Abstract
BACKGROUND AND PURPOSE:
The relationship between white matter disruption and cognitive dysfunction of patients with mTBI in the chronic stage remains unclear. The aim of this study was to identify white matter integrity by using DTI in patients with mTBI without morphologic traumatic abnormalities seen with conventional imaging and to evaluate the association of such regions with cognitive function.
MATERIALS AND METHODS:
Diffusion tensor images from 51 consecutive patients with mTBI without morphologic traumatic abnormalities on conventional MRI were processed, and FA maps were generated as a measure of white matter integrity. All subjects underwent cognitive examinations (MMSE and WAIS-R FIQ). Correlations between the skeletonized FA values in the white matter and the cognitive function were analyzed by using regression analysis.
RESULTS:
In patients with mTBI, significantly decreased FA value clusters in the white matter compared with the healthy controls were found in the superior longitudinal fasciculus, superior frontal gyrus, insula, and fornix. Cognitive examination scores positively correlated with FA values in a number of regions in deep brain structures, which were anatomically close or physiologically intimate to the regions with significant FA value reduction, in patients with mTBI.
CONCLUSIONS:
The present study shows that patients with mTBI in the chronic stage have certain regions with abnormally reduced white matter integrity in the brain. Although the clinical and pathologic-anatomic correlation of these findings remains to be elucidated, these brain regions are strongly suggested to be related to chronic persistent cognitive impairments in these patients.
An estimated 1–2 million people sustain a nonfatal TBI each year in the United States by various means, including crashes in motor vehicles, sports, and assaults.1 Approximately 80% of these injuries are classified as mild2,3 with loss of consciousness lasting <30 minutes, an initial GCS score of 13–15, or posttraumatic amnesia lasting <24 hours.3 Despite its frequency, pathophysiologic, neurophysiologic, and neuropsychologic mechanisms of mTBI remain poorly understood.4,5 Clinically, TBI is associated with symptoms of impaired cognitive function, memory disturbance, decreased activity, failures of emotional control, easy anger, carelessness, excessive tenacity, planning failure for execution, and excessive dependence. These symptoms often cause social problems in families, schools, work places, and other communities.6
Neural tissue damage following mTBI is referred to as DAI. It is often subtle and difficult to detect.7 Diagnostic imaging of mTBI can increase our understanding of the clinical symptoms and help determine treatment strategies. In particular, DTI is sensitive to the diffusion characteristics of water (such as the principal diffusion direction and diffusion anisotropy) and has been developed as a tool to investigate the integrity of brain tissues such as white matter tracts8 and to uncover discrete axonal injury.9 Diffusion anisotropy describes how variable the diffusion is in different directions and is most commonly quantified in a ratio of axial-to-radial diffusivity known as fractional anisotropy. In general, FA values are highest in major white matter tracts (the maximum theoretic value of 1) and lower in the gray matter, while approaching zero in the CSF.10 Variations in FA within white matter structures as an indicator of DAI reflect multiple factors, including myelination, axon attenuation, axonal membrane integrity, axon diameter, and intravoxel coherence of fiber orientation.11 A recent study showed the complexity of the relationship between FA and cognitive function in patients with TBI who were classified as having severe, moderate, or mild cases and those with or without microbleeds.12 The relationship between FA and cognitive function when restricted to patients with mTBI without microbleeds is still not well-understood.
To perform voxelwise correlation analysis, we used TBSS, which is a fully automated whole-brain analysis technique that uses voxelwise statistics on diffusion metrics but simultaneously minimizes the effects of misalignment by using a conventional voxel-based analysis method.13,14 The purpose of this study was to identify white matter integrity by using skeletonized maps that were processed by using TBSS in patients with mTBI without morphologic traumatic abnormalities on conventional MR imaging and to evaluate the correlation between FA values in the white matter and cognitive function by using regression analysis.
