Abstract
BACKGROUND AND PURPOSE:
Cerebellar and brain stem atrophy are important features in SCA3, whereas SCA6 has been regarded as a “pure” cerebellar disease. However, recent neuropathologic studies have described additional brain stem involvement in SCA6. We, therefore, aimed to investigate the occurrence and impact of regional infratentorial brain volume differences in patients with SCA3 and SCA6.
MATERIALS AND METHODS:
Thirty-four patients with genetically proved SCA (SCA3, n = 17; SCA6, n = 17) and age-matched healthy control subjects (n = 51) were included. In all subjects, high-resolution T1-weighted images were acquired with a 1.5T MR imaging scanner. Individual brain stem and cerebellar volumes were calculated by using semiautomated volumetry approaches. For all patients with SCA, clinical dysfunction was scored according to the ICARS. Multiple regression analysis was used to identify the contribution of regional volumes to explain the variance in clinical dysfunction in each SCA genotype.
RESULTS:
Cerebellar volumes were lower in patients with SCA6 compared with controls and with those with SCA3. In contrast to controls, brain stem volume loss was observed in patients with SCA3 (P < .001) and, to a lesser extent, in those with SCA6 (P = .027). Significant linear dependencies were found between ICARS and cerebellum volume (SCA3: R2 = 0.29, P = .02; SCA6: R2 = 0.29, P = .03) and between ICARS and brain stem volume (SCA3: R2 = 0.49, P = .002; SCA6: R2 = 0.39, P < .01) in both subtypes. Both cerebellar and brain stem atrophy contributed independently to the variance in clinical dysfunction in SCA6, while in SCA3, only brain stem atrophy was of relevance.
CONCLUSIONS:
Our current findings in accordance with recent neuroradiologic and pathoanatomic studies suggest brain stem and cerebellar volume loss as attractive surrogate markers of disease severity in SCA3 and SCA6.
SCA comprises a genetically and clinically heterogeneous group of autosomal dominant disorders, linked genetically to CAG triplet repeat expansions in the most common subtypes: SCA1, SCA2, SCA3, and SCA6.1 Their cardinal feature is progressive ataxia caused by degeneration of the cerebellum. In some subtypes, additional parts of the central and peripheral nervous system can be involved as shown by histopathologic and imaging studies.1–6 Particularly, MR imaging has been proved to detect additional extracerebellar involvement in vivo. Several prior MR imaging studies have demonstrated atrophy in SCA6, mainly of the cerebellum,7–9 whereas additional involvement of the cerebral cortex, basal ganglia, pons, and medulla has been identified by using MR imaging in SCA3.1,4,5,10,11 However, there is increasing evidence of widespread neurodegeneration in SCA6 affecting the brain stem and the primary motor cortex, as shown recently in neuropathologic studies.12,13
So far, only one study has assessed brain stem atrophy in SCA6, applying quantitative MR imaging methods. Those results were limited by the small number of patients, and the impact of those findings was not discussed further.5 In general, structural MR imaging has become a valuable tool for studying the extent of neurodegeneration in SCA in vivo. Therefore, taking previous neuropathologic findings in SCA6 into account, the present study aimed to explore the degenerative alterations evoked by SCA6 in the cerebellum and brain stem by using MR imaging−based volumetry and to determine the impact of these findings on clinical disability, compared with findings in SCA3.
Materials and Methods
Patients
A total of 34 patients with genetically proved spinocerebellar ataxia (SCA3, n = 17; SCA6, n = 17) was studied (Table 1). The age-matched control group consisted of 51 healthy subjects (control group for SCA3: mean age, 49.5 ± 11.0 years [range, 33–71 years]; control group for SCA6: mean age, 63.7 ± 5.9 years [range, 56–73 years]; male/female ratio, 28:23) with no history of neurologic or psychiatric diseases. The following clinical parameters were surveyed for all patients: Degree of disability was scored according to the ICARS, which consists of the 4 subscales: I) posture and gait disturbances, II) kinetic functions, III) speech disorders, and IV) oculomotor disorders.14
Table 1:
Demographic and clinical characteristics of patients with SCA3 and SCA6 (mean ± SD, range)
| SCA3 (n = 17) | SCA6 (n = 17) | Difference of Mean SCA3 vs SCA6a | |
|---|---|---|---|
| Age (yr) | 49.8 ± 11.5 (27–67) | 65.8 ± 6.5 (52–75) | P = .008b |
| Sex (M/F) | 10:7 | 11:6 | |
| Disease duration (yr) | 6.4 ± 4.8 (0–18) | 8.9 ± 5.7 (1–23) | N.S. |
| Age at onset (yr) | 43.4 ± 12.3 (19–62) | 55.6 ± 8.7 (41–67) | P = .004b |
| CAG repeat length | 68.7 ± 3.5 (58–73) | 21.6 ± 0.7 (21–22) | N.A. |
| ICARS | 34.7 ± 10.8 (17–60) | 30.6 ± 10.9 (15–46) | N.S. |
| I) Posture and gait | 11.0 ± 7.8 (2–29) | 9.0 ± 5.2 (3–23) | P = .043c |
| II) Kinetic functions | 13.0 ± 4.8 (2–24) | 13.0 ± 6.1 (5–32) | N.S. |
| III) Speech | 3.0 ± 1.5 (0–5) | 3.0 ± 1.6 (0–6) | N.S. |
| IV) Oculomotor | 4.0 ± 1.5 (0–6) | 5.0 ± 1.2 (2–6) | N.S. |
Statistically significant differences between the SCA3 and SCA6 patient groups under consideration of the disease duration; analysis of variance (GLM model) with disease duration as a covariable.
