValue | Added R2 |
|---|---|---|---|
| Measurements obtained <12 hr (R2 = 75.50, F = 80.09**) | |||
| PLIC damage | 5.36 | 8.95** | 75.50 |
| Constant | 0.64 | ||
| Measurements obtained at 72 hr (R2 = 85.62, F = 74.39**) | |||
| m-NIHSS | 0.75 | 5.72** | 79.00 |
| PLIC damage | 2.28 | 3.39* | 6.62 |
| Constant | −1.58 | ||
| Measurements obtained at day 30 (R2 = 90.10, F = 236.72**) | |||
| m-NIHSS | 0.96 | 15.39** | 90.10 |
| Constant | −0.40 | ||
P< .01;
P< .001.
Association between the Region of the CST Affected in the First 3 Days after Stroke and FA Indexes at Day 30
Our previous study demonstrated that mean FA values along the affected CST were significantly lower than the normal contralateral side only at day 30 after stroke onset (P < .001), and these values were lower than the corresponding FA values obtained at admission and at day 3. Moreover, the decrease in mean FA values correlated positively with the motor deficit at 30 days after stroke.19 Combined involvement of the PLIC and CS or CR at day 30 was not significantly associated with decreased FA indexes (P = 0.445 for the interaction between PLIC and CS; P = .830 for PLIC and CR). Hence, axonal damage reflected as decreased FA ratio values at day 30 was also secondary to PLIC damage. There was no association between infarct volume and WD (r = −0.221, P = .090).
Discussion
We sought to determine whether acute stroke damage to specific CST regions evident at DTT can predict limb motor outcome on a categoric scale based on the m-NIHSS. We found that the involvement of the PLIC alone or in combination with other specific CST regions in the first 12 hours after stroke was strongly associated to severe motor deficits in the first 12 hours and poor motor functional outcome at day 90. Although damage to the CS and CR at day 3 was also associated with poor motor outcome at day 90, PLIC damage in the first 12 hours after stroke was clearly the best predictor of motor deficits and of their severity.
Predictors of motor outcome proposed include location and extension of the stroke specifically within the CST, grade of initial motor deficit, and infarct volume. Our findings corroborate previous studies that found motor outcome is strongly dependent on the integrity of the CST and that the involvement of regions such as the PLIC with more attenuated and organized corticofugal tract fibers is associated with poor long-term recovery after stroke.6 Shelton and Reding14 found that the probability of recovery of upper limb movement at 2 months decreased progressively with the involvement of the MC, CR, or internal capsule. In turn, Schiemanck et al6 found that infarcts involving the internal capsule, alone or in combination with other areas, were associated with a significantly lower probability of hand motor deficit rather than infarcts in the MC, subcortex, or CR. We also found that axonal injury of the CST affected by stroke (as determined by decreased FA values in the pons) in the acute stage was only associated with PLIC damage.
Although it seems logical that larger lesions would correlate with greater deficits,33 we found no correlation between infarct volume and motor outcome at day 90. Motor deficit was present only when critical motor regions were involved, suggesting that large lesions do not necessarily predict poor outcome and that location of the lesion might be more predictive than its size. Whereas subcortical strokes are normally smaller than cortical strokes, they are also more likely to involve both primary MC and PMC fibers, and patients with subcortical infarcts have worse motor outcome than those with cortical stroke.14 These findings may indicate that the extent of damage specifically within the CST is a major determinant of motor deficit.
Previous structural imaging studies designed to predict motor recovery based on lesion location within the CST used conventional axial MR imaging sections and hand-drawn CST masks.6,8 Using T2 changes to assess lesions may not accurately reflect specific neuronal damage, because lesions can be patchy and edema can contribute to T2 signal intensity hyperintensity. Conventional T2-weighted MR imaging provides excellent contrast between white and gray matter but provides no information about fiber direction.34 In contrast, DTT clearly depicts the trajectory of the CST, making it possible to evaluate the topography and extent of tissue damage, particularly in acute stroke.31 We found strong interrater agreement, indicating the reliability and validity of DTT as a lesion mapping technique for this purpose. Recently, some DTI studies have reported that motor outcome could be predicted by using anatomic relationships between the stroke lesion and CST damage on DTT in patients with intracerebral hemorrhage, CR infarct, and lacunar infarcts.15–20,35 Jang et al17 demonstrated that DTT performed at an early stage of pontine infarct (mean DTT scanning, 15 days; range, 5–30 days) is useful for predicting motor outcome. Similarly, another study reported that the degree of CST involvement on DTT within 3 days of stroke onset was strongly correlated with the severity of motor deficit and functional recovery at 3 months in patients with an acute lenticulostriate infarct.19 To our knowledge, ours is the first prospective study to examine consecutive patients with DTT within the first 12 hours after MCA stroke onset.
