A group of 22 patients with type 2 diabetes underwent MR spectroscopy of the deep gray matter nuclei and their metabolite ratios of NAA/Cr and Cho/Cr were assessed and correlated with laboratory findings. Metabolite ratios were different in the left and right lenticular nuclei and in the thalami, suggesting that diabetes affects these differently with the former being more vulnerable than the latter. The metabolic abnormalities preceded anatomic lesions.
Abstract
BACKGROUND AND PURPOSE:
Previous studies by using proton MR spectroscopy found metabolite abnormalities in the cerebral cortex and white matter of patients with type 2 diabetes mellitus. The present study was undertaken to detect metabolite differences in the lenticular nuclei and thalamus in patients with T2DM.
MATERIALS AND METHODS:
Twenty subjects with T2DM and 22 age-matched control subjects underwent single-voxel MR spectroscopy in the left and right lenticular nuclei and left and right thalami. NAA/Cr and Cho/Cr ratios were calculated. Brain lactic acid, fasting blood glucose, and glycosylated hemoglobin levels were also monitored.
RESULTS:
The NAA/Cr ratio was lower in the left lenticular nuclei of subjects with T2DM (P = .007), whereas the Cho/Cr ratio was increased in both the and right lenticular nuclei (P = .001). The NAA/Cr ratio was negatively correlated with FBG in the left (r = −0.573, P = .008) and right nuclei (r = −0.564, P = .010). It was also negatively correlated to HbA1c in the left (r = −0.560, P = .010) and right (r = −0.453, P = .045) nuclei. The Cho/Cr ratio was positively correlated with these variables (P < .05). No significant differences in NAA/Cr or Cho/Cr ratios were observed in the thalamus of patients with T2DM. Lactic acid was not detected in any of the patients in the study.
CONCLUSIONS:
The different metabolic statuses of the lenticular nuclei and thalamus suggest different effects of T2DM in each of these brain nuclei, with the lenticular nuclei being more vulnerable than the thalamus. The abnormal metabolic status was observed before lesions had appeared in these brain areas.
Proton MR spectroscopy can be used to determine the resonance peaks of many kinds of brain metabolites and neurotransmitters but is most often used to monitor NAA, Cho, Cr, and lactic acid.1 NAA is a marker for neurons and axons, reflecting the number and functional status of neurons.2 Cho, mainly distributed in the glial cells, is involved in cell membrane composition and myelin formation. Cr is associated with energy metabolism.2
Type 2 diabetes mellitus is an extremely prevalent metabolic disease, which is associated with a variety of acute and chronic complications. Previous works have shown that DM causes metabolic changes in the brain, especially in the cerebral cortex and white matter.3–5
Most studies suggest that diabetes affects either the number or the function of central neurons, which is mirrored by a reduction in NAA levels and a lower NAA/Cr ratio.4–6 Studies investigating the effects of diabetes on Cho metabolism have produced inconsistent results. Studies by Kreis and Ross5 and Biessels et al6 reported no difference in Cho/Cr ratios in diabetic rats or in brain tissue from patients with diabetes. However, Sahin et al7 found evidence of a lower Cho/Cr ratio in the parietal and frontal lobe white matter in patients with T2DM, which was inversely related to HbA1c levels. Other works demonstrated an increase in the Cho/Cr ratio in the left occipital lobe gray matter of patients with T2DM but found no significant difference in the Cho/Cr ratio between patients and controls in the bilateral frontal and left parietal lobe white matter.8
While many studies have investigated the effects of diabetes in the brain cortex, few have investigated its effect in specific brain nuclei such as the lenticular nuclei and thalamus. Previous studies report that motor defects such as hemichorea may occur in patients with diabetes with lesions in the striatum. MR imaging indicated that this may be caused by lesions in the lenticular nuclei and was evidenced by lower NAA/Cr ratios and increased Cho/Cr in these patients.9,10 On the basis of these findings, it may be speculated that T2DM might result in metabolic abnormalities in the basal ganglia, even in the absence of visible lesions in those areas.