Materials and Methods
Patients
Fifty-one consecutive patients (28 men and 23 women; mean age, 37.1 ± 10.2 years; range, 21–58 years) with cognitive, behavioral, or emotional impairments in a chronic stage were enrolled in this study (Table 1). They all had mTBI due to crashes in motor vehicles (an initial GCS score: 13–15 points) and were outpatients at Kizawa Memorial Hospital from August 2004 to June 2009. The study excluded those patients with intracranial surgery and those with a history of penetrating head injury, open skull fracture, neurologic disease unassociated with the injury, mental retardation, psychiatric disease, or alcohol or substance abuse. All of the enrolled patients were at least 6 months postinjury (average, 35.1 ± 3.7 months; range, 6–88 months) at the time of the study and underwent neurologic and cognitive examinations, including the MMSE and WAIS-R FIQ, as well as neuroimaging within 2 weeks after consultation. Patients had no morphologic abnormalities such as large focal neuroradiologic lesions, hydrocephalus, or brain atrophy on MR imaging. Age-matched control volunteers (30 men and 20 women; mean age, 35.8 ± 13.4 years; range, 22–60 years) were recruited (Table 1). Patients and control participants who were left-handed were also excluded.
Table 1:
Subject characteristicsa
| Patients (n = 51) | Controls (n = 50) | |
|---|---|---|
| Age (yr) | 37.1 ± 10.2 | 35.8 ± 13.4 |
| Male sex (%) | 55.0 | 60.0 |
| Initial GCS (range) | 14.8 ± 0.6 (13–15) | |
| Time postinjury (mo) | 35.1 ± 26.3 | |
| MMSE | 27.9 ± 2.4 | |
| FIQ (WAIS-R) | 87.4 ± 18.5 |
Expressed as mean ± SD.
The study was approved by our institutional research ethics committee and was in accordance with the Declaration of Helsinki. All subjects provided informed written consent.
Data Acquisition and Imaging Parameters
Whole-brain conventional MR imaging and DTI were acquired on a 1.5T MR imaging scanner (Signa; GE Healthcare, Milwaukee, Wisconsin) by using a quadrature transmit-receive head coil. The conventional MR imaging protocol included T2-weighted fast spin-echo (TR/TE/ NEX = 2600 ms/107 ms/20), T1-weighted FLAIR (TR/TE/TI/NEX = 1850 ms/34 ms/805 ms/1), T2-weighted FLAIR (TR/TE/TI/NEX = 8000 ms/115 ms/2000 ms/1), and T2* gradient-recalled echo sequences (TR/TE/NEX/flip angle = 1550 ms/18 ms/1/20°). In all sequences, a total of 16 contiguous sections were acquired in the axial plane with 6-mm section thickness, 512 × 256 matrix size, and a 240 × 240 mm FOV.
Diffusion tensor images were acquired with a single-shot echo-planar sequence (TR/TE/NEX = 10,000 ms/80 ms/4, section thickness = 3 mm, matrix size = 128 × 128, and FOV = 250 × 250 mm). Diffusion gradients were set in 6 noncollinear directions by using 2 b-values (b = 0 and 1000 s/mm2).
Statistical Analysis
For DTI, diffusion-weighted volume images were registered to the b=0 image by affine transformations to minimize distortion due to motion and eddy currents and then brain-extracted by using the Brain Extraction Tool from the FMRIB Software Library Image Processing Toolbox (http://www.fmrib.ox.ac.uk/fsl/),15 and FA images were generated by using the Diffusion Toolbox.16
Voxelwise analysis of FA images was performed by using TBSS in the FMRIB Software Library.13 Image analysis by using TBSS involves the following steps: 1) nonlinear alignment of all FA images to the common FMRIB58 FA template space, 2) affine-transformation of the aligned images into 1 × 1 × 1 mm3 MNI152 space (a normalized/averaged brain atlas developed by the Montreal Neurological Institute), 3) averaging of the aligned FA images to create a 4D mean FA image, 4) thinning of the mean FA image to create a mean FA “skeleton ” representing the center of all white matter tracts and thereby removing partial volume confounds; and 5) thresholding of the FA skeleton at FA ≥ 0.3 to suppress areas of extremely low mean FA and to exclude regions with considerable interindividual variability.
For voxelwise analysis of skeletonized FA images, patients with mTBI were compared with control volunteers. A randomization procedure with voxel-based thresholding and 5000 permutations was used to perform group analysis statistics. The nonparametric 2-sample t test was used to detect differences between patients with mTBI and controls.
