Abstract
BACKGROUND:
In 2003, Higashida et al proposed the Thrombolysis In Cerebral Infarction scale to evaluate angiographic intracranial flow. Our aim is to review how subsequently published studies define TICI.
MATERIALS AND METHODS:
We used the ISI Web of Knowledge and SciVerse Scopus databases to search for “TICI” and “thrombolysis in cerebral infarction” and for articles that cited the original TICI paper from January 2004 through May 2012. Articles were categorized according to their definition of the TICI categories, typically grades 0–4, with grade 2 (partial reperfusion) subdivided into 2a and 2b, and rate of contrast entry to the perfused area. In addition, we catalogued the type of redefinitions of TICI subcategory 2 and additions of new categories.
RESULTS:
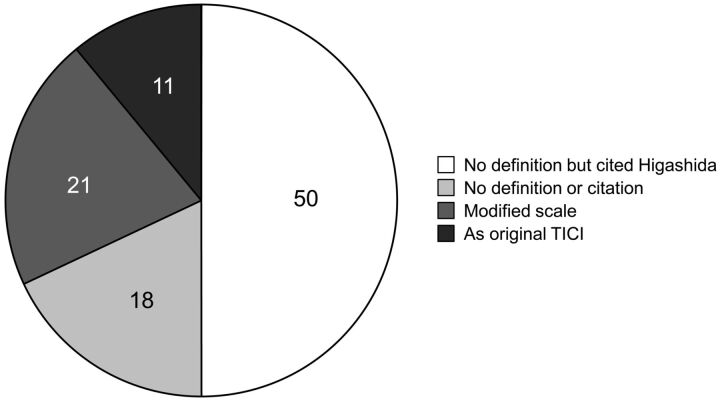
Of 236 articles screened, 74 were included. Eight (11%) explicitly followed the TICI scale as originally defined. Thirty-seven (50%) cited Higashida but did not define their scale. Fifteen (21%) used and explained modified scales. Thirteen (18%) used the term TICI, but did not define the scale and did not cite Higashida. Eighteen (24%) specified a 2a subcategory. Nine defined grade 2a as <67% filling, 6 defined it as <50%, and 3 did not offer a percentage. Two studies added a 2c subcategory. Fifty-two (70%) used a cutoff level to define “successful reperfusion.” Of these, 65% used TICI ≥2, 33% used TICI ≥2b, and 2% used TICI = 3.
CONCLUSIONS:
There is substantial variability in the definition and/or application of the TICI scale in the literature. This variability could considerably impact our understanding of results of revascularization studies.
The Thrombolysis in Myocardial Infarction scale is a widely applied, graded response scale for assessment of treatment outcome in the coronary arteries.1 In 2003, Higashida et al2 proposed a seemingly simple modification of the TIMI scale to evaluate intracranial perfusion assessed in cerebral angiography. This new scale, the Thrombolysis in Cerebral Infarction scale, was intended to standardize the grading of angiographic outcomes, particularly for trials of endovascular treatment of acute ischemic stroke (Table 1). As originally described, TICI categories span from no perfusion (grade 0) to complete perfusion (grade 3). The “partial perfusion” category (grade 2) is defined as cases in which contrast passes the obstruction but with rates of entry and washout slower than normal and is subdivided into 2 subcategories, 2a and 2b. Although the TICI scale has achieved fairly rapid acceptance into the medical literature, the scale was somewhat arbitrarily created and has not been validated or tested systematically.