In the present study, we used 1H-MR spectroscopy to observe whether the metabolism within the lenticular nuclei and thalamus was altered in patients with T2DM and to explore the impact of T2DM on brain nuclei.
Materials and Methods
Study Design
Subjects were selected for the study from the Department of Neurology, Second Affiliated Hospital of Dalian Medical University, between March 2011 and December 2011. The subjects included 20 patients (7 men and 13 women) with T2DM (age range, 51–85 years) and 22 control subjects (9 men and 13 women) between 52 and 80 years of age.
Patients with T2DM met the 1999 World Health Organization diagnostic criteria for diabetes11 and were using oral medication or insulin to control blood glucose. The duration of T2DM ranged from 1 month to 30 years. Those who came to the hospital during the same time but without DM were selected as control subjects. All of the subjects enrolled in the study were right-handed. None of the subjects had coexisting systemic diseases, head trauma, cognitive dysfunction, thyroid dysfunction, alcohol dependence, hemichorea, infectious diseases of the central nervous system, or epilepsy. Those with impaired glucose tolerance or impaired fasting glucose regulation were excluded from the study. None of the subjects had a history of hypoglycemia.
Institutional ethics approval was obtained from the Second Affiliated Hospital of Dalian Medical University. All study subjects provided informed consent before entering the study.
Measurement of Brain Metabolites
Studies were performed on a 1.5T Signa MR imaging system (GE Healthcare, Milwaukee, Wisconsin). Conventional axial, sagittal, and coronal spin-echo scans and axial fast spin-echo scans, including T1- and T2-weighted images, were obtained before spectroscopy to rule out any lesions or structural abnormalities. T2-weighted imaging was performed by using the following parameters: TR/TE, 4300/105.3 ms; k-space matrix, 320 × 256; FOV, 24 × 24 cm; section thickness, 6 mm; space, 1 mm. The axial section showing the most anterior extent of the anterior margin of the genu of the corpus callosum was chosen as the reference image on which to center the voxels for both locations.12
Single-voxel spectroscopy was performed on all subjects with a circularly polarized head coil by using a point-resolved spectroscopy sequence (TR/TE, 1000/144 ms) with 128 averages. Due to the less complex spectra and increased reproducibility of the measurements, the long-TE point-resolved spectroscopy sequence was chosen as the primary pulse sequence. Voxels were placed in the bilateral lenticular nuclei (10 × 10 × 10 mm) and thalamus (10 × 10 × 10 mm), respectively, avoiding the CSF of the lateral ventricles, sulci, and cistern. Voxel-based shimming was performed to optimize field homogeneity before acquiring the spectra. Water suppression was performed by applying a chemical shift selective saturation-pulse technique provided by the vendor. Postprocessing of the spectral data was performed by using vendor-provided software (FuncTool 2, GE Healthcare).
The integral values of the metabolite peaks of NAA, Cho, and Cr were observed at 2.12, 3.29 and 3.11 ppm, respectively. Metabolites ratios were obtained for NAA/Cr and Cho/Cr.
Fasting blood glucose and glycosylated hemoglobin levels were monitored in all subjects.
Statistical Analysis
Statistical analyses were performed by using the Statistical Package for the Social Sciences, Version 16.0 for Windows (SPSS, Chicago, Illinois). Data were presented as means and SDs. The Shapiro-Wilk test was used for the normality of all continuous variables. An independent t test was used for comparison of the metabolite ratios between the T2DM group and the controls, and the Mann-Whitney U test was used to assess the differences of FBG and HbA1c between the 2 groups. Categoric data were compared by using analysis of variance. Correlations were estimated by using Spearman rank correlation coefficients. P < .05 was considered statistically significant.