The randomization procedure and a simple regression analysis were used to investigate the relationship between white matter structural integrity and cognitive function. These statistical analyses were thresholded at a probability of P < .01, with the extent threshold set at 8 voxels. Fiber tracts corresponding to a particular cluster were identified in reference to the white matter atlas.17 The correlation between cognitive variables and FA values was examined by plotting an individual subject's peak FA values from significant regions against his or her scores on the cognitive tests (MMSE, WAIS-R FIQ).
Results
FA Differences between mTBI and Healthy Control Groups
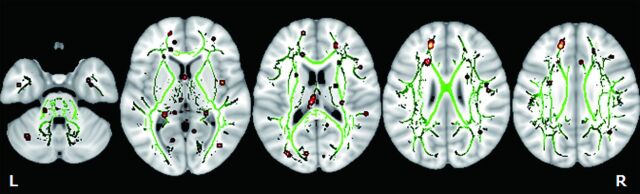
Results of TBSS comparative analysis of DTI between the mTBI and healthy control groups yielded several clusters of voxels with significantly (P < .01) decreased FA on the white matter skeleton, which were located in the right superior longitudinal fasciculus, left superior frontal gyrus, right insula, and left fornix (Fig 1). For each cluster, the anatomic location, the total number of voxels and the z score are listed in Table 2. There were no significant increases in FA values of patients with mTBI compared with controls.
Fig 1.
TBSS analysis of the white matter skeleton. Voxels demonstrating significantly (P < .01) decreased FA values for the subjects with mTBI compared with the control group are shown in red-yellow. Voxels are thickened into local tracts and overlaid on the white matter skeleton (green). Further cluster details are given in Table 2.
Table 2:
Anatomic location of decreased FA clusters in the mTBI group compared with controls
| Hemisphere | Anatomic Location | MNI Coordinates |
Z Score | No. of Voxels | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Right | Superior longitudinal fasciculus | 33 | −4 | 22 | 4.1 | 8 |
| Left | Superior frontal gyrus | −16 | 40 | 27 | 3.86 | 24 |
| Right | Insula | 41 | −1 | 6 | 3.74 | 9 |
| Left | Fornix | −4 | −22 | 17 | 3.38 | 11 |
Correlation between FA Values and Cognitive Examinations
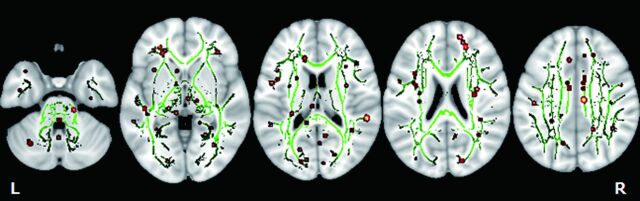
Cognitive scores of the patients are summarized in Table 1. Mean ± SD MMSE and FIQ scores in the mTBI group were 27.9 ± 2.4 and 87.4 ± 18.5, respectively. Mini-Mental State Examination scores were correlated positively with FA values in the right supramarginal gyrus, left inferior longitudinal fasciculus, right superior parietal lobule, right sagittal stratum, left middle frontal gyrus, right superior frontal gyrus, left cerebellum, right cingulum, and right superior occipital gyrus in the mTBI group (Fig 2). These results are summarized in Table 3. The correlations of FA values to MMSE scores for all regions showing significant (P < .01) associations are shown (On-line Fig 1). There were no regions where FA values negatively correlated with MMSE scores in the brain.
Fig 2.
TBSS analysis of the white matter skeleton. Voxels demonstrating FA values correlated significantly (P < .01) to MMSE are shown in red-yellow. Voxels are thickened into local tracts and overlaid on the white matter skeleton (green). Further cluster details are given in Table 3.
