Abstract
Background
Sepsis is a frequently lethal state, commonly associated with left ventricular (LV) dysfunction. Right ventricular (RV) dysfunction in sepsis is less well understood.
Research Question
In septic patients, how common is RV dysfunction, and is it associated with worse outcomes?
Study Design and Methods
We measured echocardiographic parameters on critically ill patients with severe sepsis or septic shock within the first 24 hours of ICU admission. We defined RV dysfunction as fractional area change (FAC) less than 35% or tricuspid annulus systolic plane excursion (TAPSE) less than 1.6 cm. We defined LV systolic dysfunction as ejection fraction (EF) less than 45% or longitudinal strain greater than -19%. Using logistic regression, we assessed the relationship between 28-day mortality and presence of RV dysfunction and LV systolic dysfunction, controlling for receipt of vasopressors, receipt of fluid, mechanical ventilation, and the acute physiology and chronic health evaluation (APACHE II) score.
Results
We studied 393 patients. RV and LV dysfunction were common (48% and 63%, respectively). Mean echocardiographic values were: RV end-diastolic area, 22.4 ± 7.0 cm2; RV end-systolic area, 14.2 ± 6.0 cm2; RV FAC, 38 ± 11%; TAPSE, 1.8 ± .06 cm; RV longitudinal strain, -15.3 ± 6.5%; LV EF, 60% ± 14%; LV longitudinal strain, -16.5% ± 6.0%. Patients with RV dysfunction had higher 28-day mortality (31% vs 16%, P = .001). In our multivariable regression model, RV dysfunction was associated with increased mortality (OR, 3.4; CI, 1.7-6.8; P = .001), and LV systolic dysfunction was not (OR, 0.63; CI, 0.3 -1.2; P = .32)
Interpretation
Right ventricular dysfunction is present in nearly half of studied septic patients and is associated with over threefold higher 28-day mortality.
Key Words: echocardiography, preload, right ventricle, septic cardiomyopathy
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation score, version 2; E/e’, ratio of early diastolic mitral inflow velocity to early diastolic mitral annular tissue velocity; EF, ejection fraction; FAC, fractional area change; LV, left ventricle; RV, right ventricle; SCM, septic cardiomyopathy; TAPSE, tricuspid annulus systolic plane excursion; TTE, transthoracic echocardiography
Sepsis and septic shock are clinical syndromes characterized by multi-organ dysfunction due to dysregulated inflammation in response to infection.1 Cardiac dysfunction in sepsis, also known as “septic cardiomyopathy (SCM),” is common and associated with increased mortality.2, 3, 4, 5, 6 When severe, SCM may contribute to a cascade of interdependent organ failure.7 Early studies of SCM pathophysiology focused primarily on systolic and diastolic dysfunction of the left ventricle (LV), but increasing attention to the right side has shown that the right ventricle (RV) may play an important and possibly independent role in SCM and its downstream effects.8
Although the RV has less muscle mass than the left, the two ventricles are connected both in series and via a common septum—leading to ventricular interdependence.9 In steady state, the RV matches the LV cardiac output to maintain left-sided filling and forward flow. During sepsis, the combination of increased RV afterload, decreased RV filling, or poor contractility may impair the RV ability to keep pace with the LV.8
Early studies of RV dysfunction in sepsis using thermodilution and radionucleotide ventriculography demonstrated reduced RV ejection fraction in patients with sepsis and particularly septic shock, but there was no clear correlation with survival.10, 11, 12, 13 More contemporary investigations have moved to transthoracic echocardiography to characterize RV dysfunction. In contrast to the LV, the RV is more challenging to image. Consequently, there remains a lack of consensus and of which echo parameters should be used to define RV dysfunction.14
Several recent studies have assessed the RV in sepsis, using a variety of functional and dimensional measurements, although they are generally smaller and assessed the heart later in the course of sepsis.15,16 RV subcostal wall thickness, pulse Doppler myocardial performance index, and RV ratio of early diastolic mitral inflow velocity to early diastolic mitral annular tissue velocity (E/e′) were found to be highly abnormal in patients with septic shock, but no difference was found between survivors and nonsurvivors.15 Harmankaya et al16 employed tissue Doppler in patients with sepsis, severe sepsis, and septic shock and found a correlation between tissue Doppler and severity of sepsis and in-hospital mortality.16 Most recently, a large study by Vallabhajosyula et al17 applied the multimodal ASE criteria for RV dysfunction and found a high prevalence of isolated RV dysfunction associated with long-term (1-year) mortality.17,18 Given the variability of these results, a more reliable definition of RV dysfunction in sepsis and septic shock is needed for use in both the research and clinical arenas.