Significant with P< .01.
Significant with P < .05.
Informed consent was obtained from all patients and volunteers. The study was approved by the local medical ethics committee.
MR Imaging
Brain MR imaging was performed by using a 1.5T scanner (Magnetom Symphony; Siemens, Erlangen, Germany) to obtain sagittal high-resolution T1-weighted (magnetization-prepared rapid acquisition of gradient echo) scans of the whole brain, with the following parameters: TE, 3.93 ms; TR, 1900 ms; TI, 1100 ms; flip angle, 15°; number of excitations, 1; resolution, 1 × 1 × 1.5 mm; matrix, 256 × 256, 128 sagittal sections.
Image Postprocessing
Image data processing was performed by a single trained operator (L.E.) blinded to subject details by using semiautomated volumetric methods as part of the NeuroQLab software (Fraunhofer-MeVis, Bremen, Germany). The reliability and usefulness of this approach have been shown in previous studies for different types of neurodegenerative diseases, including SCA and multiple sclerosis.15,16 All volumes were derived from individual standardized T1-weighted 3D MR imaging datasets.
Cerebellar volumetry was implemented by using a previously described semiautomated method, which has been validated for its reproducibility and usefulness in SCA.17 This postprocessing procedure requires little user interaction; typically the steps for 3D segmentation and volumetry of the cerebellum take <10 minutes. Briefly summarized, after manually defining the upper and lower borders of the cerebellum on the T1-weighted 3D dataset, a cerebellum mask is refined by multiple modified watershed transforms, providing a separation between the brain stem and cerebellum, which is performed anatomically, where the cerebellar peduncles are thinnest. Once the cerebellum mask has been obtained, the histogram of the original data within this mask is analyzed. This automatic histogram analysis is a modification of the model-based whole-brain volumetry method described earlier.16 It has been adapted to the specific shape of cerebellar image-intensity characteristics to robustly yield cerebellar volumes. The histogram model used comprises a voxel-wise weighting factor for partial volume effects occurring at the boundary between cerebellar tissue and CSF, such that cerebellar volume reduction due to successive sulcal widening, even if only minimal, can be sensitively measured.
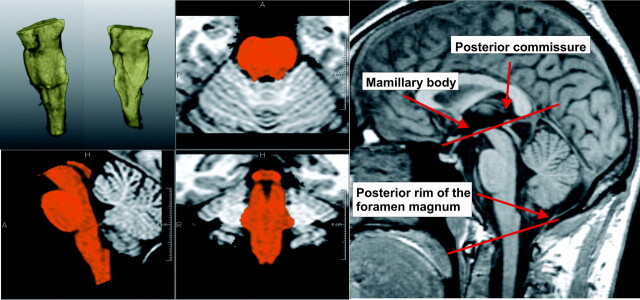
For brain stem segmentation, the same software tool was used, and its capability of classifying cerebellar and brain stem tissue was used to segment the brain stem and assess brain stem volumes (Fig 1). Borders of the brain stem were defined according to the method proposed by Luft et al.18 Briefly, in a first step, a slab with parallel upper and lower boundaries containing the brain stem was cut from the 3D MR imaging dataset. The superior boundary of the brain stem was defined as the para-axial plane between the mamillary bodies and the posterior commissure. The inferior boundary of the brain stem was defined as a parallel plane transecting the posterior rim of the foramen magnum. Also, the ICC was measured for further normalization of CNS volumes.
Fig 1.
Example of quantitative brain stem volumetry. Upper left: 3D segmentation of the brain stem. Lower left and middle: brain stem segmentation in orthogonal views. The borders of the brain stem are defined according to the method proposed by Luft et al.18 Right: midsagittal view showing anatomic landmarks.
Reproducibility of Brain Stem Volumetry
Intrarater and inter-rater reproducibility of brain stem volumetry was evaluated on a group of 16 healthy individuals.
To evaluate the intrarater reproducibility, one investigator who was blinded to subject identities repeated the measurements at least 1 week apart. Similarly, inter-rater reproducibility was assessed by 2 independent investigators. Scan-rescan reproducibility was investigated on a subgroup of 10 subjects who underwent MR imaging twice, either examined on different visits within 1 week or completely repositioned between subsequent MR imaging examinations.
Reproducibility for the group of subjects was expressed as the RC, based on the method proposed by Bland and Altman.19 The RC was calculated as the mean of the absolute differences of the data pairs divided by the mean of all volumetric values.
Statistical Analysis
Statistical analyses were calculated with the Statistical Package for the Social Sciences, Version 16 (SPSS, Chicago, Illinois).
Normal distribution of the volumetric data in each subgroup (healthy controls and patients with SCA3 and SCA6) was confirmed by Kolmogorov-Smirnov testing. The criteria of normal distribution were not fulfilled for all clinical parameters within the subgroups, particularly the ICARS subscore groups and the CAG repeat length.