Table 3:
Anatomic locations showing a positive correlation of MMSE and FA values in patients with mTBI
| Label | Hemisphere | Anatomic location | MNI Coordinates |
Z Score | No. of Voxels | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| A | Right | Supramarginal gyrus | 54 | −36 | 17 | 4.9 | 23 |
| B | Left | Inferior longitudinal fasciculus | −26 | 12 | −9 | 4.22 | 22 |
| C | Right | Superior parietal lobule | 13 | −64 | 40 | 3.97 | 9 |
| D | Right | Sagittal stratum | 31 | −41 | −5 | 3.89 | 13 |
| E | Left | Middle frontal gyrus | −29 | 1 | 51 | 3.88 | 14 |
| F | Right | Superior frontal gyrus | 21 | 41 | 19 | 3.41 | 10 |
| G | Left | Cerebellum | −3 | −70 | −21 | 3.4 | 8 |
| H | Right | Cingulum | 27 | −37 | −8 | 3.35 | 8 |
| I | Right | Cingulum | 8 | −17 | 35 | 3.24 | 8 |
| J | Right | Superior occipital gyrus | 16 | −82 | 24 | 3.17 | 9 |
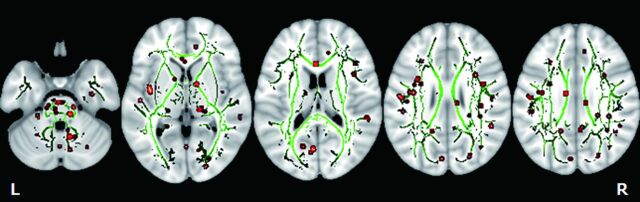
Scores from the FIQ were correlated positively with FA values in the left insula, bilateral cerebellum, right sagittal stratum, right superior temporal gyrus, left amygdala, left precuneus, right cingulum, right superior longitudinal fasciculus, right angular gyrus, right superior occipital gyrus, left cuneus, right inferior temporal gyrus, right lenticular fasciculus, and left fusiform gyrus in the mTBI group (Fig 3). These results are summarized in more detail in Table 4. The correlations of FA values to FIQ scores for all regions showing significant (P < .01) associations are shown in On-line Fig 2. There were no regions where FA values negatively correlated with FIQ scores in the brain.
Fig 3.
TBSS analysis of the white matter skeleton. Voxels demonstrating FA values correlated significantly (P < .01) to FIQ are shown in red-yellow. Voxels are thickened into local tracts and overlaid on the white matter skeleton (green). Further cluster details are given in Table 4.
Table 4:
Anatomic locations showing a positive correlation of FIQ and FA values in patients with mTBI
| Label | Hemisphere | Anatomic location | MNI Coordinates |
Z Score | No. of Voxels | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| A | Left | Insula | −39 | −3 | 3 | 6.01 | 19 |
| B | Right | Cerebellum | 29 | −69 | −33 | 4.93 | 8 |
| C | Left | Cerebellum | −6 | −55 | −45 | 4.9 | 9 |
| D | Right | Sagittal stratum | 30 | −61 | −10 | 4.79 | 8 |
| E | Right | Superior temporal gyrus | 57 | −34 | 11 | 4.59 | 12 |
| F | Left | Amygdala | −24 | 2 | −22 | 4.47 | 12 |
| G | Left | Precuneus | −5 | −29 | 44 | 4.34 | 9 |
| H | Right | Cerebellum | 6 | −68 | −35 | 4.22 | 8 |
| I | Right | Cingulum | 4 | −49 | 31 | 4.2 | 8 |
| J | Right | Superior longitudinal fasciculus | 37 | −39 | 22 | 4.1 | 9 |
| K | Right | Angular gyrus | 46 | −45 | 23 | 3.95 | 8 |
| L | Right | Superior occipital gyrus | 24 | −76 | −15 | 3.93 | 13 |
| M | Left | Cuneus | −4 | −69 | 15 | 3.53 | 10 |
| N | Right | Cerebellum | 37 | −62 | −33 | 3.43 | 9 |
| O | Right | Inferior temporal gyrus | 42 | 11 | −24 | 3.41 | 8 |
| P | Left | Lenticular fasciculus | −14 | 3 | −7 | 3.39 | 9 |
| Q | Left | Fusiform gyrus | −34 | −29 | −18 | 3.36 | 8 |
Discussion
Pathologic studies have demonstrated that DAI is characterized by focal lesions predominantly in the corpus callosum, fornix, dorsolateral quadrant, quadrants of the rostral brain stem, and the cerebellum with microscopic evidence of damage to axons.18,19 Diffuse axonal injury is regarded as a principal pathology causing posttraumatic cognitive impairments, and it is seen in patients with TBI without focal morphologic lesions in the brain. The histopathology of these cases represents collections of hemosiderin-laden macrophages in the perivascular space and an increase in the number of macrophages in the white matter, particularly in the section taken from the frontal lobe.20
