Abstract
BACKGROUND AND PURPOSE:
The goal of endovascular treatment of cerebral bifurcation aneurysms is to achieve safe coiling of the sac along with preserving patency of the diverging branches. Our purpose was evaluate procedural safety and efficacy as well as the long-term durability of endovascular treatment of bifurcation aneurysms with double stent–assisted coiling.
MATERIALS AND METHODS:
One hundred ninety-one consecutive patients with bifurcation aneurysms were included in this series. Technical failure occurred in 3 aneurysms (1.5%); 188 patients with 193 aneurysms treated with double stent–assisted coiling were retrospectively evaluated; 113 aneurysms were located at middle cerebral artery bifurcation, 42 at the anterior communicating artery, 22 at the basilar artery bifurcation, and the remaining 16 at the internal carotid artery bifurcation; 132 were small (<10 mm), 56 were large (10–25 mm), and 5 were giant (>25 mm).
RESULTS:
The technical success rate of double-stent application was 98.5% (193 aneurysms). In total, there were 5 procedural complications with an associated rate of 2.7%, one of which led to death (0.5%). Delayed ischemic stroke occurred in 2 patients (1.1%). Overall, permanent morbidity occurred in 2 patients, with associated rate of 1.1%. Follow-up was obtained in 186 aneurysms (96.4%), and recanalization occurred in 4 aneurysms (2.2%). In subgroup analysis, the recanalization rate was 3.8% for large aneurysms and 40% for giant aneurysms. No recanalization occurred in small aneurysms.
CONCLUSIONS:
Dual stent–assisted coiling of cerebral aneurysms is a feasible and safe procedure. It may offer a curative solution with long-term durability for treatment of wide-neck small and large aneurysms.
In early practice, the application and technical success of endovascular treatment of cerebral aneurysms with detachable coils were widely affected by morphologic factors, including shape and dome-to-neck ratio.1 Nevertheless, the use of adjunctive materials such as remodeling balloons,2 the neck-bridge device,3 and, thereafter, self-expanding stents,4 has resulted in significant expansion of patient selection criteria.
Easy and safe navigation, mostly through a microcatheter without a need for an exchange procedure, is the most important feature of the self-expanding stents currently in use, compared with the balloon-expandable coronary stents. Although the initial idea behind the use of a stent was to obtain a mechanical scaffold across the neck of the broad-based aneurysms, subsequent laboratory data have shown the benefit of hemodynamic alterations in the aneurysm sac before and after stent deployment.5,6
Bifurcation aneurysms may present a therapeutic challenge with unfavorable geometric configurations. The double stent–assisted coiling technique, defined by Chow et al,7 offers maintenance of patency of involved branches with deployment of stents in each particular vessel, along with creation of a new bifurcation point across the neck of the related aneurysm. Likewise, in sidewall aneurysms, laboratory data revealed flow alterations after placement of stents in the Y-configuration at the neck of a bifurcating aneurysm model.8 Moreover, double-stent placement in Y-configuration may provide sufficient flow reduction even without endosaccular coiling and may result in aneurysm thrombosis in selected situations, as reported in a recently published series.9
Herein, we report our immediate treatment results and long-term clinical and angiographic follow-up data on 183 bifurcation aneurysms treated with the double stent–assisted coil embolization technique.
Materials and Methods
This retrospective case series included 188 consecutive patients with 193 intracranial bifurcation aneurysms treated with the double stent–assisted coil embolization technique between September 2006 and August 2011 (Table 1). DSAC was deemed necessary in bifurcation aneurysms 1) when the origins of the branching arteries could not be preserved otherwise (including balloon assistance or single-stent placement); 2) when there was no identifiable aneurysm neck and therefore it was necessary to create a barrier for neck construction; and 3) when the aneurysm could not be packed fully otherwise and was likely to recur, particularly those of large size. Twenty-one patients had prior treatment or failed attempts; 167 patients received double stent–assisted coiling as the initial treatment. Of these 21 patients, 19 had recanalized aneurysms after balloon remodeling–assisted and/or single stent–assisted coiling; 1 patient had a clip remnant and the other had a failed surgical clipping attempt. Most of the patients presented with headache. Only 3 patients who presented with subarachnoid hemorrhage were included in this series; 1 of these patients were treated within the acute phase of SAH caused by coil protrusion into the parent artery, and the remaining 2 were treated in the subacute phase after appropriate premedication with antiplatelet (anti-aggregating) agents.