Table 1:
The original Thrombolysis in Cerebral Infarction perfusion scale2
| Category | Title | Description |
|---|---|---|
| Grade 0 | No Perfusion | No antegrade flow beyond the point of occlusion. |
| Grade 1 | Penetration with Minimal Perfusion | The contrast material passes beyond the area of obstruction but fails to opacify the entire cerebral bed distal to the obstruction. |
| Grade 2 | Partial Perfusion | The contrast material passes beyond the obstruction and opacifies the arterial bed distal to the obstruction. However, the rate of entry of contrast into the vessel distal to the obstruction and/or its rate of clearance from the distal bed are perceptibly slower than its entry into and/or clearance from comparable areas not perfused by the previously occluded vessel. |
| Grade 2a | Only partial filling (less than two-thirds) of the entire vascular territory is visualized. | |
| Grade 2b | Complete filling of all of the expected vascular territory is visualized but the filling is slower than normal. | |
| Grade 3 | Complete Perfusion | Antegrade flow into the bed distal to the obstruction occurs as promptly as into the obstruction and clearance of contrast material from the involved bed is as rapid as from an uninvolved other bed of the same vessel or the opposite cerebral artery. |
Although the TICI scale has been criticized because of confusing internal inconsistencies,3 it is still widely used in the literature.4 Modifications of the TICI scale have been subsequently proposed, such as a change in the definition of the 2a subcategory (to indicate <50% filling of the vascular territory) or by the addition of a 2c subcategory.5 Our current aim is to review published studies that use the TICI scale to describe how the TICI scale is defined and utilized across studies.
Materials and Methods
We performed a search of the medical literature by using the ISI Web of Knowledge and SciVerse Scopus databases. We searched for the terms “TICI” and “thrombolysis in cerebral infarction.” We also used these databases to search for all articles from January 2004 through May 2012 that cited the original TICI paper. In addition, we reviewed the reference list from all identified articles to identify other papers by using graded response scales for cerebral perfusion, whether or not these papers referenced or utilized the original TICI paper.
In the initial description of the TICI scale in 2003, grade 0 indicates no perfusion as evidenced by no antegrade flow beyond the point of arterial occlusion.2 Grade 1 is defined as penetration with minimal perfusion” and applies when the “contrast material passes beyond the area of obstruction but fails to opacify the entire cerebral bed distal to the obstruction.” Grade 2 is broadly defined as partial perfusion, which occurs when the contrast material passes beyond the obstruction, opacifies the distal arterial bed, but the rate of entry of contrast and/or its rate of clearance from the vascular bed are slower than comparable areas not perfused by the previously occluded vessel. The opposite cerebral artery or the arterial bed proximal to the occlusion can be used for comparison rates. Grade 2 is subdivided into 2a and 2b. A grade of 2a indicates partial filling (less than two-thirds) of the entire vascular territory and 2b indicates complete filling of the expected vascular territory, but with a perceptibly slower filling rate than normal. Finally, grade 3 is defined as complete perfusion and applies when “antegrade flow into the bed distal to the obstruction occurs as promptly as into the obstruction and clearance of the contrast material from the involved bed is as rapid as from an uninvolved other bed of the same vessel or the opposite cerebral artery.”2
With our literature search, we identified a total of 236 articles. We excluded articles that did not relate to the TICI scale (115 articles) and articles that were in languages other than English or that were not accessible in full length (49 articles). We qualitatively assessed whether the definition of TICI in each article adhered to the original definition of the TICI scale and evaluated the articles that were cited when TICI was described. We then accordingly categorized the articles into 4 groups: 1) articles that explicitly stated the scale and followed the original TICI scale completely, 2) articles that did not explicitly define the scale but cited the original TICI paper, 3) articles that defined a modified scale, and 4) articles that used TICI but did not define the scale and did not cite the original TICI paper. We also catalogued the type and number of definitions of subcategory 2a and noted if a subcategory of TICI was used as a threshold for “successful revascularization.” This study was exempt from institutional review board review.
Results
Of 74 total included articles, 8 (11%) followed the original TICI scale completely and explicitly stated the categories.6–13 One article claimed to have followed the scale completely but did not state the categories.14 Thirty-seven (50%) articles did not explicitly define the scale but still cited the original paper by Higashida et al.15–51 Modifications of the TICI scale were used in 15 (20%) articles.4,5,52–65 Of these, 8 cited only the original TICI paper, 4 cited the original TICI paper and other papers, and 3 cited only other papers. Thirteen (18%) articles used TICI but did not define the scale and did not cite the original TICI paper.13,30,56,66–75 These results are depicted in Fig 1.
Fig 1.
Distribution of definition and citation of the TICI scale in the literature. Articles in the English literature that use the TICI (Thrombolysis in Cerebral Infarction) grading scale, distributed according to definition and citation of TICI.