Results
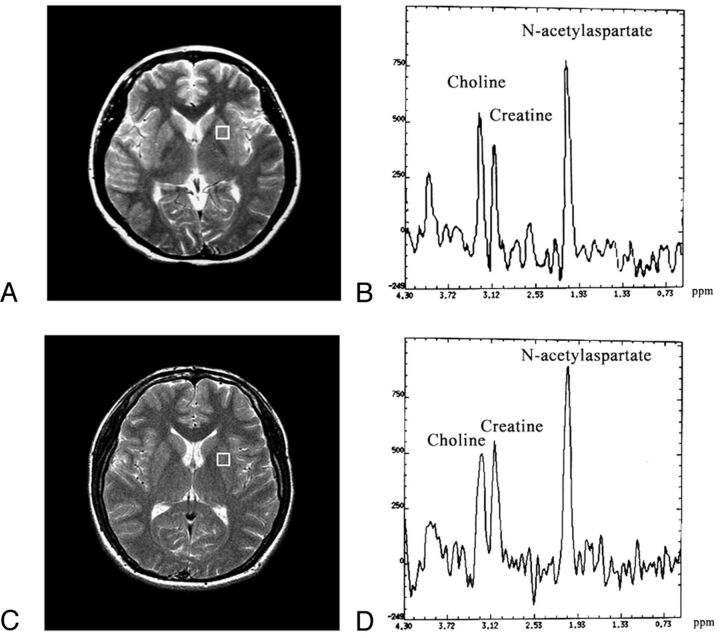
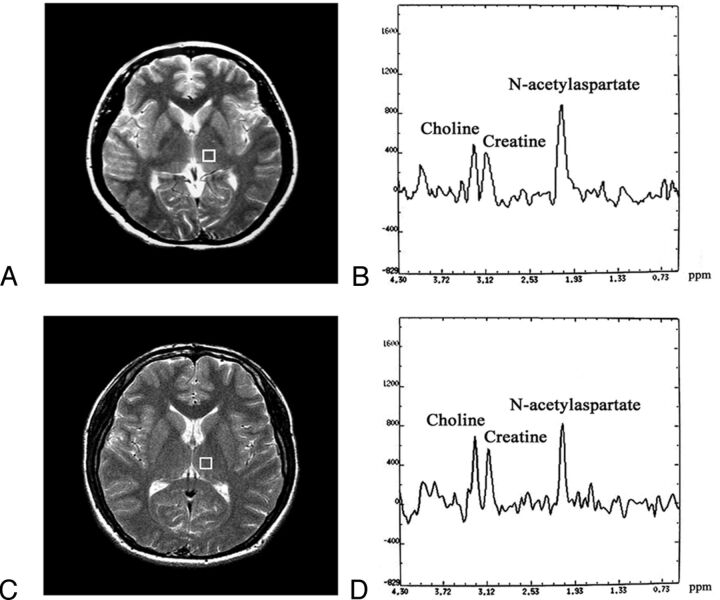
Figures 1 and 2 show the regions of interest in the right lenticular nucleus and thalamus together with corresponding MR spectra for Cho, Cr, and NAA.
Fig 1.
A and C, Regions of interest used for 1H-MR spectroscopy in a patient with T2DM (female, 57 years of age) and a control subject (female, 58 years of age). B, Spectrum of the patient with T2DM obtained from the left lenticular nucleus. D, Spectrum of the control subject obtained from the left lenticular nucleus.
Fig 2.
A and C, Region of interest in the left thalamus used for 1H-MR spectroscopy in the same patients in Fig 1 with T2DM and control subject. B and D, Spectra of the patient with T2DM is above and the control subject is below.
FBG in patients with T2DM was in the range of 5.85–15.96 mmol/L, and in the control group, it ranged from 5.05 to 5.90 mmol/L (P = .000). The HbA1c was also statistically significantly higher in the T2DM group (5.8%–14.3%) than in the control group (4.4%–5.6%, P = .000). There was no significant difference in the occurrence of high blood pressure between the 2 groups (85% versus 77.3%).
Metabolite ratios for the right and left lenticular nuclei and thalamus are shown in Table 1. The NAA/Cr ratio in the left lenticular nuclei was significantly lower in the patients with T2DM than in healthy control subjects (P = .007), but no significant difference in this ratio was found between patients and controls in the right lenticular nuclei (P = .071).