Table 1:
Patient and aneurysm characteristics
| No of Patients | 188 |
| Sex | |
| Female | 111 |
| Male | 77 |
| Age, y | |
| Mean | 50.3 |
| Minimum | 15 |
| Maximum | 72 |
| No. of treated aneurysms | 193 |
| Aneurysm location (%) | |
| Anterior | 171 (88.6) |
| MCA | 113 |
| AcomA | 42 |
| ICA bifurcation | 16 |
| Posterior | 22 (11.4) |
| Basilar bifurcation | 22 |
| Aneurysm size (%) | |
| Small, <100 mm | 132 (68.4) |
| Large | 56 (29) |
| Giant | 5 (2.6) |
One hundred thirteen aneurysms were located at middle cerebral artery bifurcation; 42 at the anterior communicating artery; 22 at the basilar artery bifurcation; and remaining 16 at the internal carotid artery bifurcation. Of the 193 aneurysms, 132 were small, 56 were large, and 5 were giant. Two of the giant aneurysms showed partial thrombosis. All giant and large aneurysms had neck size wider than 4 mm. All small aneurysms had dome-to-neck ratio ≤1.5 (Fig 1–3).
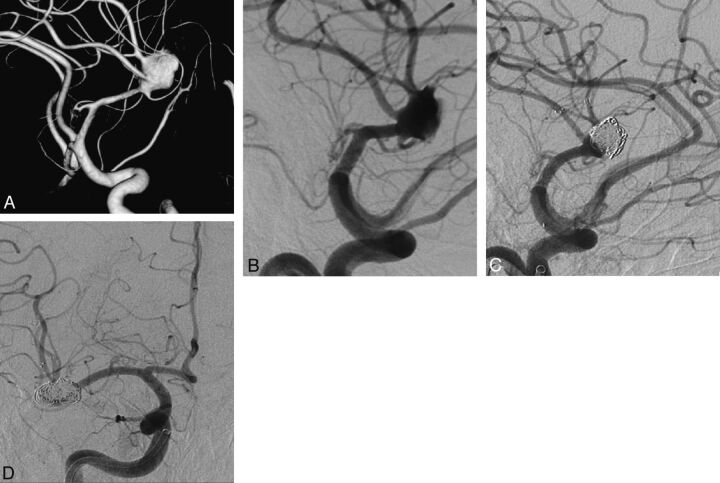
Fig 1.
A, 3D view of a complex right MCA aneurysm involving both superior and inferior trunks. B, Right internal carotid artery oblique angiogram demonstrating the complex right MCA aneurysm. C, Postembolization oblique DSA view after both superior and inferior trunks were stented in Y-configuration with suboptimal aneurysm packing. D, One-year control angiography in anterior-posterior view revealing the reconstruction of MCA bifurcation and complete occlusion of the aneurysm.
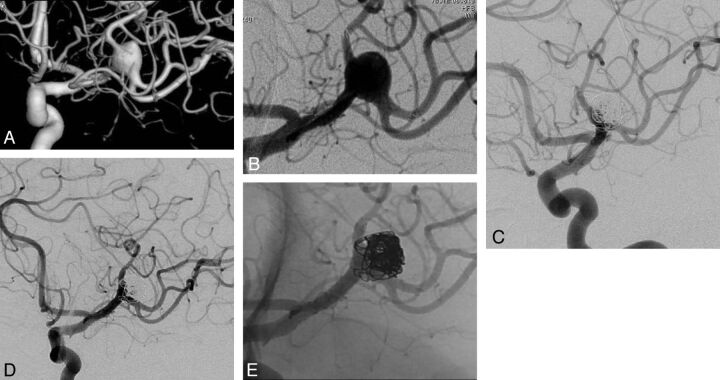
Fig 2.
A, 3D view of a complex left MCA aneurysm with both superior and inferior trunks of MCA originating from the aneurysm sac. B, Preoperative DSA revealing both trunks of left MCA stented in Y-configuration, creating a neck. C, Six-month control DSA showing stable aneurysm occlusion. D and E, Two-year control angiography with subtracted (D) and nonsubtracted (E) views confirming long-term aneurysm occlusion and MCA bifurcation reconstruction.