The age dependence of each volumetric parameter of the control group was modeled by linear regression to correct for natural aging effects. An age-correction operation for natural aging on each CNS volume was performed by linear scaling according to the results of the linear regression of the control group on all data. It was assumed that the age dependence of the patient data is composed of a disease-related fraction and a part that is due to natural aging and that is parallel to the effects in the healthy control group. Thus, the age correction also compensated for the natural age-related proportion of atrophy in the CNS structures in the patient groups. All data were scaled to a mean age of 50 years. To compensate for head-size differences, which can be due to sex and body size, we corrected all volumetric results by normalization according to the ICC.20
Correlation analysis between the volumetric results and the clinical parameters and disease duration and the CAG repeat length in the SCA3 and in the SCA6 group was assessed by Spearman ρ correlation analysis. To take into account disease duration–related effects, we assessed comparisons of the SCA groups' clinical data and comparisons of the clinical data with the CNS volumes by an analysis of variance with disease duration as a covariable and SCA groups as a fixed factor.
The Student t test was used to examine the group differences between the control group and the patients with SCA. Analyses to assess associations between the total ICARS score, the ICARS subscores, and the volumetric results were performed. Linear regression was analyzed between the total ICARS score, and the CNS volumes. Because ICARS score and herein-quantified CNS volumes can be dependent on disease duration, all analyses were repeated with disease duration as a covariable to check for pseudoassociations. Correlations between ICARS subgroups I to IV and CNS volumes were assessed by an ordinal regression analysis with disease duration as a covariate. An ordinal regression analysis was chosen because the value range of the ICARS subscore groups is limited.
In total, sex-specific differences in the normalized results were not found. Therefore, differentiation with respect to sex was not considered in the further analysis.
To find the CNS volumes, which are most strongly associated with clinical dysfunction (as expressed by the total ICARS score) in each SCA genotype, we chose a multiple regression analysis with forward stepwise selection. The criteria used in the regression for inclusion (Pin) and exclusion (Pout) of the variables according to the probability of the F-value were Pin = .05 and Pout = .1. Performance of the model was reported by the percentage of the explained variance of the ICARS score. The multiple regression was corrected for disease duration.
Results
Clinical Measures
Demographic and clinical characteristics of patients with SCA are summarized in Table 1. Patients with SCA6 were older than patients with SCA3. Also, the mean age at disease onset was significantly higher in patients with SCA6 than in those with SCA3. There was no significant difference between groups regarding disease duration and total ICARS score. Taking the corresponding ICARS subscores into account, we found significant differences between both SCA subtypes only for subscore I (posture and gait disturbances), derived mainly by more restricted walking ability in SCA3 compared with SCA6 (P = .015). Disease duration was found to be correlated to total ICARS score in both subtypes; however, no correlation was found between either the CAG repeat length or age at disease onset and ICARS score in either groups (Table 2).
Table 2:
Correlation of disease severity and CNS volumes with disease duration and CAG repeat length
| Disease Duration |
CAG Repeat Length |
|||
|---|---|---|---|---|
| SCA3 | SCA6 | SCA3 | SCA6 | |
| ICARS score | ρ = 0.598 | ρ = 0.632 | N.S. | N.S. |
| P = .011a | P = .006b | |||
| Cerebellar volume | ρ = −0.506 | ρ = −0.623 | N.S. | N.S. |
| P = .038a | P = .017a | |||
| Brain stem | ρ = −0.51 | N.S. | N.S. | N.S. |
| volume | P = .036a | |||
Significant with P < .05.
Highly significant with P < .01.
Age Correction
After normalization to the ICC, significant correlations with age were found for all CNS volumes. These results are in good agreement with other studies concerning brain volume changes in natural aging.21,22 Therefore, age correction for natural aging effects, as described above, was applied to all individual volume results.
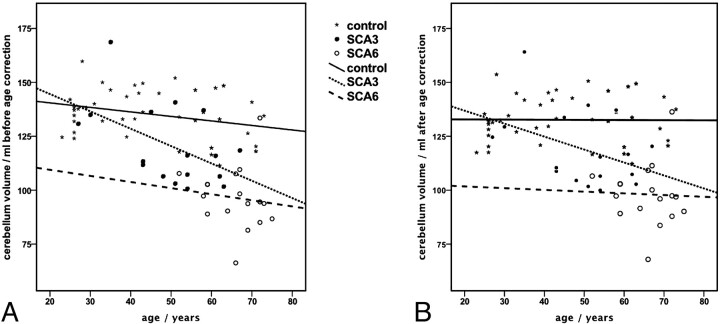
Figure 2 demonstrates the effect of the age correction on the cerebellar volume. Linear regression within the subgroups shows that the age dependence disappeared within the control group (R2 of linear regression before age correction, 0.079; after age correction, <0.0001) and decreased within the SCA patient groups (R2 of linear regression before age correction: SCA3, 0.255; SCA6, 0.016; after age correction: SCA3, 0.161; SCA6, 0.002). We found analog effects for the age correction of the brain stem volumes (data not shown). For the clinical parameters ICARS, ICARS subscore groups, and CAG, which are pure disease-related measures, a correction for natural aging effects was not feasible. In the further analysis steps, only age-corrected cerebellum and brain stem volumes were included.
Fig 2.