Table 1:
NAA/Cr and Cho/Cr ratios (mean ± SD) in the lenticular nuclei and thalamus of control subjects and patients with T2DM
| Left |
Right |
|||||
|---|---|---|---|---|---|---|
| Controls (n = 22) | T2DM (n = 20) | Pa | Controls (n = 22) | T2DM (n = 20) | Pb | |
| Lenticular nuclei | ||||||
| NAA/Cr | 1.61 ± 0.19 | 1.41 ± 0.26 | .007 | 1.57 ± 0.20 | 1.45 ± 0.24 | .071 |
| Cho/Cr | 0.99 ± 0.19 | 1.23 ± 0.24 | .001 | 0.98 ± 0.16 | 1.18 ± 0.20 | .001 |
| Thalamus | ||||||
| NAA/Cr | 1.70 ± 0.16 | 1.64 ± 0.16 | .222 | 1.72 ± 0.15 | 1.65 ± 0.18 | .221 |
| Cho/Cr | 1.13 ± 0.15 | 1.17 ± 0.16 | .479 | 1.15 ± 0.15 | 1.21 ± 0.16 | .236 |
Comparison of metabolite ratios in the left nuclei between patients and controls.
Comparison of metabolite ratios in the right nuclei between patients and controls.
The Cho/Cr ratios in the left and right lenticular nuclei were both significantly higher in patients with T2DM than in controls (P = .001 for both sides). No statistically significant difference in NAA/Cr or Cho/Cr ratios between patients with T2DM and controls was found in the bilateral thalami.
In both patients and controls, metabolite ratios in the left brain nuclei and thalamus were not statistically significantly different from those on the right side of the brain. Lactic acid was not observed in the nuclei of any of the patients.
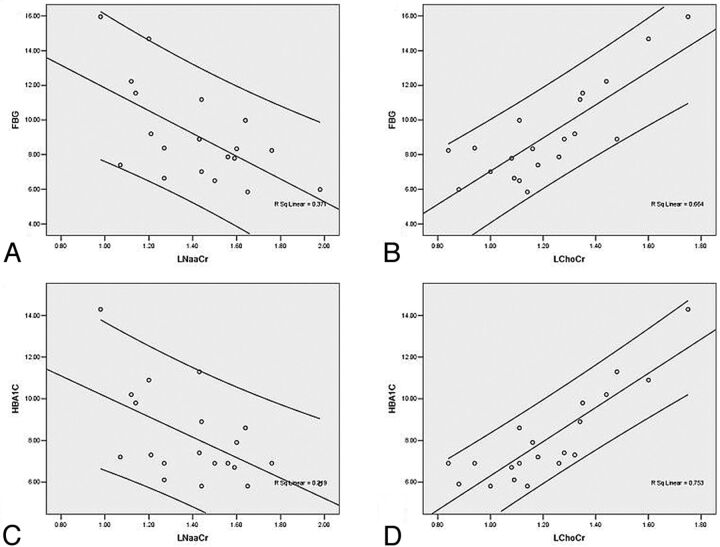
HbA1c and FBG were negatively correlated with NAA/Cr ratios in the left and right lenticular nuclei of patients in the T2DM group (Table 2). There was also a positive correlation between the Cho/Cr ratio and HbA1c and FBG in the T2DM group. Figure 3 shows the significant correlations between the metabolite ratios in the left lenticular nucleus and FBG as well as HbA1c.
Table 2:
Spearman rank correlations between lenticular nuclei metabolite ratios and FBG and HbA1c in patients with T2DM
| Left |
Right |
|||||||
|---|---|---|---|---|---|---|---|---|
| NAA/Cr |
Cho/Cr |
NAA/Cr |
Cho/Cr |
|||||
| r | P Value | r | P Value | r | P Value | r | P Value | |
| FBG (mmol/L) | −0.573 | .008 | 0.735 | .000 | −0.564 | .010 | 0.608 | .004 |
| HbA1c (%) | −0.560 | .010 | 0.838 | .000 | −0.453 | .045 | 0.619 | .004 |
Fig 3.