Longitudinal strain measurement is a relatively new echocardiographic measurement, suspected to be less susceptible to changes in function due to ventricular loading conditions and perhaps a more sensitive marker of ischemic ventricular dysfunction compared with traditional two-dimensional echocardiographic assessments.19 RV strain may have some value in assessing systolic function in systolic heart dysfunction and pulmonary hypertension as well as other clinical scenarios, but its utility in sepsis is less clear.20, 21, 22 In this retrospective observational study, we sought to characterize the role of RV dysfunction in early sepsis and septic shock, with the addition of RV strain assessment.
Study Design and Methods
Study Design
This is a secondary analysis of a prospectively identified cohort of ICU patients admitted between October 2012 and November 2015 at Intermountain Medical Center, an academic tertiary care hospital. These patients were admitted to one of two ICUs (one medical, one medical-surgical), where transthoracic echocardiography (TTE) is commonly performed on patients with severe sepsis or septic shock (more so in patients with shock) at the time of ICU admission. The protocol was approved by the Intermountain Institutional Review Board (#1009957) with a waiver of informed consent.
Patients
We screened patients admitted with severe sepsis or septic shock defined by the then-current American College of Chest Physicians/Society of Critical Care Medicine consensus criteria.23 Patients met criteria for inclusion if they (1) were at least 18 years of age; (2) had a clinically suspected infection; (3) had two or more systemic inflammatory response syndrome criteria; and (4) had either septic shock (systolic BP < 90 mm Hg despite an IV fluid challenge of ≥20 mL/kg or infusion of any dose of vasopressor medications) or severe sepsis (defined in this study as serum lactate ≥4 mmol/L).
Transthoracic Echocardiography
TTEs were performed using a Philips iE-33 (Philips Medical Systems) machine for clinical indications. Patients were excluded if their TTE occurred more than 24 hours after onset of sepsis or if the image quality was poor. All TTEs were performed by a registered diagnostic cardiac sonographer. Studies were interpreted and formatted by an advanced cardiac sonographer (T. D. O.) followed by a consensus interpretation from two level-II echocardiographers (C. K. G., M. J. L.).
We measured common echocardiographic parameters, including LV ejection fraction (EF), myocardial performance index, RV fractional area change (FAC), and tricuspid annulus systolic plane excursion (TAPSE) from M-mode, among others. We adhered to imaging standards as recommended by the American Society of Echocardiography.18,24 We used Image Arena (TomTec) to perform speckle tracking for ventricular strain. We selected standard apical four-chamber views for strain analysis.24 We selected the best available single cardiac cycle regarding image quality and measured longitudinal strain of the endocardium. We rejected images because of poor image quality if we could not track two or more adjacent segments in the apical four-chamber view. For RV strain, we measured the free wall, excluding the septum.25 All echocardiographic interpretations were blinded to all clinical data at time of image analysis. Clinicians did not follow any specific hemodynamic management protocol based on echocardiographic findings.
Before analyzing any data, we defined RV dysfunction as having either an RV FAC of < 35% or a TAPSE of < 1.6 cm. We selected these parameters based on their ease of measurement and their ubiquity in clinical practice, and we chose thresholds based on published guidelines.18,24 We defined LV systolic dysfunction as an EF < 45% or global longitudinal strain of -19% or higher (strain is measured with negative percentages, where a lower value indicates better systolic function). Our threshold for strain was based on prior published upper limits of normal.26 We defined LV diastolic dysfunction as patients with E/e′ ≥ 13.27 We defined LV dysfunction as the presence of either LV systolic or diastolic dysfunction.
Clinical Data
We collected demographic information, vital signs, mechanical ventilation parameters, and vasopressor infusion rates at the time of the TTE. We converted vasopressor infusion rates of vasopressors into norepinephrine-equivalent dosing, per previously described methods.28 The cause of sepsis was determined by chart review. We calculated total volume of IV fluid administered in the 6 hours before and 6 hours after the TTE. We calculated Acute Physiology and Chronic Health Evaluation II (APACHE II)29 score at the time of ICU admission. We also measured ICU length-of-stay, Sequential Organ Failure Assessment30 scores at the time of ICU admission and 72 hours after ICU admission. Our primary clinical outcome was 28-day all-cause mortality.