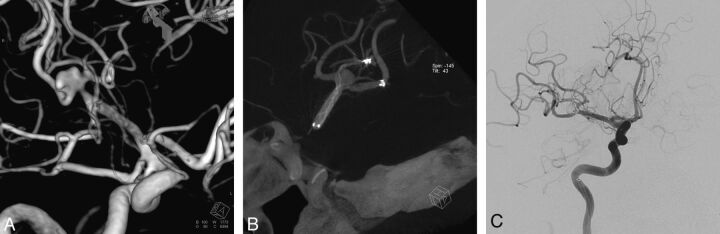
Fig 3.
A, 3D view of wide-neck right middle cerebral artery aneurysm. B, Flat-panel CT angiography showing the alignment of Enterprise and Solitaire stents in Y-configuration. C, Postembolization oblique DSA view with total packing of the aneurysm with coils.
Five patients with AcomA aneurysms, treated with X-configured DSAC embolization, had been reported previously in a technical note.10 According to the configuration or shape of the 2 stents, 183 aneurysms were treated with Y-configuration; 8 with AcomA aneurysms and 1 with fenestrated basilar artery aneurysms treated with X-configuration. One internal carotid artery bifurcation had T-configured double stents, one of which was deployed horizontally from the contralateral side.
Strict anti-aggregation and intraprocedural anticoagulation protocols were administered in all but 1 case that had an unplanned DSAC in the acute phase of SAH. Accordingly, double antiplatelet therapy with acetylsalicylic acid (300 mg per day) and clopidogrel were initiated 1 week before the procedure with a loading dose of 300–600 mg clopidogrel, followed by 75 mg daily. Thrombocyte inhibition levels were confirmed by means of VerifyNow (Accumetrics, San Diego, California) and a standard thrombocyte aggregation test. The procedure was not performed if the patient did not have a platelet inhibition level of 30% at minimum. If the patient had resistance to clopidogrel after use for 1 week or the second test showed that the patient was still a low responder, the anti-aggregation medicine was switched to ticlopidine. After the control angiogram was obtained at the sixth month, clopidogrel was discontinued and acetylsalicylic acid was to be taken life-long. Anticoagulation protocol during the procedure consisted of intravenous administration of heparin with close monitoring of blood activated clotting time levels.
Technique
All procedures were performed under general anesthesia with biplane flat-panel angiography; a 90-cm 6F introducer sheath was used in combination with 6F guiding catheters. DSAC consists of deployment of 2 self-expanding stents; the second is placed to pass through the interstices of the first stent, 1 in each branch coming off from the sac or neck of the broad-based bifurcation aneurysm. In this technique, with the crossing stent wires, a new bifurcation point is created below the neck of the aneurysm (redirecting the blood flow toward the relevant branches) for safe coiling of the sac, obviating the risk of coil protrusion to the parent artery. Selection of the branch to be catheterized first is extremely important for the success of the technique; that is, the angle between the proximal vessel and the diverging branch is the most important factor for decision-making—the one that has a sharper angle must be stented before the one with a wider angle. The second criterion is the orientation of the aneurysm neck; that is, the side involved by the aneurysm neck for a wider segment should be stented first.
According to our technique, a microcatheter (Prowler Select Plus, Codman & Shurtleff, Raynham, Massachusetts; Rebar, ev3, Irvine, California) with an inner size of 0.021 inches was used in combination with various guidewires. Afterward, a 0.010-inch microcatheter (SL 10, Boston Scientific, Fremont, California; Echelon 10, ev3) was placed into the aneurysm sac over various guidewires before deployment of the first stent. The first stent was then deployed within the branch to extend to the main vessel, covering the neck of the aneurysm partially. The microcatheter was passed through one of the struts of the first stent, and the second stent was placed to extend from the other branch to the main artery to cover the remaining neck segment.