Imaging modalities such as CT, T1WI, and T2WI often show diffuse atrophy of the brain and ventricular enlargement in a chronic stage of TBI.21,22 Even in severe cases of diffuse TBI, identified morphologic changes due to brain lesions are scarcely detectable by routine neuroimaging examinations; however, diffuse brain atrophy is often the only diagnostic clue for DAI in the chronic stages. In general, FLAIR and DWI are deemed to provide more diagnostic information related to DAI in comparison with T1WI or T2WI. In some cases, FLAIR imaging can reveal more morphologic changes due to nonhemorrhagic DAI lesions following TBI in the subcortical regions and adjacent to the ventricles, and these changes are more clearly seen compared with T2WI.23 However, conventional MR imaging, including FLAIR, may actually underestimate the existence of minute hemorrhagic DAI lesions in a number of cases. Furthermore, these findings do not directly delineate the true extent of the traumatic axonal damage. T2*-weighted imaging at high field strengths and SWI have recently been reported to be more sensitive diagnostic techniques for minute hemorrhagic lesions compared with conventional T2* gradient-recalled echo sequences. SWI is a useful tool for the identification of traumatic microbleeds and hemorrhagic DAI lesions, even in the chronic stage of TBI.24–26
However, conventional MR imaging, including FLAIR, as well as T2*-weighted imaging and SWI may still underestimate the existence of minute DAI lesions in some cases. Evaluation of FA values obtained from DTI images is another promising neuroradiologic technique for detecting minute brain lesions due to DAI. We have previously reported the significant relationship between white matter integrity and cognitive functions in certain areas of the brain following TBI.27
In the acute or subacute stages of TBI, 80%–100% of patients with mTBI have ≥1 neurocognitive symptom, mostly including headache, slowed thinking, and impaired attention and memory, attributable to their injury.28,29 Nearly half of these patients demonstrate a gradual, though sometimes incomplete, recovery 3 months postinjury; and in 80%–90%, these symptoms are improved by 6–12 months after injury.30–33 As a result, nearly 10%–20% of patients with mTBI have sustained cognitive, emotional-behavioral, and physical impairments at 1 year postinjury. Their persistent symptoms are difficult to treat and may lead to vocational disability.34–36
Whether these symptoms are attributed to morphologic brain lesions caused by brain impact in patients with mTBI is still controversial. A human postmortem study of patients with mTBI who died from other causes showed multifocal axonal injury, which involved the corpus callosum and fornix in all patients, by using an immunostaining technique.37 Alternatively, there are meta-analytic studies that concluded that individuals with chronically impaired cognitive test performances were either faking their deficits or that the cause was not brain-based in patients with mTBI.38–40
This is the first study to evaluate white matter abnormalities by comparing DTI from patients with mTBI without any focal morphologic abnormality on conventional MR imaging and healthy control subjects by using TBSS analysis. The results indicated that there were some regions, the right superior longitudinal fasciculus, left superior frontal gyrus, right insula, and left fornix, with significantly decreased FA values compared with those in healthy controls, which might be attributed to a minute morphologic abnormality in the damaged brains of patients with mTBI. Additionally, the results showed that the location of these regions was mostly concordant with those in the previous neuropathologic studies.
Furthermore, our results showed a number of white matter regions that were significantly related to MMSE and FIQ in the brain, which suggests that cognitive function generally involves multiple white matter pathways—that is, these cognitive tests were not related to a single region in the brain. These results are not surprising because the MMSE, which is a general neurologic examination with testing of crude cognitive function, and the FIQ, which is an index showing a wide range of cognitive functions, are tests for evaluating human cognitive functions comprehensively. These brain regions were shown to be anatomically close or physiologically intimate to the regions with significant FA value reduction in this study. Therefore, we cannot necessarily neglect the hypothesis that the chronic persistent cognitive impairments following mTBI may be caused by morphologic brain lesions.