Statistical Analysis
We compared clinical outcomes of patients with and without RV dysfunction using the χ2 test for proportions and Wilcoxon rank-sum tests as appropriate. The primary outcome was 28-day mortality, with ventilator-free days, change in Sequential Organ Failure Assessment from day 3 to day 1, and several organ-specific organ failure-free days as secondary outcomes, which were controlled for multiple comparisons using a false discovery rate of 5%. We used multivariable logistic regression to assess the relationship between 28-day mortality, RV dysfunction, and LV systolic dysfunction, controlling for APACHE II, receipt of mechanical ventilation, amount of IV fluid administered in the 6 hours antecedent to the echocardiogram, and norepinephrine-equivalent dose of vasopressors. We performed secondary regression analyses using RV strain as a covariate, adjusted for the same clinical covariates mentioned previously. Finally, we performed sensitivity analyses to determine a threshold for normal vs abnormal RV strain with respect to the presence of RV dysfunction. Analyses were performed using the R Statistical Package, version 3.5.1.31
Results
We screened 1,053 patients with sepsis or septic shock admitted to one of the study ICUs during the study period. TTE was performed within the first 24 hours in 393 patients who presented to these ICUs with sepsis or septic shock, 38% of whom received vasopressors and 26% of whom received mechanical ventilation (Table 1). The median time from ICU admission to TTE was 2.3 hours. Overall 28-day mortality in the cohort was 24%. Median ICU length of stay was 2.8 days (interquartile range, 1.8-5.1).
Table 1.
Summary Statistics of Relevant Echocardiographic and Clinical Parameters
| Variable | Mean (SD) | Dead (n = 93) | Alive (n = 300) | P |
|---|---|---|---|---|
| RV end-diastolic area, cm2 | 22.39 (7.03) | 22.31(6.88) | 22.41 (7.09) | .91 |
| RV end-systolic area, cm2 | 14.19 (5.97) | 14.88 (6.5) | 14 (5.81) | .32 |
| RV fractional area change, % | 37.51 (11.07) | 34.63 (12.96) | 38.3 (10.38) | .03 |
| RV basal diameter (4ch), cm | 4.14 (0.83) | 4.19 (0.92) | 4.13 (0.79) | .56 |
| TAPSE, cm | 1.81 (0.55) | 1.59 (0.48) | 1.88 (0.55) | <.001 |
| IVC maximum dimension, cm | 2.19 (0.53) | 2.08 (0.41) | 2.22 (0.56) | .99 |
| IVC minimum dimension, cm | 1.72 (0.57) | 1.65 (0.56) | 1.74 (0.58) | .29 |
| IVC collapsibility, % | 28.27 (23.14) | 27 (22.12) | 28.62 (23.46) | .64 |
| Right atrial area (4ch), cm2 | 18.01 (6.95) | 17.28 (5.55) | 18.23 (7.32) | .21 |
| RV global longitudinal strain, % | −15.32 (6.5) | −14.99 (6.76) | −15.44 (6.43) | .67 |
| RV free-wall strain, % | −17.87 (7.34) | −16.85 (7.23) | −18.18 (7.33) | .25 |
| aRV myocardial performance index | 0.62 (0.29) | 0.66 (0.35) | 0.6 (0.27) | .47 |
| aRV tissue Dopplers | 12.6 (4.24) | 12.96 (4.39) | 12.48 (4.21) | .59 |
| bPA systolic pressure, mm Hg | 42.0 (10.0) | 42.2 (9.7) | 41.4 (11.1) | .60 |
| LV ejection fraction, % | 60.26 (12.98) | 60.63 (14.77) | 59.97 (13.31) | .64 |
| Left atrial volume index, mL/m2 | 30.11 (14.95) | 30.49 (18.32) | 29.95 (13.79) | .73 |
| LV E/e’ | 12.60 (5.88) | 13.20 (7.96) | 12.44 (5.16) | .80 |
| LV global longitudinal strain, % | −16.62 (5.75) | −15.85 (5.99) | −16.71 (6.04) | .31 |
| LV myocardial performance index | 0.64 (0.22) | 0.64 (0.22) | 0.64 (0.21) | .88 |
| Clinical parameters | ||||
| Age, y | 62.65 (16.39) | 65.1 (15.41) | 62.0 (16.51) | .12 |
| APACHE II | 25.83 (10.31) | 32.97 (10.22) | 23.68 (9.45) | <.0001 |
| Elixhauser Comorbidity Index | 9.49 (3.86) | 10.53 (3.98) | 9.17 (3.77) | .005 |
| Fluid receipt 6 hours before echo, mL | 2,153 (1551) | 2,286 (1737) | 2,102 (1482) | .63 |
| Receipt of vasopressors during echo; No. (%) | 151 (38%) | 39 (42%) | 112 (37%) | .46 |
| Norepinephrine equivalent dose at echo (among those receiving pressors), μg/kg/min | 0.22 (0.25) | 0.41 (0.64) | 0.13 (0.15) | .007 |
| Ventilated during echo; No. (%) | 104 (26%) | 32 (34%) | 72 (24%) | .06 |
| Pao2/Fio2 Ratio | 238 (133) | 240 (155) | 271 (136) | .11 |
Characteristics and comparisons between patients alive and dead at 28 days are displayed as well. Tests of significance are not adjusted for multiple comparisons. IVC = inferior vena cava; LV = left ventricle; PA = pulmonary artery; RAA = right atrial area; RV = right ventricle; TAPSE = tricuspid annular systolic plane excursion.