Eighteen (24%) of articles mentioned the rate of contrast filling in their use of TICI. Most modifications of TICI eliminated the subcategories of 2a and 2b. Only 18 (24%) articles specified a 2a subcategory. Of these, 9 defined 2a as filling of <67% of the affected vascular territory (compatible with the original TICI) and 6 defined 2a as filling of <50% of the affected vascular territory. A 2c subcategory was added in 2 articles, and a category 4 was added in 1 article. Examples of the variability in definitions of TICI categories are detailed in Table 2.
Table 2:
Varying definitions of TICI grades in the literature
| Category | Definition |
|---|---|
| Grade 0 | No flow |
| No canalization | |
| Complete occlusion | |
| No recanalization/reperfusion | |
| Grade 1 | Minimal recanalization (<20%) |
| Minimal flow (very slow) without significant flow distal to the occlusion site | |
| Limited or no reperfusion | |
| Distal movement of thrombus without reperfusion | |
| Perfusion past initial occlusion, but limited distal branch | |
| Filling | |
| Grade 2 | Partial recanalization—recanalization of some but not all of the occluded arteries |
| Incomplete recanalization/reperfusion | |
| Near-normal flow, with flow distal to the occlusion but not filling the distal branches normally | |
| Grade 2a | Perfusion of <50% of the MCA distribution |
| Partial filling of the entire vascular territory | |
| Partial perfusion with incomplete distal filling of <50% of expected territory | |
| Partial filling of the entire vascular territory | |
| Grade 2b | Partial perfusion with incomplete distal branch filling of ≥50–99% of the expected territory |
| Complete filling, but the filling is slower than normal | |
| Perfusion of half or greater of the vascular distribution of the occluded artery | |
| Grade 2c | Near-complete perfusion without clearly visible thrombus but with delay in contrast run-off |
| Grade 3 | Full perfusion with filling of all distal branches, including M3, M4 |
| Normal flow | |
| Partial recanalization with >50% reperfusion | |
| Full perfusion with normal filling of distal branches in a normal hemodynamic fashion | |
| Grade 4 | Complete recanalization/reperfusion |
Most articles (n = 52, 70%) defined a threshold within the TICI scale that indicated “successful revascularization” as one of the study end points. Of these, 34 (65%) used TICI ≥2, 17 (33%) used TICI ≥2b (although only 1 of these studies defined a precise cutoff for 2b; 67% filling of the vascular territory), and 1 used TICI = 3. These thresholds for successful angiographic revascularization were prespecified in the methods in 40 (77%) of these articles.
Discussion
The term “TICI” connotes a standard and widely accepted metric of revascularization, analogous to the ubiquitous TIMI outcome for coronary revascularization. In the current study, we found substantial variability in how the term “TICI” scale is both defined and used in the recent English literature. Far from being a consistent and universal scale, we noted that only a small minority of studies, by use of the term “TICI” when reporting outcomes after revascularization, actually used the original TICI scale. Furthermore, many studies failed to provide sufficient detail to allow the reader to understand exactly what categories were used. Finally, the definition of successful revascularization varied widely among studies. These current findings are relevant for several reasons.
First, our understanding of the current literature has the potential to be greatly affected by these findings. The modification that changed the cutoff point between TICI subcategories 2a and 2b has particular relevance because a grade on the TICI scale ≥2b was used as an end point to define successful reperfusion in one-third of the articles that specified this end point in our study. Second, the definition of TICI will affect study design for future trials of endovascular therapy for acute ischemic stroke. The TICI grading scale is increasingly used to define end points of revascularization success in studies. If we define success as achieving a certain grade of TICI (eg, TICI 2b) but we do not have consistent grading systems, we cannot compare or combine results of clinical studies. To achieve enough patients for studies to be powered adequately, it is necessary for investigators from different centers to collaborate together. Without a standardized grading scale, this will not be possible. It is essential that we communicate clearly with consistent terminology.