Effect of the correction for natural aging on the cerebellum volume. Lines represent the linear regression within the control group and SCA subgroups of age. Cerebellar volumes before (A) and after (B) age correction; age dependence is fully compensated in the control group and reduced in the patient groups.
Volumetry of CNS Structures
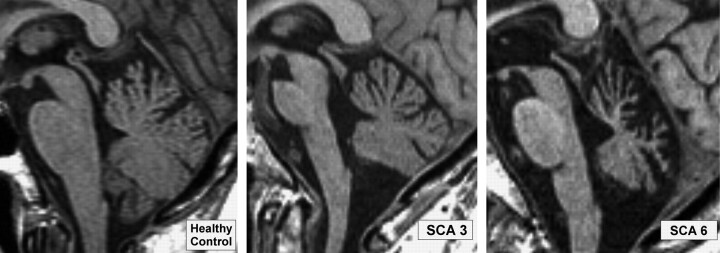
The absolute volumes of the cerebellum and brain stem in our control subjects were in good agreement with the literature.4,23 Group differences for CNS volumetry are given in Table 3. Cerebellar volume was significantly lower in patients with SCA6 compared with controls and with patients with SCA3. In comparison with healthy subjects, patients with SCA3 exhibited significantly lower cerebellar volumes. As expected, significant volume loss was found in the brain stem in patients with SCA3 and, to a lesser extent, in those with SCA6. Figure 3 illustrates typical MR imaging findings in SCA3 and SCA6 in contrast to a healthy control subject.
Table 3:
Cerebellum and brain stem volumes of patients and control subjects
| SCA3 | SCA6 | Control Subjects | |
|---|---|---|---|
| Cerebellum volume (ml) | |||
| Mean ± SD | 119.0 ± 17.2 | 98.1 ± 14.4 | 132.6 ± 11.3 |
| P | 004ab | 006 (SCA3)bc, <.001 (SCA6)b | |
| Brain stem volume (ml) | |||
| Mean ± SD | 21.5 ± 3.8 | 27.3 ± 2.5 | 28.8 ± 2.0 |
| P | <.001ab | <.001 (SCA3),bc .027 (SCA6)d | |
Group differences between SCA3 and SCA6 assessed by variance analysis with disease duration as a covariate (GLM model).
Highly significant with P < .01.
Group differences between control groups and patient groups assessed by Student ttests.
Significant with P < .05.
Fig 3.
Sagittal T1-weighted images of the brain stem in patients with SCA and in a healthy control subject. Brain stem atrophy is clearly visible in SCA3 and to a lesser extent in SCA6. Note the marked atrophy of the cerebellum in SCA6.
Brain Stem Reproducibility Measures
Reproducibility of brain stem volumetry was accurate as shown by the RC for intra- (1.22%) and inter-rater reproducibility (1.5%). The accuracy of this method was further verified by high scan-rescan reproducibility (RC, 1.66%).
Correlations between CNS Volumes and Disease Duration and CAG Repeat Length
The Spearman correlation revealed a significant inverse correlation between disease duration and cerebellar and brain stem volumes in SCA3 (Table 2). In SCA6, disease duration was inversely correlated only to cerebellar volume. In both SCA groups, the age at disease onset was in general not significantly associated with atrophy of the cerebellum or the brain stem. Only in SCA6, we found a tendency for an inverse correlation between brain stem volume and age at disease onset (ρ = −0.483, P = .080). Correlation analysis with CAG repeat length was not appropriate in SCA6 because the CAG range was very restricted in this group. In SCA3, no significant correlations between CNS volumes and CAG repeat length were found.
Associations between CNS Volumes and Clinical Disability
CNS Volumes and ICARS.
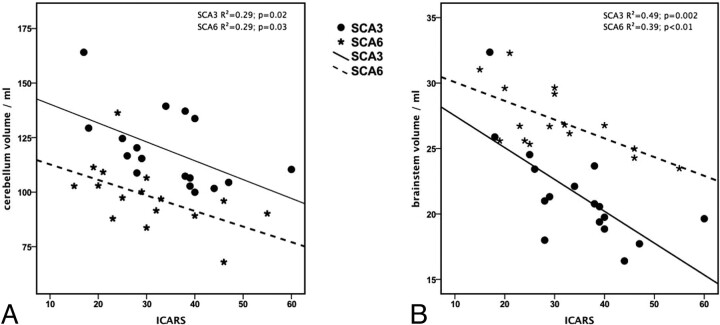
Associations between the ICARS and CNS volumes with and without adjusting for disease duration are summarized in Table 4. Linear regression between total scores of ICARS and CNS volumes revealed significant linear dependencies between ICARS and cerebellar volume and between ICARS and brain stem volume in patients with SCA3 and SCA6 (Fig 4).