Significant correlations for the lenticular nucleus (left side) between metabolite ratios and FBG as well as HbA1c. A and B, Correlations between FBG and NAA/Cr and Cho/Cr. C and D, Correlations between HbA1c and NAA/Cr and Cho/Cr.
Discussion
Diabetes mellitus is a metabolic disorder with alterations of carbohydrate metabolism and biochemical metabolism of the brain.3 Our findings show lower levels of NAA/Cr in the left lenticular nucleus and increased Cho/Cr levels in both the left and right lenticula of subjects with T2DM. Both FBG and HbA1c were negatively correlated with the NAA/Cr ratio in the left nuclei as well as the right, while the Cho/Cr ratio was positively correlated. No significant differences in NAA/Cr or Cho/Cr ratios were observed in the thalamus of patients with T2DM.
Lower NAA/Cr levels observed in the left lenticular nucleus of patients with T2DM suggest that the number or the functional state of the neuron in the lenticular nucleus was affected by diabetes. This was consistent with previous studies reporting that diabetes could affect the neuronal function, resulting lower NAA or NAA/Cr ratios in some regions of the brain.4,5
The increased Cho/Cr ratios in the lenticular nuclei may have been related to ≥1 of the following reasons: First, long-term high blood glucose levels could cause blood viscosity to increase and then disturb the macro- and microvascular function and structure, which might result in vessel dysfunction and cerebral arteriosclerosis.13,14 The abnormal vascular function might further contribute to hypoperfusion, ischemia, and hypoxia. Energy depletion caused by ischemia would increase the Cho concentration by reducing the synthesis and enhancing the hydrolysis of acetylcholine.15,16
Second, choline is a mixture of molecular sources predominantly found in cell membranes.17 Therefore, a second explanation for the increased Cho levels is that hypoxia resulting from local hypoperfusion may cause a breakdown of myelin and decomposition or hydrolysis of cell membranes.9
Finally, increased Cho in T2DM may be a result of reactive glial proliferation, which may occur as a secondary response to neuronal dysfunction in the presence of relative tissue hypoxia.18
Despite the metabolic changes observed in the lenticular nuclei, we were unable to demonstrate any significant differences in Cho/Cr ratios in the thalamus, suggesting that dysfunction of cellularmembrane metabolites or abnormalities of neuronal energy metabolites do not occur in this part of the brain.
In the study of Tiehuis et al,19 HbA1c was found to have no significant effect on the metabolite ratios in the corona radiata. We did, however, find that the NAA/Cr ratio in the lenticular nuclei of patients with T2DM was negatively related to FBG and HbA1c, while Cho/Cr was positively related to these 2 variables. These findings are partly supported by previously published results that suggested that neuronal damage was more serious in patients with diabetes with poor glycemic control than in well-controlled patients.7 It is thought that chronic high blood glucose levels alter neuron metabolism, protein synthesis, and expression, as well as the apoptosis of a gene that may accelerate neuronal degeneration.20 The positive association between Cho/Cr and FBG and HbA1c also suggests that the Cho levels may be increased under conditions of high blood glucose and hypoxia, resulting from the sequence of events described above.
Our finding that differences of metabolites were observed only in the lenticular nucleus but not in the thalamus suggests that the lenticular nucleus might be more vulnerable than the thalamus because of differences in vascular anatomy. The lenticular nuclei are supplied by the penetrating arteries called lenticulostriate arteries, which originate from the middle cerebral arteries. These are penetrating end arteries that lack collaterals. This pattern of vascular anatomy may make the lenticular nuclei particularly sensitive to the changes of blood flow and more susceptible to metabolic change. The thalamus is supplied by a more robust arterial network that includes the posterior perforating arteries (the tuberthalamic artery, thalamogeniculate artery, and anterior choroidal artery) as well as the medial and lateral posterior choroidal arteries. The collateral circulation of the thalamus is also abundant. Blood supply to the thalamus is mainly from the posterior cerebral cycle. The thalamus is also partly supplied by the anterior cerebral cycle, because the anterior choroidal artery, which is involved in the blood supply of subthalamus, also arises from the middle cerebral artery. The specialized blood supply to the thalamus might make it less sensitive to changes in blood flow, and consequently, its tolerance to ischemia is better than that of the lenticular nucleus.