To our knowledge, our study is the first to specifically describe the varying definitions of the TICI scale as it is reported in the literature. Others, however, have previously called attention to the confusion surrounding the TICI scale.3,76 In 2007, Tomsick76 acknowledged confusion about the different revascularization scales. He noted the inconsistent descriptions and applications in the literature; some focus on recanalization, some focus on reperfusion, and others confusingly (and erroneously) use the terms interchangeably. Letters denoting acronyms for different scales are littered throughout the literature and include the TICI, TIMI, TIBI (Thrombolysis in Brain Ischemia), and AOL (Arterial Occlusive Lesion) scales. In a previous review of the TICI scale, the inherent inconsistencies within the original TICI scale itself were identified.3 For example, there is no applicable TICI grade for a case in which greater than two-thirds but less than “complete” filling of the vascular territory is visualized. In addition, there is no applicable TICI grade for a partially revascularized territory with normal rate of distal opacification, a scenario not uncommonly encountered.3
The TIMI scale—unlike the TICI scale—has not been the subject of frequent modifications. The definition of the TIMI scale throughout the abundant cardiology literature has not been systemically evaluated, but there is general consensus that when used for the evaluation of myocardial perfusion before and after coronary reperfusion therapies, it is used consistently. In the mid1990s, a quantitative assessment of coronary flow called the corrected TIMI Frame Count (CTFC) was reported in an attempt to standardize the scale,77 but the original semiqualitative TIMI scale has continued to be the standard used by interventional cardiologists. However, the TIMI scale cannot be easily applied to the more complex cerebral arteries. One review found that 7 different operationalized versions of the TIMI scale have been used in major stroke trials, emphasizing again the need for a single, uniform, consistent scale for grading of perfusion in cerebral arteries.78
This study has several limitations. Some articles from our literature search were not reviewed because of a lack of accessibility of full-length articles or because they were written in languages other than English, creating a selection bias. However, increasing the number of studies we reviewed may have increased the observed variability in TICI definitions. Also, the categories into which articles were divided were subjectively chosen and were evaluated by only 2 investigators.
Further opportunities to refine our grading scales and further our understanding of brain reperfusion abound. Weaknesses in current grading scales for cerebral perfusion are not limited to confusing terminology. Vessel recanalization in the treatment of acute ischemic stroke has been shown to be associated with favorable clinical functional outcomes, but when reperfusion is only partial, the clinical relevance of the use of different TICI grade 2 subdivisions is not known. Furthermore, there are few data regarding the intra-observer and interobserver variability when applying the TICI scale to angiography results. It also remains unclear whether it is appropriate to apply TICI to the posterior circulation and whether the degree of collateral flow—particularly in cases with distal M3–4 occlusions—modifies the effect of revascularization (as measured by TICI) on clinical outcomes.
Scales are designed to aid in the objective description of angiographic results, standardize data for research studies, and assist in outcome prediction.79 We hope that by clarifying what we mean by “TICI,” we will be better able to evaluate the efficacy of revascularization therapies for acute ischemic stroke in the future.
Conclusions
There is substantial variability in how the TICI scale is defined and applied in the cerebrovascular literature. Few studies provide sufficient detail for readers to understand what is meant by each TICI grade. Because TICI score is increasingly used as an outcome measure in studies of revascularization therapies in acute ischemic stroke, this variability has the potential to considerably impact results and our understanding of these therapies.
ABBREVIATIONS:
- TICI
Thrombolysis in Cerebral Infarction
- TIMI
Thrombolysis in Myocardial Infarction
Footnotes
Disclosures: David Kallmes—UNRELATED: Consultancy: ev3;* Grants/Grants Pending: MicroVention,* Sequent Medical,* ev3,* Benvenue Medical;* Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Codman* (*money paid to institution).