The stents used for double-stent placement in this series are given in Table 2. In the beginning of our experience, only the commercially available Neuroform stents (Boston Scientific), which had an open-cell design, were used in the first 6 cases, as in the technique first described by Chow et al.7 Since then, with the introduction of the self-expanding stents that could be navigated through a microcatheter and after we succeeded in in vitro testing of Enterprise (Cordis Neurovascular) and Solitaire (ev3) stents for the technique of dual-stent placement in an intersecting configuration, we modified the double-stent application technique. These stents were preferred to augment flow redirection. In this series, double Enterprise stents of 4.5-mm diameter with combinations of variable lengths (ie, 22, 28, or 37 mm) were used in 126 aneurysms. Double Solitaire stents with different combinations of 4 × 20 mm and 3 × 30 mm were used in 32 aneurysms; Enterprise and Solitaire stents were combined in 29 aneurysms.
Table 2:
Stent types used in dual-stent reconstruction
| Type of Stent | Total (%) | MCA | AcomA | Basilar | ICA |
|---|---|---|---|---|---|
| Double Neuroform | 6 (3.1) | 2 | 0 | 2 | 2 |
| Double Enterprise | 126 (65.3) | 79 | 30 | 11 | 6 |
| Double Solitaire | 32 (16.6) | 12 | 7 | 7 | 6 |
| Enterprise + Solitaire | 29 (15.1) | 20 | 5 | 2 | 2 |
| Total | 193 | 113 | 42 | 22 | 16 |
The aneurysms were coiled with a variety of bare platinum coils; however, softer coils were preferred to avoid kick-back of the microcatheter, which would result in recatheterization of the aneurysm sac. We did not suggest dense packing of the aneurysm sac because we thought that dual-stent placement would create a flow-diverging effect.
Results
DSAC was attempted in 196 aneurysms, and technical failure occurred in 3 aneurysms (1.5%), resulting in a technical success rate of 98.5%. The first stent was deployed in these 3 patients uneventfully; however, the second stent could not be placed, but we were able to complete the endovascular treatment with coiling successfully with no complication, and the control angiography revealed stable complete occlusion of the relevant aneurysms despite the first stent partly covering the neck initially. No problems were encountered regarding stent opening within the bifurcation branches despite the mismatch between stent and artery diameters.
There were 5 procedural complications (5/188; 2.7%), 1 of which led to death resulting from intraprocedural rupture, with an associated mortality rate of 0.5%. Aneurysm rupture occurred in 2 other patients during coiling, but these patients were discharged without clinical consequences. Acute in-stent thrombus was observed in 1 patient and resolved completely with intravenous tirofiban injection. In the remaining patient, loss of consciousness developed with sudden onset 12 hours after the uneventful embolization procedure of an unruptured aneurysm, and the immediate CT scan revealed SAH of unknown origin. This patient was discharged with left-side moderate paresis and became independent the sixth month after the initial treatment (modified Rankin Scale 1).
Transient ischemic symptoms occurred in 2 patients within 30 days; diffusion-weighted MR imaging revealed small diffusion lesions, and their symptoms resolved with the addition of low-molecular-weight heparin to anti-aggregation therapy. Delayed ischemic stroke occurred in 2 patients (1.1%) as the result of unauthorized early cessation of clopidogrel. Overall, cumulative, procedure-related hemorrhagic, and delayed ischemic complications occurred in 9 (4.8%) patients, of which death occurred in 1 patient (0.5%) and permanent morbidity in 2 patients (1.1%).
According to the immediate posttreatment angiograms, 49 of 193 (25.4%) aneurysms were completely obliterated (Raymond class I); 63 of 193 (32.6%) had a neck remnant (Raymond class II), and the remaining 81 of 193 (42%) aneurysms had incomplete obliteration (Raymond class III).
Until August 2011, 186 of 193 aneurysms were out of at least 6 months for follow-up, and control angiography was performed in all, at 6 months to 2 years; the results were evaluated with respect to initial occlusion grade and aneurysm size. According to the latest follow-up, 152 aneurysms had initial control angiography at the sixth month; 34 aneurysms had initial control angiography at the first year. Of those that had initial follow-up, 87 aneurysms had an additional 2-year control. In these 186 aneurysms, recurrence was noted in 4 aneurysms, with an overall recanalization rate of 2.2%. All 4 of these aneurysms received the dual stent–assisted coiling as a retreatment after the initial treatment with the balloon-remodeling technique or single stent–assisted coiling. In this series, there was no recanalization seen in aneurysms in which DSAC was performed as the initial treatment.