Table 4:
Linear regression between ICARS total score and CNS volumes: simple linear regression and linear regression including disease duration as a covariate
| SCA | Linear Regression Type | Coefficient B (95% CI) | R2 | P | |
|---|---|---|---|---|---|
| Cerebellar volume | 3 | Simple | −0.341 (−0.631 to −0.543) | 0.29 | .024a |
| Including DD | −0.19 (−0.50 to −0.11) | 0.46 | .20 | ||
| 6 | Simple | −0.407 (−0.758 to −0.057) | 0.30 | .026a | |
| Including DD | −0.33 (−0.76 to −0.14) | 0.31 | .15 | ||
| Brain stem volume | 3 | Simple | −2.01 (−3.141 to −0.879) | 0.49 | .002b |
| Including DD | −1.51 (−2.7 to −0.31) | 0.60 | .02a | ||
| 6 | Simple | −2.732 (−4.626 to −0.837) | 0.39 | .008b | |
| Including DD | −2.37 (−4.3 to −0.44) | 0.46 | .02a |
Significant with P < .05
Significant with P < .01.
Fig 4.
Linear regression graphs illustrating the relationship between ICARS score and cerebellar (A) and brain stem (B) volume for both subtypes.
To exclude spurious associations via the dependency on disease duration, we repeated the regression analysis with disease duration as a covariate (Table 4). In both subtypes, the linear associations with total ICARS scores remained significant for brain stem volume and diminished for cerebellar volume, when including disease duration.
CNS Volumes and ICARS Subscores.
The associations between the CNS volumes and the 4 ICARS subscores were assessed by ordinal regression analyses, in which the pseudo-R2 represents a goodness-of-fit measure of the regression. Table 5 details the results of the ordinal regression between ICARS subscores and CNS volumes with and without including disease duration as a covariate:
Posture and Gait. Posture and gait functions showed linear dependency on cerebellar and brain stem volume in both SCA3 and SCA6. When taking disease duration as a covariate into account, we found that these associations were no longer significant.
Kinetic Functions. Kinetic functions depended linearly on brain stem volume only in the SCA6 group. This association was still significant when taking disease duration into account.
Speech. The impairment of speech showed a significant linear association with the volume of the cerebellum in the SCA6 group. When taking disease duration as a covariate into account, we found that this association was no longer significant. No associations were found in SCA3.
Oculomotor Functions. The impairment of oculomotor functions in the SCA3 group was significantly linear-dependent on the volumes of the cerebellum and the brain stem. This significance ceased for the brain stem volume when including disease duration as a covariate, whereas dependency of the oculomotor subscore on cerebellar volume remained significant. In SCA6, when we took disease duration into account, oculomotor function was significantly linear-dependent on brain stem volume.
Table 5:
Ordinal regression between ICARS subscore groups and CNS volumes: simple ordinal regression and ordinal regression including disease duration (DD) as a covariate
| Regression Type | SCA | Cerebellum |
Brain Stem |
||
|---|---|---|---|---|---|
| Simple Ps.R2P | Including DD Ps.R2P | Simple Ps.R2P | Including DD Ps.R2P | ||
| I) Posture and gait | 3 | 0.35 0.018a | 0.45 0.10 | 0.23 0.025a | 0.41 0.11 |
| 6 | 0.41 0.010a | 0.43 0.062 | 0.23 0.044a | 0.36 0.10 | |
| II) Kinetic functions | 3 | 0.05 0.32 | 0.065 0.72 | 0.14 0.14 | 0.14 0.19 |
| 6 | 0.21 0.053 | 0.27 0.29 | 0.29 0.025a | 0.40 0.048a | |
| III) Speech functions | 3 | 0.08 0.26 | 0.49 0.62 | 0.03 0.52 | 0.51 0.32 |
| 6 | 0.30 0.036a | 0.43 0.11 | 0.07 0.27 | 0.41 0.521 | |
| IV) Oculomotor functions | 3 | 0.46 0.007b | 0.57 0.048a | 0.30 0.03a | 0.49 0.18 |
| 6 | 0.04 0.37 | 0.05 0.42 | 0.17 0.10 | 0.24 0.046a | |
Significant with P < .05.
Significance with P < .01.
Multiple Regression Analysis
Stepwise multiple regression analysis for both SCA subtypes included ICARS total score as a dependent variable and disease duration, brain stem volume, and cerebellar volume as independent variables. In SCA3, the brain stem volume explained 49% of the variance of ICARS score. The other variables were not able to improve the model (Table 6). In SCA6, the cerebellum alone explained 26% of the variance (OR = −0.41, 95% CI = −0.76 to −0.06, P = .026). However, the stepwise inclusion of cerebellar and brain stem volumes together explained 55% of the variance of ICARS score, indicating that the inclusion of brain stem volume in SCA6 independently improves the model (Table 7). Brain stem volume alone explained 38% of the variance in ICARS score in SCA6.