REFERENCES
- 1.TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial: phase I findings. N Engl J Med 1985;312:932–36 [DOI] [PubMed] [Google Scholar]
- 2.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109–137 [DOI] [PubMed] [Google Scholar]
- 3.Kallmes DF. TICI: if you are not confused, then you are not paying attention. AJNR Am J Neuroradiol 2012;33:975–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Interventional Management of Stroke (IMS) II Study. Stroke 2007;38:2127–35 [DOI] [PubMed] [Google Scholar]
- 5.Noser EA, Shaltoni HM, Hall CE, et al. Aggressive mechanical clot disruption: a safe adjunct to thrombolytic therapy in acute stroke? Stroke 2005;36:292–96 [DOI] [PubMed] [Google Scholar]
- 6.Kim EY, Heo JH, Lee SK, et al. Prediction of thrombolytic efficacy in acute ischemic stroke using thin-section noncontrast CT. Neurology 2006;67:1846–48 [DOI] [PubMed] [Google Scholar]
- 7.Kulcsar Z, Bonvin C, Pereira VM, et al. Penumbra system: a novel mechanical thrombectomy device for large-vessel occlusions in acute stroke. AJNR Am J Neuroradiol 2010;31:628–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KY, Han SW, Kim SH, et al. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre- and post-thrombolytic angiography in acute ischemic stroke patients. Stroke 2007;38:192–93 [DOI] [PubMed] [Google Scholar]
- 9.Schumacher HC, Meyers PM, Higashida RT, et al. Reporting standards for angioplasty and stent-assisted angioplasty for intracranial atherosclerosis. J Vasc Interv Radiol 2009;20:S451–73 [DOI] [PubMed] [Google Scholar]
- 10.Suh DC, Kim JK, Choi CG, et al. Prognostic factors for neurologic outcome after endovascular revascularization of acute symptomatic occlusion of the internal carotid artery. AJNR Am J Neuroradiol 2007;28:1167–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau AY, Wong EH, Wong A, et al. Significance of good collateral compensation in symptomatic intracranial atherosclerosis. Cerebrovasc Dis 2012;33:517–24 [DOI] [PubMed] [Google Scholar]
- 12.Liebeskind DS, Cotsonis GA, Saver JL, et al. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab 2011;31:1293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth C, Papanagiotou P, Behnke S, et al. Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions. Stroke 2010;41:2559–67 [DOI] [PubMed] [Google Scholar]
- 14.Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol 2011;69:963–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almekhlafi MA, Hu WY, Hill MD, et al. Calcification and endothelialization of thrombi in acute stroke. Ann Neurol 2008;64:344–48 [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Mori T, Izumoto H, et al. MR imaging-based localized intra-arterial thrombolysis assisted by mechanical clot disruption for acute ischemic stroke due to middle cerebral artery occlusion. AJNR Am J Neuroradiol 2011;32:748–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kole M, Amin B, Marin H, et al. Intracranial angioplasty and stent placement for direct cerebral revascularization of nonacute intracranial occlusions and near occlusions. Neurosurg Focus 2009;26:E3. [DOI] [PubMed] [Google Scholar]
- 18.Mordasini P, Frabetti N, Gralla J, et al. In vivo evaluation of the first dedicated combined flow-restoration and mechanical thrombectomy device in a swine model of acute vessel occlusion. AJNR Am J Neuroradiol 2011;32:294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueira RG, Schwamm LH, Buonanno FS, et al. Low-pressure balloon angioplasty with adjuvant pharmacological therapy in patients with acute ischemic stroke caused by intracranial arterial occlusions. Neuroradiology 2008;50:331–40 [DOI] [PubMed] [Google Scholar]
- 20.Raychev R, Ovbiagele B. Endovascular therapy of acute ischemic stroke. Exp Opin Pharmacother 2011;12:913–30 [DOI] [PubMed] [Google Scholar]
- 21.Sugg RM, Noser EA, Shaltoni HM, et al. Intra-arterial reteplase compared to urokinase for thrombolytic recanalization in acute ischemic stroke. AJNR Am J Neuroradiol 2006;27:769–73 [PMC free article] [PubMed] [Google Scholar]
- 22.Castano C, Dorado L, Guerrero C, et al. Mechanical thrombectomy with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke 2010;41:1836–40 [DOI] [PubMed] [Google Scholar]