The follow-up results of 186 aneurysms with respect to immediate postoperative angiography results are shown in Table 3. There was no recanalization in the group, which had had class I occlusion initially. All aneurysms that had had class III occlusion in the immediate postoperative angiography showed further occlusion to class I at control angiography. The 4 aneurysms that showed recanalization had class II occlusion initially.
Table 3:
Angiographic occlusion rates at 6-month follow-up
| Occlusion Rate | Angiographic Occlusion at Initial Control Angiography |
||||
|---|---|---|---|---|---|
| No. of Initial Occlusions | No. of Follow-Up | Class I | Class II | Class III | |
| Class I | 49 (25.4) | 46 | 46 | 0 | 0 |
| Class II | 63 (32.6) | 60 | 52 | 4 | 4 |
| Class III | 81 (42) | 80 | 80 | 0 | 0 |
| Total | 193 (100) | 186 | 178 | 4 | 4 |
In regard to the aneurysm size, follow-up data revealed the following results.
1) One hundred twenty-eight of 132 of the small aneurysms had control angiography; no recanalization occurred. 2) Control angiography was available in 53 of 56 large aneurysms; recanalization occurred in 2 aneurysms (3.8%). 3) In 5 giant aneurysms, all with follow-up, 2 had recanalization (40%).
Discussion
To preserve the patency of the branching arteries while achieving a complete occlusion of bifurcation aneurysms, devices such as remodeling balloons,2 the neck-bridge device,3 and stents4 have been used adjunctively for endovascular treatment.
Having the capability of adapting their shape to the anatomy of arterial bifurcation, supercompliant balloons may provide safer and denser packing because they secure the neck and enable better coil conformation compared with conventional coiling.3 However, with aneurysms having too wide a neck, a single balloon may not be sufficient to protect from coil herniation, even when a round balloon is positioned at the neck instead of the balloon catheter with its tip positioned in one particular branch. A double balloon, placed in each branch, crossing the neck of the aneurysm, may be a solution, but, this technique has the disadvantage of having 3 microcatheters in the same artery simultaneously.
The TriSpan neck-bridge device (Boston Scientific) is an important tool that was used to achieve safe coil occlusion of bifurcation aneurysms while preserving the parent artery.2 The device had the advantages of easy placement through a microcatheter, single material remained within the sac, and it did not require the use of antiplatelet agents. However, TriSpan is not commercially available presently, and the published data are limited to the series about the initial experience without any data regarding the long-term efficacy.11
More recently, self-expanding stents have come into clinical use; well-defined advantages of these devices are improvement of packing densities while preserving coil protrusion into the parent artery12 and alteration of intra-aneurysmal hemodynamics.6,8 Likewise, the flow alterations obtained with sidewall aneurysms in laboratory investments after stent placement5 and the magnitude of the velocity of the jet entering the sac, has been reduced after placement of 2 Neuroform stents at the aneurysm neck in Y-configuration in the bifurcating aneurysm model.8 The effects of stents were shown to be more apparent at the end of the cardiac cycle by reducing shear stress in the sac almost by 40%.8 Furthermore, stents act as a biologic system by inducing endothelization over the struts, which leads to healing of the vessel wall also at the segment of broad-based aneurysm location.13 Placement of 2 intersecting stents across the aneurysm neck, even without additional coiling, may result in complete occlusion or reduction in filling of the aneurysms in some cases.9 Thus, the flow diversion effect of double-stent placement in Y-configuration is confirmed in vivo as well.