Conclusions
The present study shows that patients with mTBI in the chronic stage have multiple regions with abnormally reduced FA values in the superior longitudinal fasciculus, superior frontal gyrus, insula, and fornix. The cognitive examination scores positively correlated with FA values in a number of regions, including the basal ganglia and limbic system, which were anatomically close or physiologically intimate to the regions with significant FA-value reduction, in the patients with mTBI. Although the clinical and pathologic-anatomic correlation of these findings remains to be elucidated, the regions with abnormally reduced FA values are strongly suggested to be related to chronic persistent cognitive impairments in these patients.
Supplementary Material
Acknowledgments
We are grateful to S. Fukuyama, MD, Y. Kasuya, and R. Okumura for technical support on neuroradiology, and to S. Uduyama, N. Mizumoto, and Y. Hibino for support on the neuropsychological examinations.
ABBREVIATIONS:
- DAI
diffuse axonal injury
- FA
fractional anisotropy
- FIQ
full-scale intelligence quotient
- GCS
Glasgow Coma Scale
- MMSE
Mini-Mental State Examination
- MNI
Montreal Neurological Institute
- mTBI
mild traumatic brain injury
- TBI
traumatic brain injury
- TBSS
tract-based spatial statistics
- WAIS-R
Wechsler Adult Intelligence Scale-Revised
References
- 1. Sosin DM, Sniezek JE, Thurman DJ. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj 1996; 10: 47– 57 [DOI] [PubMed] [Google Scholar]
- 2. Kraus JF, Nourjah P. The epidemiology of mild, uncomplicated brain injury. J Trauma 1988; 28: 1637– 43 [DOI] [PubMed] [Google Scholar]
- 3. Holm L, Cassidy JD, Carroll LJ, et al. Summary of the WHO Collaborating Centre for Neurotrauma Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2005; 37: 137– 41 [DOI] [PubMed] [Google Scholar]
- 4. Jennett B. The problem of mild head injury. Practitioner 1978; 221: 77– 82 [PubMed] [Google Scholar]
- 5. Zappala G, Thiebaut de Schotten M, Eslinger PJ. Traumatic brain injury and the frontal lobes: what can we gain with diffusion tensor imaging? Cortex 2012; 48: 156– 65 [DOI] [PubMed] [Google Scholar]
- 6. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci 2011; 13: 287– 300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maller JJ, Thomson RH, Lewis PM, et al. Traumatic brain injury, major depression, and diffusion tensor imaging: making connections. Brain Res 2010; 64: 213– 40 [DOI] [PubMed] [Google Scholar]
- 8. Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 2003; 4: 469– 80 [DOI] [PubMed] [Google Scholar]
- 9. Rimrodt SL, Peterson DJ, Denckla MB, et al. White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex 2010; 46: 739– 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Masutani Y, Aoki S, Abe O, et al. MR diffusion tensor imaging: recent advance and new techniques for diffusion tensor visualization. Eur J Radiol 2003; 46: 53– 66 [DOI] [PubMed] [Google Scholar]
- 11. Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 2002; 15: 435– 55 [DOI] [PubMed] [Google Scholar]
- 12. Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011; 134 (pt 2): 449– 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith SM, Johansen-Berg H, Jenkinson M, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc 2007; 2: 499– 503 [DOI] [PubMed] [Google Scholar]
- 14. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487– 505 [DOI] [PubMed] [Google Scholar]
- 15. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23 (suppl 1): S208– 19 [DOI] [PubMed] [Google Scholar]
- 16. Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003; 50: 1077– 88 [DOI] [PubMed] [Google Scholar]
- 17. Oishi K, Faria AV, van Zijl PCM. MRI Atlas of Human White Matter. 2nd ed. London, UK: Academic Press; 2011 [Google Scholar]
- 18. Adams JH, Doyle D, Graham DI, et al. Deep intracerebral (basal ganglia) haematomas in fatal non-missile head injury in man. J Neurol Neurosurg Psychiatry 1986; 49: 1039– 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adams JH, Graham DI, Murray LS, et al. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol 1982; 12: 557– 63 [DOI] [PubMed] [Google Scholar]