- 23.Deguchi I, Dembo T, Fukuoka T, et al. Usefulness of MRA-DWI mismatch in neuroendovascular therapy for acute cerebral infarction. Eur J Neurol 2012;19:114–20 [DOI] [PubMed] [Google Scholar]
- 24.Deshmukh VR, Fiorella DJ, Albuquerque FC, et al. Intra-arterial thrombolysis for acute ischemic stroke: preliminary experience with platelet glycoprotein IIb/IIIa inhibitors as adjunctive therapy. Neurosurgery 2005;56:46–54 [DOI] [PubMed] [Google Scholar]
- 25.Fesl G, Patzig M, Holtmannspoetter M, et al. Endovascular mechanical recanalisation after intravenous thrombolysis in acute anterior circulation stroke: the impact of a new temporary stent. Cardiovasc Intervent Radiol 2012;35:1326–31 [DOI] [PubMed] [Google Scholar]
- 26.Froehler MT, Tateshima S, Duckwiler G, et al. The hyperdense vessel sign on CT predicts successful recanalization with the Merci device in acute ischemic stroke. J Neurointervent Surg 2013;5:289–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauck EF, Ogilvy CS, Siddiqui AH, et al. Direct endovascular recanalization of chronic carotid occlusion: should we do it? Case report. Neurosurgery 2010;67:E1152–59 [DOI] [PubMed] [Google Scholar]
- 28.Sandhu GS, Parikh PT, Hsu DP, et al. Outcomes of intra-arterial thrombolytic treatment in acute ischemic stroke patients with a matched defect on diffusion and perfusion MR images. J Neurointervent Surg 2012;4:105–09 [DOI] [PubMed] [Google Scholar]
- 29.Jankowitz B, Aghaebrahim A, Zirra A, et al. Manual aspiration thrombectomy: adjunctive endovascular recanalization technique in acute stroke interventions. Stroke 2012;43:1408–11 [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Hwang YH, Kim YS, et al. Direct thrombus retrieval using the reperfusion catheter of the Penumbra system: forced-suction thrombectomy in acute ischemic stroke. AJNR Am J Neuroradiol 2011;32:283–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SJ, Ha YS, Ryoo S, et al. Sulcal effacement on fluid attenuation inversion recovery magnetic resonance imaging in hyperacute stroke: association with collateral flow and clinical outcomes. Stroke 2012;43:386–92 [DOI] [PubMed] [Google Scholar]
- 32.Kwon TH, Kim BM, Nam HS, et al. Carotid stenting in acute ischemic stroke patients with intraluminal thrombus. Neuroradiology 2011;53:773–78 [DOI] [PubMed] [Google Scholar]
- 33.Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011;42:1237–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loh Y, Liebeskind DS, Shi ZS, et al. Partial recanalization of concomitant internal carotid-middle cerebral arterial occlusions promotes distal recanalization of residual thrombus within 24 h. J Neurointervent Surg 2011;3:38–42 [DOI] [PubMed] [Google Scholar]
- 35.Machi P, Costalat V, Lobotesis K, et al. Solitaire FR thrombectomy system: immediate results in 56 consecutive acute ischemic stroke patients. J Neuroint Surg 2012;4:62–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machi P, Lobotesis K, Maldonado IL, et al. Endovascular treatment of tandem occlusions of the anterior cerebral circulation with Solitaire FR thrombectomy system: initial experience. Eur J Radiol 2012;81:3479–84 [DOI] [PubMed] [Google Scholar]
- 37.Mordasini P, Hiller M, Brekenfeld C, et al. In vivo evaluation of the Phenox CRC mechanical thrombectomy device in a swine model of acute vessel occlusion. AJNR Am J Neuroradiol 2010;31:972–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park MS, Kim JT, Yoon W, et al. Intra-arterial thrombolysis after full-dose intravenous tPA via the “Drip and Ship” approach in patients with acute ischemic stroke: preliminary report. Chonnam Med J 2011;47:99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parrilla G, Garcia-Villalba B, Espinosa de Rueda M, et al. Hemorrhage/contrast staining areas after mechanical intra-arterial thrombectomy in acute ischemic stroke: imaging findings and clinical significance. AJNR Am J Neuroradiol 2012;33:1791–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prothmann S, Lockau H, Dorn F, et al. The Phenox clot retriever as part of a multimodal mechanical thrombectomy approach in acute ischemic