Lactate resulting from the mismatch between glycolysis and oxygen supply is a product of anaerobic metabolism, and it is a hallmark for the detection of cerebral ischemia.21 However, none of the patients with T2DM in our study had lactic acid detected. We speculated that this was because ischemia resulting from T2DM was not enough to cause anaerobic metabolism. However, this speculation needs to be proved by further study.
Our finding that significant differences in NAA/Cr ratios between patients and controls were only present in the left lenticular nucleus suggests that the metabolic changes of the left lenticular nucleus play a more important role in T2DM. It is probable that the left hemisphere is more vulnerable than the right because of a “dominant hemisphere” effect. All subjects in the study were right-handed, which might explain why metabolite changes were more obvious in the left hemisphere. We speculated that the dominant hemisphere is more likely to be in the state of energy shortage because its energy requirements might be higher. Under hypoxic conditions, this would make this hemisphere more susceptible to nerve tissue damage, metabolic abnormalities, and dysfunction.
CONCLUSIONS
1H-MR spectroscopy contributed to the early detection of brain damage. The abnormal metabolic status was observed in the brain areas that appeared with normal structure in MR imaging. Different metabolic statuses of the lenticular nuclei and thalamus were observed, suggesting different effects of T2DM in each of these brain nuclei, with the lenticula being more vulnerable than the thalamus. However, we could not show now whether the perfusion of these 2 areas was different. Further study is needed to verify the perfusion state of both areas.
ABBREVIATIONS:
- T2DM
type 2 diabetes mellitus
- DM
diabetes mellitus
- FBG
fasting blood glucose
- HbA1c
glycosylated hemoglobin
Footnotes
Disclosures: Yongzhong Lin—RELATED: Grant: Natural Science Foundation of Liaoning Province of China (No. 20102049),* Special Funds of the National Natural Science Foundation of China (No. 30950025),* Science and Technology Planning Project of Liaoning Province of China (No. 2010225009),* and National Science and Technology Major Project of China (No. 2012ZX09503001-003),* Fees for Participation in Review Activities such as Data Monitoring Boards, Statistical Analysis, Endpoint Committees and the Like; Payment for Writing or Reviewing the Manuscript; Provision of Writing Assistance, Medicines, Equipment, or Administrative Support: Natural Science Foundation of Liaoning Province of China (No. 20102049). Changkai Sun—RELATED: Grant, Support for Travel to Meetings for the Study or Other Purposes: National Natural Science Foundation of China (No. 30950025),* Consulting Fee or Honorarium; Payment for Writing or Reviewing the Manuscript: Science and Technology Project of Liaoning Province of China (No. 2010225009),* Fees for Participation in Review Activities such as Data Monitoring Boards, Statistical Analysis, Endpoint Committees and the Like; Provision of Writing Assistance, Medicines, Equipment, or Administrative Support; Other: National Science and Technology Major Project of China (No. 2012ZX009503001-003).* *Money paid to the institution.
This work was supported by the Science and Technology Planning Project of Dalian (No. 2010E15SF182), the Natural Science Foundation of Liaoning Province of China (No. 20102049), and the Special Funds of the National Natural Science Foundation of China (No. 30950025).