In the multiple regression analysis, the best model for predicting motor outcome at day 90 in the acute stage was PLIC damage by infarct on DWI alone (not in combination with the clinical parameters); therefore, PLIC damage could be considered an early imaging predictor of poor motor outcome. Several studies have demonstrated that the grade of initial motor deficit is the most important determinant of motor recovery.1–5 In this respect, at day 3 we found that the clinical assessment is the most useful predictor of motor outcome and that adding information about PLIC damage increases the accuracy of the prognosis. Our findings are in line with those obtained by Feys et al4, who analyzed the site of the lesion on CT and MR imaging between 5 and 29 days after stroke (median, 10) and obtained arm motor scores 13 to 37 days after stroke (median, 22). These authors found that arm recovery at 2 months was best predicted by a combination of the motor performance (R2 = 59.21) and purely subcortical lesion location (R2 = 5.31) and that motor recovery at 12 months was best predicted by clinical tests alone (R2 = 53.11) when clinical scores were measured at 2 months after stroke.
Clinical assessment in the acute setting has some limitations. First, it can be difficult to assess the grade of paresis clinically in uncooperative or severely cognitively impaired patients, and clinical findings are occasionally inconclusive or questionable with respect to motor outcome. Second, the ischemic penumbra evidenced by perfusion-diffusion mismatch (not evaluated in the current study) can produce symptoms that are clinically indistinguishable from those produced by the infarct core.36 The ischemic penumbra represents severely hypoperfused tissue around an infarct core; the neurons in the penumbra are supposedly structurally intact but functionally inactive, so penumbral areas are potentially salvageable.37 In our sample, the ischemic penumbra could explain why some patients without CST involvement by infarct presented motor deficits in the acute stage and why the initial motor deficit did not correlate with motor outcome, though the effects of the ischemic penumbra are limited to the acute phase and have no direct effect on the long-term outcome. Hence, if perfusion is restored to penumbral areas and disturbances disappear (eg, at day 3) and the DWI abnormality does not involve the CST, the outcome will be good despite high m-NIHSS score on admission.
Our results show that DTT can be useful in the clinical scenario, making it possible to determine the damage to specific regions of motor pathways in patients with acute stroke consistently, easily, and quickly. Including DTT in acute stroke protocols may generate valid prognostic information because motor outcome seems strongly influenced by CST damage, in particular at the level of the PLIC. In this scenario, DTT could improve the accuracy of prognosis and help improve management in individual stroke patients.
Several limitations to our study should be emphasized. First, we considered long-term clinical follow-up (90 days) because though motor recovery seems to occur predominantly in the first few months after stroke, some patients show considerable recovery in later phases.1 However, although several longitudinal cohort studies and randomized controlled trials found that most of the overall improvement in motor functions occurred within the first month after stroke, some degree of motor recovery continued in some patients in later phases for up to 6 months, especially in subgroups with high motor severity score on admission (59.57% of patients with motor deficit in our cohort). Second, the aim of this study was to design a simple and easy method to evaluate different CST regions qualitatively (affected or not) in the acute stroke scenario; thus, we did not consider quantitative data such as the proportion of damaged fibers, that may have improved the accuracy of our predictions.10,11 Nevertheless, our results indicate that DTT performed within hours of stroke onset is useful for determining which patients are likely to suffer long-term motor deficits. Importantly, this approach eliminates the need for more advanced postprocessing techniques that are more time-consuming and require greater specialization, so it can be applied more widely and benefit more patients. Finally, DTI reflects the averaged water diffusion property within a voxel, which is considered an indirect indicator of the axons; therefore, this approach may oversimplify the model of the axonal structures.31
Conclusions
DTT should be incorporated in MR imaging protocols for acute stroke because determining the damage to specific regions of motor pathways can help predict motor outcome. Our study lends support to the idea that motor outcome is highly dependent on lesion location and the extent to which acute stroke affects the CST. In particular, PLIC damage could be considered an early imaging predictor of poor motor outcome. These findings have implications for the use of lesion mapping techniques in the prognosis of motor outcome after stroke and for establishing more effective criteria for enrolling and evaluating patients in experimental rehabilitation programs. Further research should focus on improving the accuracy of predictions of motor outcome after stroke based on early imaging predictors, with special attention to the prognostic value of DTI.