stroke: single center experience in 56 patients. Sci World J [Epub ahead of print 24 April 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Psychogios MN, Kreusch A, Wasser K, et al. Recanalization of large intracranial vessels using the Penumbra system: a single center experience. AJNR Am J Neuroradiol 2012;33:1488–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth C, Junk D, Papanagiotou P, et al. A comparison of 2 stroke devices: the new Aperio clot-removal device and the Solitaire AB/FR. AJNR Am J Neuroradiol 2012;33:1317–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siemonsen S, Lobel U, Sedlacik J, et al. Elevated T2-values in MRI of stroke patients shortly after symptom onset do not predict irreversible tissue infarction. Brain 2012;135:1981–89 [DOI] [PubMed] [Google Scholar]
- 44.Sauvageau E, Levy EI. Self-expanding stent-assisted middle cerebral artery recanalization: technical note. Neuroradiology 2006;48:405–08 [DOI] [PubMed] [Google Scholar]
- 45.Stampfl S, Hartmann M, Ringleb PA, et al. Stent placement for flow restoration in acute ischemic stroke: a single-center experience with the Solitaire stent system. AJNR Am J Neuroradiol 2011;32:1245–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatum J, Farid H, Cooke D, et al. Mechanical embolectomy for treatment of large vessel acute ischemic stroke in children. J Neurointervent Surg 2013;5:128–34 [DOI] [PubMed] [Google Scholar]
- 47.Alhazzaa M, Murphy A, Lum C, et al. Angioplasty as an adjuvant therapy for the treatment of acute ischemic stroke. Can J Neurol Sci 2011;38:593–99 [DOI] [PubMed] [Google Scholar]
- 48.Yin NS, Benavides S, Starkman S, et al. Autopsy findings after intracranial thrombectomy for acute ischemic stroke: a clinicopathologic study of 5 patients. Stroke 2010;41:938–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu L, Liebeskind DS, Jahan R, et al. Thrombus branching and vessel curvature are important determinants of middle cerebral artery trunk recanalization with Merci thrombectomy devices. Stroke 2012;43:787–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribo M, Molina CA, Alvarez B, et al. Intra-arterial administration of microbubbles and continuous 2-MHz ultrasound insonation to enhance intra-arterial thrombolysis. J Neuroimaging 2010;20:224–27 [DOI] [PubMed] [Google Scholar]
- 51.Kulcsar Z, Bonvin C, Lovblad KO, et al. Use of the Enterprise intracranial stent for revascularization of large vessel occlusions in acute stroke. Klinische Neuroradiologie [Epub ahead of print 28 Feb 2010] [DOI] [PubMed] [Google Scholar]
- 52.Belisle JG, McCollom VE, Tytle TL, et al. Intraarterial therapy for acute ischemic strokes. J Vasc Interv Radiol 2009;20:327–33 [DOI] [PubMed] [Google Scholar]
- 53.King S, Khatri P, Carrozella J, et al. Anterior cerebral artery emboli in combined intravenous and intra-arterial rtPA treatment of acute ischemic stroke in the IMS I and II trials. AJNR Am J Neuroradiol 2007;28:1890–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levy EI, Mehta R, Gupta R, et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol 2007;28:816–22 [PMC free article] [PubMed] [Google Scholar]
- 55.Seifert M, Ahlbrecht A, Dohmen C, et al. Combined interventional stroke therapy using intracranial stent and local intraarterial thrombolysis (LIT). Neuroradiology 2011;53:273–82 [DOI] [PubMed] [Google Scholar]
- 56.Ribo M, Molina C, Alvarez B, et al. Buying time for recanalization in acute stroke: arterial blood infusion beyond the occluding clot as a neuroprotective strategy. J Neuroimaging 2009;19:188–90 [DOI] [PubMed] [Google Scholar]
- 57.Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol 2008;29:582–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe M, Mori T, Imai K, et al. Endovascular interventions for patients with serious symptoms caused by embolic carotid T occlusion. Neurol Med Chir (Tokyo) 2011;51:282–88 [DOI] [PubMed] [Google Scholar]
- 59.Yoo AJ, Verduzco LA, Schaefer PW, et al. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke 2009;40:2046–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ikushima I, Ohta H, Hirai T, et al. Balloon catheter disruption of middle cerebral artery thrombus in conjunction with thrombolysis for the treatment of acute middle cerebral artery embolism. AJNR Am J Neuroradiol 2007;28:513–17 [PMC free article] [PubMed] [Google Scholar]