Right ventricular tissue Doppler was not systematically recorded in all patients, so the myocardial performance index and s’ were assessed only in 123 patients.
Pulmonary artery systolic pressure was measurable in only 122 patients.
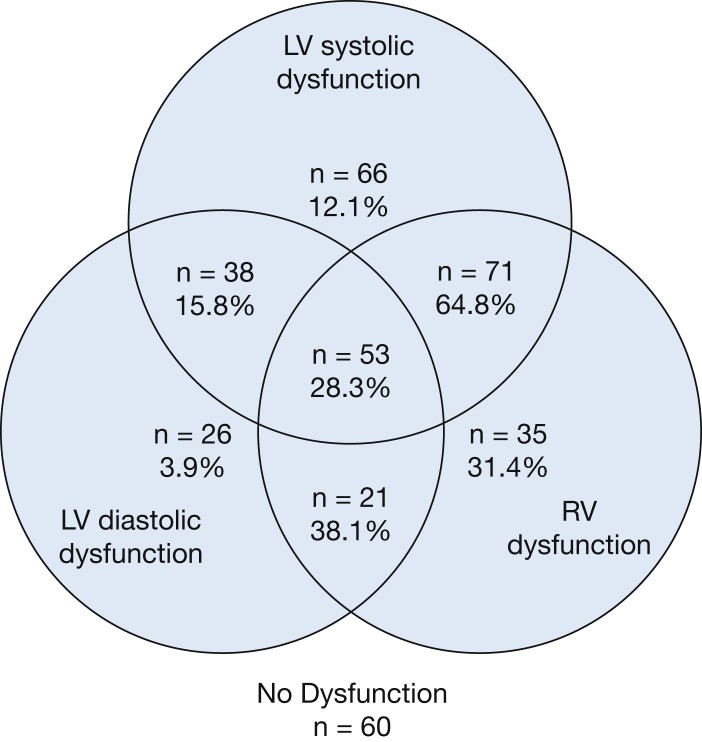
Echocardiographic findings are presented in Table 1. We observed a mean (±SD) RV fractional area change of 38 (±11)% and a TAPSE of 1.8 (±0.6) cm. Despite an average EF of 60%, LV strain was an average of -17%, and myocardial performance index also was frequently abnormal, with an average of 0.64 (cited normal values are < 0.5).32 We were able to categorize RV status in 375 patients, 181 (48%) of whom had RV dysfunction. Similarly, LV systolic dysfunction was present in 238 (63%) of 380 categorizable patients, and LV diastolic dysfunction was present in 141 (47%) of 301 categorizable patients. Combining LV diastolic and systolic dysfunction resulted in 286 (74%) of 385 patients who had some form of LV dysfunction. We observed considerable overlap with RV and LV dysfunction (Fig 1), with only 35 (9%) having isolated RV dysfunction.
Figure 1.
Distribution of RV and LV systolic and diastolic function among the 370 patients who could be categorized for both RV and LV dysfunction, with 28-day mortality expressed in percentages. LV = left ventricular; RV = right ventricular.