- 20. Bigler ED. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J Int Neuropsychol Soc 2004; 10: 794– 806 [DOI] [PubMed] [Google Scholar]
- 21. Besenski N. Traumatic injuries: imaging of head injuries. Eur Radiol 2002; 12: 1237– 52 [DOI] [PubMed] [Google Scholar]
- 22. Meythaler JM, Peduzzi JD, Eleftheriou E, et al. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch Phys Med Rehabil 2001; 82: 1461– 71 [DOI] [PubMed] [Google Scholar]
- 23. Parizel PM, Ozsarlak, Van Goethem JW, et al. Imaging findings in diffuse axonal injury after closed head trauma. Eur Radiol 1998; 8: 960– 65 [DOI] [PubMed] [Google Scholar]
- 24. Scheid R, Walther K, Guthke T, et al. Cognitive sequelae of diffuse axonal injury. Arch Neurol 2006; 63: 418– 24 [DOI] [PubMed] [Google Scholar]
- 25. Babikian T, Freier MC, Tong KA, et al. Susceptibility weighted imaging: neuropsychologic outcome and pediatric head injury. Pediatr Neurol 2005; 33: 184– 94 [DOI] [PubMed] [Google Scholar]
- 26. Ashwal S, Babikian T, Gardner-Nichols J, et al. Susceptibility-weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury. Arch Phys Med Rehabil 2006; 87 (12 suppl 2): S50– 58 [DOI] [PubMed] [Google Scholar]
- 27. Nakayama N, Okumura A, Shinoda J, et al. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry 2006; 77: 850– 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dikmen S, Machamer J, Temkin N. Mild head injury: facts and artifacts. J Clin Exp Neuropsychol 2001; 23: 729– 38 [DOI] [PubMed] [Google Scholar]
- 29. McMillan TM, Herbert CM. Further recovery in a potential treatment withdrawal case 10 years after brain injury. Brain Inj 2004; 18: 935– 40 [DOI] [PubMed] [Google Scholar]
- 30. Dikmen S, McLean A, Temkin NR, et al. Neuropsychologic outcome at one-month postinjury. Arch Phys Med Rehabil 1986; 67: 507– 13 [PubMed] [Google Scholar]
- 31. Leininger BE, Gramling SE, Farrell AD, et al. Neuropsychological deficits in symptomatic minor head injury patients after concussion and mild concussion. J Neurol Neurosurg Psychiatry 1990; 53: 293– 96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beetar JT, Guilmette TJ, Sparadeo FR. Sleep and pain complaints in symptomatic traumatic brain injury and neurologic populations. Arch Phys Med Rehabil 1996; 77: 1298– 302 [DOI] [PubMed] [Google Scholar]
- 33. Deb S, Lyons I, Koutzoukis C, et al. Rate of psychiatric illness 1 year after traumatic brain injury. Am J Psychiatry 1999; 156: 374– 78 [DOI] [PubMed] [Google Scholar]
- 34. Bohnen N, Jolles J. Neurobehavioral aspects of postconcussive symptoms after mild head injury. J Nerv Ment Dis 1992; 180: 683– 92 [DOI] [PubMed] [Google Scholar]
- 35. Bohnen N, Jolles J, Twijnstra A. Neuropsychological deficits in patients with persistent symptoms six months after mild head injury. Neurosurgery 1992; 30: 692– 95, discussion 695–96 [PubMed] [Google Scholar]
- 36. Hofman PA, Stapert SZ, van Kroonenburgh MJ, et al. MR imaging, single-photon emission CT, and neurocognitive performance after mild traumatic brain injury. AJNR Am J Neuroradiol 2001; 22: 441– 49 [PMC free article] [PubMed] [Google Scholar]
- 37. Blumbergs PC, Scott G, Manavis J, et al. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet 1994; 344: 1055– 56 [DOI] [PubMed] [Google Scholar]
- 38. Schretlen DJ, Shapiro AM. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int Rev Psychiatry 2003; 15: 341– 49 [DOI] [PubMed] [Google Scholar]
- 39. Belanger HG, Curtiss G, Demery JA, et al. Factors moderating neuropsychological outcomes following mild traumatic brain injury: a meta-analysis. J Int Neuropsychol Soc 2005; 11: 215– 27 [DOI] [PubMed] [Google Scholar]
- 40. Frencham KA, Fox AM, Maybery MT. Neuropsychological studies of mild traumatic brain injury: a meta-analytic review of research since 1995. J Clin Exp Neuropsychol 2005; 27: 334– 51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.