- 61.Khatri P, Abruzzo T, Yeatts SD, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology 2009;73:1066–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JS, Hong JM, Kim EJ, et al. Comparison of the incidence of parenchymal hematoma and poor outcome in patients with carotid terminus occlusion treated with intra-arterial urokinase alone or with combined IV rtPA and intra-arterial urokinase. AJNR Am J Neuroradiol 2012;33:175–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li YH, Li MH, Zhao ZG, et al. Comparison of MRI-based thrombolysis for patients with middle cerebral artery occlusion <or =3 h and 3–6 h. Neurology India 2009;57:426–33 [DOI] [PubMed] [Google Scholar]
- 64.Liu W, Yin Q, Yao L, et al. Decreased hyperintense vessels on FLAIR images after endovascular recanalization of symptomatic internal carotid artery occlusion. Eur J Radiol 2012;81:1595–600 [DOI] [PubMed] [Google Scholar]
- 65.Gandini R, Pampana E, Del Giudice C, et al. Acute stroke treatment using the Penumbra endovascular mechanical thrombolysis device: a single-centre experience. La Radiologia Medica 2012;117:1199–214 [DOI] [PubMed] [Google Scholar]
- 66.Bang JS, Oh CW, Jung C, et al. Intracranial stent placement for recanalization of acute cerebrovascular occlusion in 32 patients. AJNR Am J Neuroradiol 2010;31:1222–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hallevi H, Barreto AD, Liebeskind DS, et al. Identifying patients at high risk for poor outcome after intra-arterial therapy for acute ischemic stroke. Stroke 2009;40:1780–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Costalat V, Machi P, Lobotesis K, et al. Rescue, combined, and stand-alone thrombectomy in the management of large vessel occlusion stroke using the Solitaire device: a prospective 50-patient single-center study: timing, safety, and efficacy. Stroke 2011;42:1929–35 [DOI] [PubMed] [Google Scholar]
- 69.Dababneh H, Guerrero WR, Khanna A, et al. Management of tandem occlusion stroke with endovascular therapy. Neurosurg Focus 2012;32:E16. [DOI] [PubMed] [Google Scholar]
- 70.Khatri P, Broderick JP, Khoury JC, et al. Microcatheter contrast injections during intra-arterial thrombolysis may increase intracranial hemorrhage risk. Stroke 2008;39:3283–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwon JH, Shin SH, Weon YC, et al. Intra-arterial adjuvant tirofiban after unsuccessful intra-arterial thrombolysis of acute ischemic stroke: preliminary experience in 16 patients. Neuroradiology 2011;53:779–85 [DOI] [PubMed] [Google Scholar]
- 72.Rohde S, Haehnel S, Herweh C, et al. Mechanical thrombectomy in acute embolic stroke: preliminary results with the Revive device. Stroke 2011;42:2954–56 [DOI] [PubMed] [Google Scholar]
- 73.San Roman L, Obach V, Blasco J, et al. Single-center experience of cerebral artery thrombectomy using the TREVO device in 60 patients with acute ischemic stroke. Stroke 2012;43:1657–59 [DOI] [PubMed] [Google Scholar]
- 74.Wehrschuetz M, Wehrschuetz E, Augustin M, et al. Early single center experience with the Solitaire thrombectomy device for the treatment of acute ischemic stroke. Intervent Neuroradiol 2011;17:235–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoo AJ, Chaudhry ZA, Nogueira RG, et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke 2012;43: 1323–30 [DOI] [PubMed] [Google Scholar]
- 76.Tomsick T. TIMI, TIBI, TICI: I came, I saw, I got confused. AJNR Am J Neuroradiol 2007;28:382–84 [PMC free article] [PubMed] [Google Scholar]
- 77.Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation 1996;93:879–88 [DOI] [PubMed] [Google Scholar]
- 78.Soares BP, Chien JD, Wintermark M. MR and CT monitoring of recanalization, reperfusion, and penumbra salvage: everything that recanalizes does not necessarily reperfuse! Stroke 2009;40:S24–27 [DOI] [PubMed] [Google Scholar]
- 79.Cloft HJ, Kallmes DF. Scaling back on scales with a scale of scales. AJNR Am J Neuroradiol 2011;32:219–20 [DOI] [PMC free article] [PubMed] [Google Scholar]