Dual stent–supported, or so-called “Y-configured” stent-assisted coil embolization of bifurcation aneurysms, was first defined by Chow et al7 and Perez-Arjona et al14 for treatment of wide-neck basilar artery bifurcation aneurysms, which then was followed by the application of a technique for treatment of an MCA aneurysm by Sani et al.15 These reports have pointed out the advantage of reconstruction of the bifurcation with deployment of double stents in each diverging branch, when a single stent does not adequately bridge the aneurysm neck, enabling the safe coiling of the sac without a risk of coil protrusion. The open-cell design of the stents leads to relatively easy passage through the struts of the deployed stent and almost fully opens the second stent at the cross-section of 2 stents taking Y-configuration.7 However, in our practice, with the introduction of closed-cell stents, the technique for DSAC evolved, and we began to use 2 closed-cell stents passing between the struts and through the cell of the stent. Although not proved with laboratory investigation on flow models, the idea behind the use of 2 closed-cell stents was that the intersection of stents, taking an hour-glass shape at the aneurysm neck when crossing each other, would act like a separator, diverting the flow streamlines into branches below the initial apex of the bifurcation and thus potentially decreasing the wall shear stress at the aneurysm neck. On the other hand, closed-cell design may have the disadvantage of kinking at the bifurcation point where the branches are diverging with acute angles, and this may make the passage of the microcatheter through the particular cell difficult. We failed to pass through the struts of the first stent to place the second stent in 3 cases, which may be explained by this issue. Moreover, central crimping and ovalization with luminal loss leading to incomplete stent apposition to the vessel have been documented with Enterprise stents, depending on the curvature.16
Technically, the Enterprise stent offers the advantage of having a distal wire, so that the microcatheter can be advanced over the wire after the first stent is placed. This eliminates the need to catheterize the former stent and therefore increases the safety of the technique while the risk of the microcatheter passing between the wall of the artery and stent is excluded. A second advantage is the anchoring flared (ie, 0.5 mm larger than the stent diameter) ends, which reduce the risk of stent migration during the passage of the microcatheter for deployment of the second one. On the other hand, major advantages of the Solitaire are: 1) It is fully retrievable, allowing precise placement. 2) It does not have a distal wire, that is, it can be deployed exactly at the tip of the microcatheter. Therefore, there is no need to navigate the microcatheter further for the purpose of purchasing the vessel segment for the distal wire, especially when there is a second tight curve at the diverging branch after bifurcation, which is not an infrequent anatomic feature, especially of MCAs. 3) It has a relatively larger cell size compared with the Enterprise, which eases passage of the microcatheter. However, the latter may sometimes be disadvantageous when struts do not secure the neck well. Thus, clinical practitioners should take these features into consideration when choosing the appropriate stent that would work best with the particular anatomic features.
A meta-analysis of the literature, concerning the safety and efficacy of endovascular treatment of unruptured aneurysms, revealed 4.8% unfavorable procedural outcome.17 Furthermore, it has been shown that in-hospital outcomes, regarding procedural mortality and morbidity, do not increase by the adjunctive use of stents compared with procedures performed without the use of a stent.18 A literature survey including 37 articles with 1510 aneurysms treated with stent-supported coiling revealed almost 10% thromboembolic complication rate, with an associated mortality rate of 0.6%.19 Learning curve analysis obtained in this study showed that the complication rate is affected by the operator's experience with the procedure.19
The cumulative complication rate observed in this study was not higher than the previous series. The only death included in this series (0.5%) occurred as the result of aneurysm rupture during coiling procedure, which is not uncommon with endovascular therapy. Relatively less occurrence of periprocedural ischemic complications (ie, 2 patients; 1.1%) would be attributable to strict anti-aggregation protocol that is checked twice with 2 different methods before the procedure. Nevertheless, delayed ischemic complications were observed in 2 patients (1.1%) who ceased usage of clopidogrel prematurely, implicating the importance of the drug compliance in the long term.
In this series, we had a recanalization rate of 2.2% and total occlusion rate of 97.8% at 6 months among those with follow-up (note that follow-up rate was 186/193; 96.4%) despite a relatively low level of initial total occlusion (25.4%) in the immediate postoperative angiography. Although no control group or matching series exist in the literature, dual-stent support appears to have an impact on the durability of the endosaccular packing. A literature survey revealed a recanalization rate of 14% after single stent–assisted coiling.19 Although this series is matchless for further discussion and may provoke controversy, our results inspire the augmented effect of intersecting dual-stent support resulting from flow changes. Of the 128 small wide-neck aneurysms, follow-up revealed no recanalization regardless of initial occlusion rates. Of the 53 large aneurysms, which had follow-up, recanalization was seen in 2 aneurysms, resulting in a rate of 3.8%. With conventional coiling, the recanalization rate is reported to be 20% and 35.3% for wide-neck small aneurysms and large aneurysms, respectively.20 In a large series consisting of 864 aneurysms located in the MCA bifurcation and the AcomA, the authors reported an overall recanalization rate of 12% with the adjunctive use of remodeling balloons, regardless of the aneurysm size.21 In a series consisting of 174 MCA aneurysms, a 27.2% recanalization rate was reported of the 114 aneurysms that had follow-up with angiography.22 From this perspective, the 2.2% recanalization rate that was achieved with DSAC seems promising.