Table 6:
Results of stepwise multivariate regression for SCA3 with ICARS score as the dependent variable and brain stem volume and cerebellum volume as independent variables
| Model | R2 of the Model | Included Predictor | B | OR | 95% CI of B | P Value |
|---|---|---|---|---|---|---|
| 1 | 0.489 | Intercept | 77.9 | |||
| Brain stem volume | −2.01 | −0.7 | −3.14 −0.88 | 0.02 |
Table 7:
Results of stepwise multivariate regression for SCA6 with ICARS score as the dependent variable and brain stem and cerebellum volumes as independent variables
| Model | R2 of the Model | Included Predictors | B | OR | 95% CI of B | P Value |
|---|---|---|---|---|---|---|
| 1 | 0.386 | Intercept, | 105.03 | |||
| Brain stem volume | −2.73 | −0.62 | −4.63 −0.84 | .008 | ||
| 2 | 0.553 | Intercept, | 124.67 | |||
| Brain stem volume, | −2.32 | −0.53 | −4.04 −0.58 | .012 | ||
| Cerebellar volume | −0.32 | −0.42 | −0.61 −0.02 | .039 |
Discussion
For a long time, SCA6 has been referred to as “pure” cerebellar ataxia with the characteristics of late onset, no limitation of life expectancy, and dominant involvement of the cerebellar cortex.1 However, there is increasing evidence of a widespread affection of extracerebellar structures in SCA6, such as the thalamus, midbrain, and even the brain stem as shown by recent postmortem studies.12,13
In the current volumetric MR imaging study, we found significant atrophy not only of the cerebellum but also of the brain stem in SCA6. Both cerebellar atrophy and brain stem atrophy contributed independently to the variance in clinical dysfunction in patients with SCA6. Using a larger cohort of patients with SCA6, we were able to confirm recent findings by Schulz et al5 showing that in addition to cerebellar atrophy, brain stem atrophy is of clinical relevance in patients with SCA6 and can be detected by quantitative MR imaging measurements.
So far only a few studies have focused on the assessment of brain stem volumes in SCA6, with controversial results regarding the presence and clinical relevance of brain stem atrophy.5,7,8 Although Murata et al8 found a reduction of the anteroposterior diameter of the pons in patients with SCA6, results failed to show a correlation with CAG repeat length. The relationship between the volume changes and clinical dysfunction was not tested in this study. In contrast, Butteriss et al7 did not find significant atrophy of the ventral portion of the pons in SCA6. However, in a recently published quantitative multicenter MR imaging study, brain stem atrophy was detected in a limited number (n = 10) of patients with SCA6.5 In this study, cerebellar and brain stem volumes together were the only MR imaging parameters explaining the variance of clinical dysfunction as expressed by the SARA in SCA6.
Given our results, there are 2 possible explanations of the discrepancies in the studies of Butteriss et al, 2005,7 and Murata et al, 1998.8 On one hand, brain stem tissue loss was assessed by different techniques. In the present study, as well as in the study published by Schulz et al,5 brain stem atrophy was quantified by using 3D MR imaging data. These techniques are more sensitive and less observer-dependent than planimetric approaches used in the other studies. Especially the herein-used approach for brain stem volumetry has been shown to be highly suitable and reliable for quantifying brain stem atrophy with respect to reproducibility and absolute volumetry. On the other hand, the use of different ataxia scores and low numbers of subjects might explain variable results in SCA6. Although we and Schulz et al used different rating scales to quantify clinical dysfunction, both studies revealed a relevance of the brain stem volume in SCA6. In our study, brain stem and cerebellar volumes together explained only 55% of the variance in clinical dysfunction, which was slightly lower than that recently described by Schulz et al. In our study, brain stem volume correlated significantly with ICARS in SCA6, whereas such a correlation could not be established for SARA in the study by Schulz et al. This finding might be explained by differences between both scores regarding the rating items.
The overall range of the total sum score is broader in ICARS than in SARA (100 versus 40 points), and rating results in all SARA items seem to be loaded on a single factor, whereas >1 factor is determined in the ICARS.24,25 Hence, the SARA only rates ataxia-related symptoms.25 This possibly leads to a higher contribution of cerebellar volume loss on the disease severity score, especially in diseases in which ataxia is dominant. Additional features like oculomotor dysfunction are not reflected in the SARA score. Because approximately 25% of all patients with SCA6 are affected by such extracerebellar oculomotor signs, ICARS may be superior to SARA when correlating extracerebellar MR imaging findings with clinical dysfunction.26
Closer inspection of the correlations between ICARS subscore groups and volumes of infratentorial structures revealed a significant correlation in SCA3 between cerebellar volume and oculomotor function, while in SCA6, brain stem volume correlated significantly to kinetic functions and oculomotor functions.
To the best of our knowledge, there has been only 1 MR imaging study published in which ICARS subscores have been taken into account. Richter et al27 found significant correlations between lower limb ataxia and cerebellar volume in a heterogeneous group of patients with pure cerebellar degeneration, also including 7 patients with SCA6. In our present study, we were not able to replicate this finding in a homogeneous SCA6 cohort. However, by including brain stem volumetry, we showed that mainly oculomotor and kinetic dysfunctions were associated with brain stem atrophy in this SCA genotype. Furthermore, in agreement with Richter et al, we were not able to find a significant correlation between cerebellar volumes and the oculomotor subscore of the ICARS in SCA6. However, it was surprising to observe that this correlation was noted among patients with SCA3.
Although one would expect a closer correlation between kinetic dysfunction and cerebellar atrophy in SCA6, ataxia may not be related exclusively to cerebellar hemispheric or vermian damage but may also be due to affection of its specific afferent and efferent connections (eg, within the pons).28,29 As with ataxia, oculomotor dysfunction may result as a consequence of damage in distinct functional compartments. Oculomotor dysfunction may be related to brain stem nuclei involvement, such as the superior and medial vestibular nuclei,13 or to a lesion of the cerebellum, in particular the oculomotor vermis.30,31 Unfortunately, we were not able to perform more specific morphometric analysis (eg, segmentation of cerebellar hemispheres and vermis into lobular regions), which would have strengthened our results. However, our results suggest that the clinical-pathologic correlation in SCA3 and SCA6 is more widespread (eg, affecting pontocerebellar circuits rather than being restricted to a specific neuroanatomic region).