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat Rec 2001;265:54–84 [DOI] [PubMed] [Google Scholar]
- 2.Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed 1991;4:47–52 [DOI] [PubMed] [Google Scholar]
- 3.Geissler A, Frund R, Scholmerich J, et al. Alterations of cerebral metabolism in patients with diabetes mellitus studied by proton magnetic resonance spectroscopy. Exp Clin Endocrinol Diabetes 2003;111:421–27 [DOI] [PubMed] [Google Scholar]
- 4.Kario K, Ishikawa J, Hoshide S, et al. Diabetic brain damage in hypertension: role of renin-angiotensin system. Hypertension 2005;45:887–93 [DOI] [PubMed] [Google Scholar]
- 5.Kreis R, Ross BD. Cerebral metabolic disturbances in patients with subacute and chronic diabetes mellitus: detection with proton MR spectroscopy. Radiology 1992;184:123–30 [DOI] [PubMed] [Google Scholar]
- 6.Biessels GJ, Braun KP, de Graaf RA, et al. Cerebral metabolism in streptozotocin-diabetic rats: an in vivo magnetic resonance spectroscopy study. Diabetologia 2001;44:346–53 [DOI] [PubMed] [Google Scholar]
- 7.Sahin I, Alkan A, Keskin L, et al. Evaluation of in vivo cerebral metabolism on proton magnetic resonance spectroscopy in patients with impaired glucose tolerance and type 2 diabetes mellitus. J Diabetes Complications 2008;22:254–60 [DOI] [PubMed] [Google Scholar]
- 8.Modi S, Bhattacharya M, Sekhri T, et al. Assessment of the metabolic profile in type 2 diabetes mellitus and hypothyroidism through proton MR spectroscopy. Magn Reson Imaging 2008;26:420–25 [DOI] [PubMed] [Google Scholar]
- 9.Higa M, Kaneko Y, Inokuchi T. Two cases of hyperglycemic chorea in diabetic patients. Diabet Med 2004;21:196–98 [DOI] [PubMed] [Google Scholar]
- 10.Lai PH, Chen PC, Chang MH, et al. In vivo proton MR spectroscopy of chorea-ballismus in diabetes mellitus. Neuroradiology 2001;43:525–31 [DOI] [PubMed] [Google Scholar]
- 11.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2000;23(suppl 1):S4–19 [PubMed] [Google Scholar]
- 12.Ajilore O, Haroon E, Kumaran S, et al. Measurement of brain metabolites in patients with type 2 diabetes and major depression using proton magnetic resonance spectroscopy. Neuropsychopharmacology 2007;32:1224–31 [DOI] [PubMed] [Google Scholar]
- 13.Keymel S, Heinen Y, Balzer J, et al. Characterization of macro-and microvascular function and structure in patients with type 2 diabetes mellitus. Am J Cardiovasc Dis 2011;1: 68–75 [PMC free article] [PubMed] [Google Scholar]
- 14.Jones RL, Peterson CM. Hematologic alterations in diabetes mellitus. Am J Med 1981;70:339–52 [DOI] [PubMed] [Google Scholar]
- 15.Djuricic B, Olson SR, Assaf HM, et al. Formation of free choline in brain tissue during in vitro energy deprivation. J Cereb Blood Flow Metab 1991;11:308–13 [DOI] [PubMed] [Google Scholar]
- 16.Scremin OU, Jenden DJ. Focal ischemia enhances choline output and decreases acetylcholine output from rat cerebral cortex. Stroke 1989;20:92–95 [DOI] [PubMed] [Google Scholar]
- 17.Blüml S, Seymour KJ, Ross BD. Developmental changes in choline- and ethanolamine-containing compounds measured with proton-decoupled (31)P MRS in in vivo human brain. Magn Reson Med 1999;42:643–54 [DOI] [PubMed] [Google Scholar]
- 18.Mäkimattila S, Malmberg-Ceder K, Hakkinen AM, et al. Brain metabolic alterations in patients with type 1 diabetes-hyperglycemia-induced injury. J Cereb Blood Flow Metab 2004;24:1393–99 [DOI] [PubMed] [Google Scholar]
- 19.Tiehuis A, van der Meer F, Mali W, et al. MR spectroscopy of cerebral white matter in type 2 diabetes; no association with clinical variables and cognitive performance. Neuroradiology 2010;52:155–61 [DOI] [PubMed] [Google Scholar]
- 20.Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev 2007;56:384–402 [DOI] [PubMed] [Google Scholar]
- 21.Saunders DE. MR spectroscopy in stroke. Br Med Bull 2000;56:334–45 [DOI] [PubMed] [Google Scholar]