Throughout the time of this study, a wide variety of other methods have been defined for stent application in bifurcation aneurysm treatment such as linear stent placement,23 nonoverlapping Y-stent placement,24 the waffle-cone technique,25 and so forth, which are mostly limited to anecdotal case series or a single case report. However, as explained above, our hypothesis was that crossing points of 2 closed-cell stents creates a point located below the initial apex of the bifurcation, which acts as a flow diverter and potentially induces further aneurysm thrombosis by reducing the flow. Recent work by Baharoglu et al26 and Gao et al,27 pointing out the contribution of vessel angle with respect to inflow of the aneurysm, claims that the angular configuration change after stent placement is an effective factor to reduce wall shear stress. Their work encourages our theoretic hypothesis as well. Accordingly, linear or nonoverlapping or kissing-stent techniques probably would lack this effect, and the waffle-cone technique may increase the flow toward the aneurysm sac.
ABBREVIATIONS:
- DSAC
double-stent assisted coil
- AcomA
anterior communicating artery
Footnotes
Disclosures: Saruhan Cekirge—UNRELATED: Consultancy: MicroVention, ev3; Payment for Lectures (including service on speakers bureaus): ev3; Payment for Development of Educational Presentations: ev3. Isil Saatci—UNRELATED: Consultancy: ev3; Payment for Lectures (including service on speakers bureaus): ev3; Payment for Development of Educational Presentations: ev3.
REFERENCES
- 1.Debrun GM, Aletich VA, Kehrli P, et al. Selection of cerebral aneurysms for treatment using Guglielmi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery 1998;6:1281–97 [DOI] [PubMed] [Google Scholar]
- 2.Moret J, Cognard C, Weill A, et al. Reconstruction technique in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results-apropos of 56 cases. J Neuroradiol 1997;24:30–44 [PubMed] [Google Scholar]
- 3.Raymond J, Guilbert F, Roy D. Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: initial experience. Radiology 2001;221:318–26 [DOI] [PubMed] [Google Scholar]
- 4.Fiorella D, Albuquerque FC, Deshmukh VR, et al. Usefulness of the Neuroform stent for the treatment of cerebral aneurysms: results at initial (3–6 mo) follow-up. Neurosurgery 2005;56:1191–201 [DOI] [PubMed] [Google Scholar]
- 5.Tateshima S, Tanishita K, Hakata Y, et al. Alteration of intraaneurysmal hemodynamics by placement of a self-expandable stent: laboratory investigation. J Neurosurg 2009;111:22–27 [DOI] [PubMed] [Google Scholar]
- 6.Canton G, Levy DI, Lasheras JC, et al. Flow changes caused by the sequential placement of stents across the neck of sidewall cerebral aneurysms. J Neurosurg 2005;103:891–902 [DOI] [PubMed] [Google Scholar]
- 7.Chow MM, Woo HH, Masaryk TJ, et al. A novel endovascular treatment of a wide-necked basilar apex aneurysm by using a Y-configuration, double-stent technique. AJNR Am J Neuroradiol 2004;25:509–12 [PMC free article] [PubMed] [Google Scholar]
- 8.Canton G, Levy DI, Lasheras JC. Hemodynamic changes due to stent placement in bifurcating intracranial aneurysms. J Neurosurg 2005;103:146–55 [DOI] [PubMed] [Google Scholar]
- 9.Cekirge HS, Yavuz K, Geyik S, et al. A novel ‘Y’ stent flow diversion technique for the treatment of bifurcation aneurysms without endosaccular coiling. AJNR Am J Neuroradiol 2011;32:1262–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saatci I, Geyik S, Yavuz K, et al. X-configured stent-assisted coiling in the endovascular treatment of complex anterior communicating artery aneurysms: a novel reconstructive technique. AJNR Am J Neuroradiol 2011;32:113–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keukeleire DK, Vanlangenhove P, Defreyne L. Evaluation of a neck-bridge device to assist endovascular treatment of wide-neck aneurysms of the anterior circulation. AJNR Am J Neuroradiol 2008;29:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendok BR, Parkinson RJ, Hage ZA, et al. The effect of vascular reconstruction device-assisted coiling on packing density, effective neck coverage, and angiographic outcome: an in vitro study. Neurosurgery 2007;61:835–41 [DOI] [PubMed] [Google Scholar]