In SCA6, Gierga et al13 found, in a postmortem study, that the extent of brain stem neurodegeneration correlated with the age at disease onset and disease duration. Their interpretation was that the severity of degeneration in SCA6 might be determined by these 2 factors. In contrast, our study and the MR imaging−based analysis by Schulz et al5 did not confirm such a correlation in vivo. An explanation for this discrepancy may be that especially early subtle tissue changes may not be detected by these global volumetric MR imaging approaches. Here, atrophy detection by MR imaging−based volumetry may require a more extensive tissue loss. Furthermore, most of our patients with SCA6 were studied at an earlier disease stage so that direct comparison with the postmortem study may not be feasible.
In SCA3, the degenerative process involves different parts of the brain.1,6 Brain stem atrophy in SCA3 has been assessed in various MR imaging studies, and its impact on clinical severity has been demonstrated recently.4,5,10,11,32 We found brain stem volume to be markedly decreased in patients with SCA3. Although patients with SCA3 exhibited additional volume loss in the cerebellum, atrophy of the brain stem was the strongest MR imaging marker explaining the variance of clinical dysfunction in this genotype. These results, corresponding to the findings of Schulz et al,5 were that clinical dysfunction in SCA3 was significantly correlated to brain stem involvement. Thus, brain stem volumetry may be of growing importance as a surrogate marker for disease-related disability in SCA3 in future therapeutic trails.
Because we relied on a semiautomatic volumetry approach, we did not analyze basal ganglia volumes, though the basal ganglia are frequently involved in SCA3 and this area has been intensively studied in neuropathologic and imaging studies.4–6,10 However, according to recently published MR imaging data using manual segmentation of the basal ganglia, the impact of basal ganglia atrophy on clinical dysfunction in SCA3 seems to be limited.5
Conclusions
Our current findings, in accordance with recent neuroradiologic and pathoanatomic studies, suggest brain stem and cerebellar volume loss as attractive surrogate markers of disease severity in SCA3 and SCA6. Longitudinal studies are required to further establish MR imaging volumetry as a potential biomarker to monitor interventional trials in SCA.
Acknowledgments
We thank Dr Silke Lange and Hiltrud Niggemann for their helpful comments on the statistical analysis.
Abbreviations
- B
parameter estimates (coefficient B)
- CAG
cytosine-adenine-guanine
- CI
confidence interval
- 95% CI
95% confidence interval of B (Table 6)
- CNS
central nervous system
- DD
disease duration
- ICARS
International Cooperative Ataxia Rating Scale
- GLM
general linear model
- ICC
intracranial capacity
- lntercept
log odds (logit estimate)
- N.A.
not applicable
- N.S.
not significant
- OR
odds ratio
- ρ
correlation coefficient
- Ps.R2
pseudo-R2 of the regression
- R2
coefficient of determination
- RC
repeatability coefficient
- SARA
Scale for the Assessment and Rating of Ataxia
- SCA
spinocerebellar ataxia
References
- 1. Schöls L, Bauer P, Schmidt T, et al. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol 2004; 3: 291–304 [DOI] [PubMed] [Google Scholar]
- 2. Brenneis C, Bosch SM, Schocke M, et al. Atrophy pattern in SCA2 determined by voxel-based morphometry. Neuroreport 2003; 14: 1799–802 [DOI] [PubMed] [Google Scholar]
- 3. Della Nave R, Ginestroni A, Tessa C, et al. Brain white matter damage in SCA1 and SCA2: an in vivo study using voxel-based morphometry, histogram analysis of mean diffusivity and tract-based spatial statistics. Neuroimage 2008; 43: 10–19 [DOI] [PubMed] [Google Scholar]
- 4. Klockgether T, Skalej M, Wedekind D, et al. Autosomal dominant cerebellar ataxia type I: MRI-based volumetry of posterior fossa structures and basal ganglia in spinocerebellar ataxia types 1, 2 and 3. Brain 1998; 121: 1687–93 [DOI] [PubMed] [Google Scholar]
- 5. Schulz JB, Borkert J, Wolf S, et al. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage 2010; 49: 158–68 [DOI] [PubMed] [Google Scholar]
- 6. Rub U, Brunt ER, Deller T. New insights into the pathoanatomy of spinocerebellar ataxia type 3 (Machado-Joseph disease). Curr Opin Neurol 2008;21:111–16 [DOI] [PubMed] [Google Scholar]
- 7. Butteriss D, Chinnery P, Birchall D. Radiological characterization of spinocerebellar ataxia type 6. Br J Radiol 2005; 78: 694–96 [DOI] [PubMed] [Google Scholar]
- 8. Murata Y, Kawakami H, Yamaguchi S, et al. Characteristic magnetic resonance imaging findings in spinocerebellar ataxia 6. Arch Neurol 1998; 55: 1348–52 [DOI] [PubMed] [Google Scholar]