Supplementary Material
Abbreviations
- BI
Barthel index
- CR
corona radiate
- CS
centrum semiovale
- CST
corticospinal tract
- DTI
diffusion tensor imaging
- DTT
diffusion tensor tractography
- DWI
diffusion-weighted MR imaging
- FA
fractional anisotropy
- IV
intravenous
- MC
motor cortex
- MCA
middle cerebral artery
- m-NIHSS
motor subindex scores of the National Institutes of Health Stroke Scale
- MRI
MR imaging
- mRS
modified Rankin scale
- NS
not significant
- PLIC
posterior limb of the internal capsule
- PMC
premotor cortex
- PWI
perfusion-weighted imaging
- rFA
FA ratio
- ROI
region of interest
- rpb
point-biserial correlation coefficient
- rtPA
recombinant tissue plasminogen activator
- WD
Wallerian degeneration
Footnotes
This project was partially supported by a grant from the Spanish Ministry of Health and Fondo de Investigaciones Sanitarias (PS09/00596 financial information service grant) and by the I-Know research project (027294-I-Know-STREP).
Indicates article with supplemental on-line tables at www.ajnr.org
Indicates article with supplemental on-line figures at www.ajnr.org.
References
- 1. Hendricks HT, van Limbeek J, Geurts AC, et al. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil 2002;83:1629–37 [DOI] [PubMed] [Google Scholar]
- 2. Shelton FD, Volpe BT, Reding M. Motor impairment as a predictor of functional recovery and guide to rehabilitation treatment after stroke. Neurorehabil Neural Repair 2001;15:229–37 [DOI] [PubMed] [Google Scholar]
- 3. Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair 2008;22:64–71 [DOI] [PubMed] [Google Scholar]
- 4. Feys H, Hetebrij J, Wilms G, et al. Predicting arm recovery following stroke: value of site of lesion. Acta Neurol Scand 2000;102:371–77 [DOI] [PubMed] [Google Scholar]
- 5. Kwakkel G, Kollen BJ, van der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34:2181–86 [DOI] [PubMed] [Google Scholar]
- 6. Schiemanck SK, Kwakkel G, Post MW, et al. Impact of internal capsule lesions on outcome of motor hand function at one year post-stroke. J Rehabil Med 2008;40:96–101 [DOI] [PubMed] [Google Scholar]
- 7. Pineiro R, Pendlebury ST, Smith S, et al. Relating MRI changes to motor deficit after ischemic stroke by segmentation of functional motor pathways. Stroke 2000;31:672–79 [DOI] [PubMed] [Google Scholar]
- 8. Pendlebury ST, Blamire AM, Lee MA, et al. Axonal injury in the internal capsule correlates with motor impairment after stroke. Stroke 1999;30:956–62 [DOI] [PubMed] [Google Scholar]
- 9. Chen CL, Tang FT, Chen HC, et al. Brain lesion size and location: effects on motor recovery and functional outcome in stroke patients. Arch Phys Med Rehabil 2000;81:447–52 [DOI] [PubMed] [Google Scholar]
- 10. Zhu LL, Lindenberg R, Alexander MP, et al. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010;41:910–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindenberg R, Renga V, Zhu LL, et al. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 2010;74:280–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rapisarda G, Bastings E, de Noordhout AM, et al. Can motor recovery in stroke patients be predicted by early transcranial magnetic stimulation? Stroke 1996;27:21919–26 [DOI] [PubMed] [Google Scholar]
- 13. van Kuijk AA, Pasman JW, Hendricks HT, et al. Predicting hand motor recovery in severe stroke: the role of motor evoked potentials in relation to early clinical assessment. Neurorehabil Neural Repair 2009;23:45–51 [DOI] [PubMed] [Google Scholar]
- 14. Shelton FN, Reding MJ. Effect of lesion location on upper limb motor recovery after stroke. Stroke 2001;32:107–12 [DOI] [PubMed] [Google Scholar]
- 15. Nelles M, Gieseke J, Flacke S, et al. Diffusion tensor pyramidal tractography in patients with anterior choroidal artery infarcts. AJNR Am J Neuroradiol 2008;29:488–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lai C, Zhang SZ, Liu HM, et al. White matter tractography by diffusion tensor imaging plays an important role in prognosis estimation of acute lacunar infarctions. Br J Radiol 2007;80:782–89 [DOI] [PubMed] [Google Scholar]