We observed that RV dysfunction was associated with higher mortality compared with patients without RV dysfunction (31 % vs 16%; P = .001), fewer ventilator-free days (26 vs 28; P = .001), and fewer organ-failure free days (Table 2). In contrast, mortality was lower in isolated LV dysfunction (12%; e-Tables 1, 2). Univariate regression demonstrated an association of RV dysfunction with mortality (OR, 2.42; CI, 1.48-4.00; P < .001), although we observed no such association with LV systolic dysfunction (OR, 0.92; CI, 0.57-1.50; P = .73) or LV diastolic dysfunction (OR, 0.94; CI, 0.54-1.61; P = .81). Our multivariable regression model demonstrated a strong association between RV dysfunction and mortality that persisted after adjustment for disease severity and the presence of LV systolic and diastolic dysfunction (Table 3; area under the receiver operating characteristic curve = 0.77). As a post hoc analysis, we observed that lower Pao2/Fio2 ratio was associated with increased odds of RV dysfunction (OR, 0.998; CI, 0.996-1.000; P = .01).
Table 2.
Clinical Parameters and Outcomes According to Presence or Absence of Right Ventricular Dysfunction
| Variable | RV Dysfunction (n = 181) | No RV Dysfunction (n = 194) | P |
|---|---|---|---|
| Clinical parameters | |||
| Age, y | 64.7 (16.0) | 60.9 (16.5) | .03 |
| APACHE II | 27.12 (10.13) | 24.43 (10.19) | .02 |
| Elixhauser Comorbidity Index | 10.0 (3.7) | 8.9 (3.9) | .004 |
| Fluid receipt 6 hours before echo, mL | 1,962 (2,028) | 2191 (2,184) | .24 |
| Receipt of vasopressors during echo; No. (%) | 63 (34.8) | 82 (42.3) | .17 |
| Norepinephrine equivalent dose at echo (among those receiving pressors), μg/kg/min | 0.21 (0.25) | 0.22 (0.26) | .12 |
| Ventilated during echo; No. (%) | 52 (28.7) | 47 (24.2%) | .35 |
| Pao2/Fio2 Ratio | 217 (125) | 255 (147) | .02 |
| Clinical outcomes | |||
| 28-Day mortality; No. (%) | 57 (31%) | 31 (16%) | .001 |
| Ventilator-free days | 26 (−1 to 28) | 28 (21-28) | .001 |
| 3-day Δ SOFA | −3 (−7 to −1) | −5 (−7 to −2) | .05 |
| Cardiac OFFD to day 14 | 11 (6-13) | 12 (9-13) | .008 |
| Coagulation OFFD to day 14 | 14 (10-14) | 14 (13-14) | .05 |
| Hepatic OFFD to day 14 | 14 (11-14) | 14 (13-14) | .13 |
| Renal OFFD to day 14 | 13 (6-14) | 14 (12-14) | .001 |
The test for significance is unadjusted for 28-day mortality (primary outcome), and all other P values of secondary outcomes are adjusted using the false discover rate method. Tests for significance for clinical parameters are unadjusted for multiple comparison. OFFD = organ failure-free days; RV = right ventricle; SOFA = sequential organ failure assessment.
Table 3.
Multivariable Logistic Regression for 28-Day Mortality (Adjusted for Admission APACHE II Score, Mechanical Ventilation at Time of Echocardiogram, Receipt of Fluid and Vasopressor Dose at Time of Echocardiogram)
| Variable | OR | 95% CI | P |
|---|---|---|---|
| RV dysfunction | 3.37 | (1.67-6.78) | .001 |
| LV systolic dysfunction | 0.63 | (0.32-1.24) | .32 |
| LV diastolic dysfunction | 1.26 | (0.64-2.50) | .51 |
| APACHE II | 1.09 | (1.04-1.13) | <.001 |
| Ventilated during echo | 0.48 | (0.19-1.17) | .11 |
| Pao2/Fio2 ratio | 1.00 | (1.00-1.00) | .29 |
| NEE dose (per 0.01 μg/kg/min increase) | 1.02 | (1.01-1.04) | .01 |
| Fluid 6 hours before echo, mL | 1.00 | (1.00-1.00) | .41 |
Area under receiver operating characteristic curve = 0.77. For patients on multiple vasopressors, all doses were converted to norepinephrine-equivalent doses, per previously described methods.28 APACHE = Acute Physiology and Chronic Health Evaluation; LV = left ventricle; NEE = norepinephrine equivalent.