- 13.Wanke I, Forsting M. Stents for intracranial wide-necked aneurysms: more than mechanical protection. Neuroradiology 2008;50:991–98 [DOI] [PubMed] [Google Scholar]
- 14.Perez-Arjona E, Fessler RD. Basilar artery to bilateral posterior cerebral artery Y stenting for endovascular reconstruction of wide-necked basilar apex aneurysms: report of three cases. Neurol Res 2004;26:276–81 [DOI] [PubMed] [Google Scholar]
- 15.Sani S, Lopes DK. Treatment of a middle cerebral artery bifurcation aneurysm using a double Neuroform stent Y configuration and coil embolization: technical case report. Neurosurgery 2005;57(1 Suppl): E209. [DOI] [PubMed] [Google Scholar]
- 16.Heller RS, Malek AM. Parent vessel size and curvature strongly influence risk of incomplete stent apposition in Enterprise intracranial aneurysm stent coiling. AJNR Am J Neuroradiol 2011;32:1714–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naggara ON, White PM, Guilbert F, et al. Endovascular treatment of unruptured intracranial aneurysms: systematic review and meta-analysis of the literature on safety and efficacy. Radiology 2010;256:887–97 [DOI] [PubMed] [Google Scholar]
- 18.McDonald JS, Norgan AP, McDonald RJ, et al. In-hospital outcomes associated with stent-assisted endovascular treatment of unruptured cerebral aneurysms in the USA. J Neurointerv Surg 2012; May 5, Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 19.Shapiro M, Becske T, Sahlein D, et al. Stent-supported aneurysm coiling: a literature survey of treatment and follow-up. AJNR Am J Neuroradiol 2012;33:159–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murayama Y, Nien YL, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg 2003;98:959–66 [DOI] [PubMed] [Google Scholar]
- 21.Cekirge HS, Yavuz K, Geyik S, et al. HyperForm balloon remodeling in the endovascular treatment of anterior cerebral, middle cerebral, and anterior communicating artery aneurysm: clinical and angiographic follow-up results in 800 consecutive patients. J Neurosurg 2011;114:944–53 [DOI] [PubMed] [Google Scholar]
- 22.Vendrell JF, Menjot N, Costalat V, et al. Endovascular treatment of 174 middle cerebral artery aneurysms: clinical outcome and radiologic results at long-term follow-up. Radiology 2009;253:191–98 [DOI] [PubMed] [Google Scholar]
- 23.Lubicz B. Linear stent-assisted coiling: another way to treat very wide-necked intracranial aneurysms. Neuroradiology 2011;53:457–59 [DOI] [PubMed] [Google Scholar]
- 24.Cho YD, Park SW, Lee JY, et al. Non-overlapping Y-configuration stenting technique with dual closed cell stents in wide-neck basilar tip aneurysms. Neurosurgery 2011, Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 25.Horowitz M, Levy E, Sauvageau E, et al. Intra/extra-aneurysmal stent placement for management of complex and wide-necked-bifurcation aneurysms: eight cases using the waffle cone technique. Neurosurgery 2006;58(4 Suppl 2):ONS258–262 [DOI] [PubMed] [Google Scholar]
- 26.Baharoglu MI, Schirmer CM, Hoit DA, et al. Aneurysm inflow-angle as a discriminant for rupture in sidewall cerebral aneurysms: morphometric and computational fluid dynamic analysis. Stroke 2010;41:1423–30 [DOI] [PubMed] [Google Scholar]
- 27.Gao B, Baharoglu MI, Cohen AD, et al. Stent-assisted coiling of intracranial bifurcation aneurysms leads to immediate and delayed intracranial vascular angle remodeling. AJNR Am J Neuroradiol 2012;33:649–54 [DOI] [PMC free article] [PubMed] [Google Scholar]