- 17. Jang SH, Bai D, Son SM, et al. Motor outcome prediction using diffusion tensor tractography in pontine infarct. Ann Neurol 2008;64:460–65 [DOI] [PubMed] [Google Scholar]
- 18. Cho SH, Kim DG, Kim DS, et al. Motor outcome according to the integrity of the corticospinal tract determined by diffusion tensor tractography in the early stage of corona radiata infarct. Neurosci Lett 2007;426:123–27 [DOI] [PubMed] [Google Scholar]
- 19. Konishi J, Yamada K, Kizu O, et al. MR tractography for the evaluation of functional recovery from lenticulostriate infarcts. Neurology 2005;64:108–13 [DOI] [PubMed] [Google Scholar]
- 20. Lee JS, Han MK, Kim SH, et al. Fiber tracking by diffusion tensor imaging in corticospinal tract stroke: Topographical correlation with clinical symptoms. Neuroimage 2005;26:771–76 [DOI] [PubMed] [Google Scholar]
- 21. Nucifora PG, Verma R, Lee SK, et al. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology 2007;245:367–84 [DOI] [PubMed] [Google Scholar]
- 22. Chung HW, Chou MC, Chen CY. Principles and limitations of computational algorithms in clinical diffusion tensor MR tractography. AJNR Am J Neuroradiol 2011;32:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomalla G, Glauche V, Weiller C, et al. Time course of Wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry 2005;76:266–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Puig J, Pedraza S, Blasco G, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol 2010;31:1324–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brott T, Marler JR, Olinger CP, et al. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke 1989;20:871–75 [DOI] [PubMed] [Google Scholar]
- 26. Saver JL, Johnston KC, Homer D, et al. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANITTAS Investigators. Stroke 1999;30:293–98 [DOI] [PubMed] [Google Scholar]
- 27. Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999;30:1538–41 [DOI] [PubMed] [Google Scholar]
- 28. European Stroke Initiative Executive Committee, EUSI Writing Committee Olsen TS, et al. European stroke initiative recommendations for stroke management—update 2003. Cerebrovasc Dis 2003;16:311–37 [DOI] [PubMed] [Google Scholar]
- 29. Prados F, Boada I, Feixas M, et al. A DTIWeb: a web-based framework for DTI data visualization and processing. Lect Notes Comput Sci 2007;4706:727–40 [Google Scholar]
- 30. Lazar M, Weinstein DM, Tsuruda KM, et al. White matter tractography using diffusion tensor deflection. Hum Brain Mapp 2003;18:306–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology 2004;230:77–87 [DOI] [PubMed] [Google Scholar]
- 32. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74 [PubMed] [Google Scholar]
- 33. Lövblad KO, Baird AE, Schlaug G, et al. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann Neurol 1997;42:164–70 [DOI] [PubMed] [Google Scholar]
- 34. Mamata H, Mamata Y, Westin CF, et al. High-resolution line scan diffusion tensor MR imaging of white matter fiber tract anatomy. AJNR Am J Neuroradiol 2002;23:67–75 [PMC free article] [PubMed] [Google Scholar]
- 35. Cho SH, Kim SH, Choi BY, et al. Motor outcome according to diffusion tensor tractography findings in the early stage of intracerebral hemorrhage. Neurosci Lett 2007;421:142–46 [DOI] [PubMed] [Google Scholar]
- 36. Provenzale JM, Shah K, Patel U, et al. Systematic review of CT and MR perfusion imaging for assessment of acute cerebrovascular disease. AJNR Am J Neuroradiol 2008;29:1476–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schaefer PW, Ozsunar Y, He J, et al. Assessing tissue viability with MR diffusion and perfusion imaging. AJNR Am J Neuroradiol 2003;24:436–43 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.