- 9. Satoh JI, Tokumoto H, Yukitake M, et al. Spinocerebellar ataxia type 6: MRI of three Japanese patients. Neuroradiology 1998; 40: 222–27 [DOI] [PubMed] [Google Scholar]
- 10. Murata Y, Yamaguchi S, Kawakami H, et al. Characteristic magnetic resonance imaging findings in Machado-Joseph disease. Arch Neurol 1998; 55: 33–37 [DOI] [PubMed] [Google Scholar]
- 11. Yoshizawa T, Watanabe M, Frusho K, et al. Magnetic resonance imaging demonstrates differential atrophy of pontine base and tegmentum in Machado-Joseph disease. J Neurol Sci 2003; 215: 45–50 [DOI] [PubMed] [Google Scholar]
- 12. Rub U, Brunt ER, Petrasch-Parwez E, et al. Degeneration of ingestion-related brainstem nuclei in spinocerebellar ataxia type 2, 3, 6 and 7. Neuropathol Appl Neurobiol 2006; 32: 635–49 [DOI] [PubMed] [Google Scholar]
- 13. Gierga K, Schelhaas HJ, Brunt ER, et al. Spinocerebellar ataxia type 6 (SCA6): neurodegeneration goes beyond the known brain predilection sites. Neuropathol Appl Neurobiol 2009; 35: 515–27 [DOI] [PubMed] [Google Scholar]
- 14. Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome: the Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 1997; 145: 205–11 [DOI] [PubMed] [Google Scholar]
- 15. Lukas C, Hahn HK, Bellenberg B, et al. Spinal cord atrophy in spinocerebellar ataxia type 3 and 6: impact on clinical disability. J Neurol 2008; 255: 1244–49 [DOI] [PubMed] [Google Scholar]
- 16. Lukas C, Hahn HK, Bellenberg B, et al. Sensitivity and reproducibility of a new fast 3D segmentation technique for clinical MR-based brain volumetry in multiple sclerosis. Neuroradiology 2004; 46: 906–15 [DOI] [PubMed] [Google Scholar]
- 17. Lukas C, Bellenberg B, Rexilius J, et al. A new sulcus-corrected approach for assessing cerebellar volume in spinocerebellar ataxia. European Congress of Radiology; ECR 2010, March 4–8, 2010, Vienna, Austria: [DOI] [PubMed] [Google Scholar]
- 18. Luft AR, Skalej M, Welte D, et al. A new semiautomated, three-dimensional technique allowing precise quantification of total and regional cerebellar volume using MRI. Magn Reson Med 1998; 40: 143–51 [DOI] [PubMed] [Google Scholar]
- 19. Bland M, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–10 [PubMed] [Google Scholar]
- 20. Whitwell JL, Crum WR, Watt HC, et al. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am J Neuroradiol 2001; 22: 1483–89 [PMC free article] [PubMed] [Google Scholar]
- 21. Blatter DD, Bigler ED, Gale SD, et al. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR Am J Neuroradiol 1995; 16: 241–51 [PMC free article] [PubMed] [Google Scholar]
- 22. Pfefferbaum A, Mathalon DH, Sullivan EV, et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol 1994; 51: 874–87 [DOI] [PubMed] [Google Scholar]
- 23. Luft AR, Skalej M, Schulz JB, et al. Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cereb Cortex 1999; 9: 712–21 [DOI] [PubMed] [Google Scholar]
- 24. Burk K, Malzig U, Wolf S, et al. Comparison of three clinical rating scales in Friedreich ataxia (FRDA). Mov Disord 2009; 24: 1779–84 [DOI] [PubMed] [Google Scholar]
- 25. Schmitz-Hubsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 2006; 66: [DOI] [PubMed] [Google Scholar]
- 26. Schmitz-Hubsch T, Coudert M, Bauer P, et al. Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms. Neurology 2008; 71: 982–89 [DOI] [PubMed] [Google Scholar]
- 27. Richter S, Dimitrova A, Maschke M, et al. Degree of cerebellar ataxia correlates with three-dimensional MRI-based cerebellar volume in pure cerebellar degeneration. Eur Neurol 2005; 54: 23–27 [DOI] [PubMed] [Google Scholar]
- 28. Mitoma H, Hayashi R, Yanagisawa N, et al. Gait disturbances in patients with pontine medial tegmental lesions: clinical characteristics and gait analysis. Arch Neurol 2000; 57: 1048–57 [DOI] [PubMed] [Google Scholar]
- 29. Dietrichs E. Clinical manifestation of focal cerebellar disease as related to the organization of neural pathways. Acta Neurol Scand Suppl 2008; 188: 6–11 [DOI] [PubMed] [Google Scholar]
- 30. Wessel K, Moschner C, Wandinger KP, et al. Oculomotor testing in the differential diagnosis of degenerative ataxic disorders. Arch Neurol 1998; 55: 949–56 [DOI] [PubMed] [Google Scholar]
- 31. Thier P, Dicke PW, Haas R, et al. The role of the oculomotor vermis in the control of saccadic eye movements. Ann N Y Acad Sci 2002; 978: 50–62 [DOI] [PubMed] [Google Scholar]
- 32. Lukas C, Schols L, Bellenberg B, et al. Dissociation of grey and white matter reduction in spinocerebellar ataxia type 3 and 6: a voxel-based morphometry study. Neurosci Lett 2006; 408: 230–35 [DOI] [PubMed] [Google Scholar]