RV Strain
We examined the correlation between RV strain, TAPSE, and FAC. We found modest associations between RV strain and TAPSE and RV strain and FAC (e-Appendix 1, e-Table 3, e-Fig 1). We additionally performed multivariable regression analysis of RV strain and clinically relevant covariates and found no association between RV strain and mortality after adjustment (e-Tables 4, 5). We found RV free wall strain was worse in patients with RV dysfunction (-15.9% vs -19.7%; P < .001; Table 4).
Table 4.
Quartiles of RV Free-Wall Strain for Patients With and Without RV Dysfunction
| RV Strain by RV Dysfunction | RV Dysfunction (n = 181) |
No RV Dysfunction (n = 194) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Q1 | Mean | Med | Q3 | Max | Min | Q1 | Mean | Med | Q3 | Max | |
| RV Free-wall strain | −37.7 | −21.3 | −15.9 | −15.1 | −11.0 | −3.6 | −36.3 | −25.3 | −19.7 | −20.6 | −13.8 | −3.2 |
Discussion
In a large, prospective obtained cohort of patients with sepsis and septic shock, we found that RV dysfunction (both in isolation or combined with LV systolic and diastolic dysfunction) is common and associated with greater than double the odds of short-term mortality. Neither LV systolic nor diastolic dysfunction alone were associated with mortality. RV strain, a relatively novel measurement, may offer added value in identifying RV dysfunction. Our findings support prior work demonstrating a moderate association of RV strain with TAPSE and FAC.33 Despite this association, we did not observe an association with RV strain and mortality in multivariate regression analysis.
Our findings support and expand on other findings regarding the RV in sepsis.16,17 Similar to the work by Vallabhajosyula et al,17 we observe that RV dysfunction is common in sepsis, and is associated with high mortality, independent of LV dysfunction. In contrast, however, our study identified increased short-term mortality (28 days), whereas their study showed no difference in in-hospital mortality but a 1-year survival disadvantage. This difference may be in part due to the study of very early sepsis and septic shock in our cohort (<24 hours) vs the more standard 72-hour inclusion criteria, which may be more prone to survivorship bias. Additionally, although our study observed an association between RV dysfunction and mortality, the work of Vallabhajosyula et al17 observed an association between long-term mortality and isolated RV dysfunction, but not RV dysfunction in general. Additionally, the Mayo Clinic cohort identified a larger proportion of patients with isolated RV dysfunction, compared with biventricular dysfunction. Part of the disparity in association may be attributed to differences in prevalence of isolated RV dysfunction between the two cohorts, because isolated RV dysfunction was less common in our cohort. Compared with the Mayo Clinic cohort, our study cohort had fewer patients receiving mechanical ventilation (26% vs 55%) and higher pao2/Fio2 ratio (238 vs 190), despite being at higher altitude. Because RV dysfunction may be aggravated by respiratory failure, possibly differences in the prevalence of respiratory failure may at least partially account for some of the differences noted between these two study cohorts. RV strain, which may be less susceptible to loading conditions than fractional area change or TAPSE, might be better parameters for future study of intrinsic RV dysfunction in these patients. Although RV strain appears to be reproducible and feasible, the need for additional normative data from large studies prevents recommending a definite reference range for RV strain at this time.24 A scheduled follow-up echocardiograph in survivors might have offered some insights into these patients’ RV function in the absence of respiratory failure or fluid receipt, but these data were not part of this observational study performed under waiver of written informed consent.
We suspect that cardiomyocyte dysfunction as a result of the dysregulated immune response in sepsis plays a substantial role in the observed RV dysfunction, but several alternate possibilities warrant discussion. First, because RV function is directly affected by afterload conditions in the pulmonary circulation,34 possibly RV dysfunction might be a surrogate for severity of lung disease, which is actually the cause of increased mortality. We do not have data on clinical determination of ARDS or cause of respiratory failure in our cohort, either of which might have better association with RV dysfunction than mechanical intubation or Pao2/Fio2 ratio. Interestingly, the association persists after adjustment for mechanical ventilation and Pao2/Fio2 ratio. Post hoc analyses revealed an association with RV dysfunction and Pao2/Fio2 ratio, making this seem plausible, although no association was detected between mechanical ventilation and RV dysfunctions (Table 2). Second, it is also possible that RV dysfunction is indicative of overzealous fluid or catecholamine administration, again leading to increased mortality, but in our study, no association was seen between fluid administration and mortality, and both fluid and vasopressor doses were adjusted for as covariates in the regression model. The absence of association with RV strain and mortality supports these aforementioned possibilities, and further study is needed to determine whether RV dysfunction is an epiphenomenon of abnormal loading conditions in the septic patient or whether RV dysfunction represents intrinsic cardiac dysfunction. Additionally, some clinicians may opt to make treatment decisions based on RV echocardiographic parameters, such as adjusting fluid administration or titrating ventilator settings. Although there is physiologic rationale for these interventions, our data cannot inform on the appropriateness of such interventions.
The disparate association between RV and LV dysfunction (either systolic or diastolic) and mortality in this patient population also raises questions, because prior studies have shown increased mortality with either LV systolic or diastolic dysfunction and sepsis.2, 3, 4, 5, 6 Although we used published norms, possibly the thresholds used to define LV dysfunction (EF <45% and strain > -19%) are too sensitive and identify very mild LV dysfunction, and perhaps we would observe different associations with a more specific threshold of strain, such as -16%. In contrast, the thresholds used for RV dysfunction (FAC < 35% and TAPSE < 1.6 cm) may detect severe disease only, and possibly a sicker patient subgroup. Other potential measures of RV dysfunction include s’ and myocardial performance index. These are promising parameters, but our study is limited in that RV tissue Doppler was not routinely assessed during the study period. Of the 123 patients who had tissue Doppler, we noted a correlation with s’ and TAPSE (r = 0.56, P < .0001).Although we report pulmonary artery pressure estimation from tricuspid regurgitant jet, this number is likely biased toward increased pressures, because its ascertainment requires tricuspid regurgitation, which was not present in most study patients. Although our definition for RV dysfunction does not discriminate between intrinsic RV dysfunction and echocardiographic abnormalities arising from increased RV preload and afterload, we adjusted for fluid receipt and presence of mechanical ventilation. Finally, possibly our definition of LV dysfunction, which includes either systolic or diastolic dysfunction, may misclassify some borderline patients. We limited our assessment of strain to the six segments of apical four-chamber, rather than 16 myocardial segments, and our determination of diastolic dysfunction. Our determination of diastolic dysfunction used a surrogate for filling pressure (E/e′) rather than the standard definition, which is challenging to apply in critically ill patients.27 Additionally, although LV diastolic dysfunction has been associated with mortality in septic patients,35 we did not observe a relationship in this cohort, perhaps because of the strong signal observed with RV dysfunction in the model and a low prevalence of isolated LV diastolic dysfunction, or the association between diastolic parameters and fluid receipt. Additionally, our cohort may differ from other studies regarding timing of the echocardiography study or fluid receipt. When compared with patients who were enrolled in the ProCESS trial, a contemporaneous study of septic patients, patients in our cohort had higher APACHE II scores and greater vasopressor use and receipt of mechanical ventilation.36
Our study is limited by its retrospective nature. The chief limitation of this study is that we do not have adequate pre-sepsis information on these patients to determine whether the RV dysfunction is a manifestation of the changes of sepsis, or whether these patients had preexisting RV dysfunction. As demonstrated in Table 2, patients with RV dysfunction were older, with greater disease severity and comorbidities. Possibly these relationships may partially confound the association between RV dysfunction and mortality. Additionally, premorbid LV systolic and diastolic dysfunction, lung disease, or sleep- disordered breathing, which could also affect RV function, were not collected. Our definitions for severe sepsis and septic shock,23 although appropriate at the time of study, were subsequently replaced by the Sepsis-3 definitions,37 meaning this cohort may not precisely align with contemporary patient cohorts defined to have sepsis or septic shock. Our definition for systolic and diastolic dysfunction might have results in misclassification of some patients with borderline values. Most of the patients were medical ICU patients; therefore, our findings may not be generalizable to ICUs with different patient populations. Because the study hospital underwent changes in its electronic medical record in 2016, the study cohort was limited to data collected earlier and therefore may fail to account for recent secular changes in sepsis treatment. Our assessment of mortality did not include cause of death. Finally, although RV dysfunction appears to be common in sepsis and septic shock, possibly our reported incidence may be overestimated because of selection bias. Although the study hospital commonly performs echocardiography in patients with septic shock, it is not routine and is less commonly performed in septic patients who are hemodynamically stable. Likely many of the septic patients who were not studied were healthier and less likely to have ventricular dysfunction.
Interpretation
RV dysfunction is common in patients with sepsis and septic shock and is associated with increased 28-day mortality, even after adjusting for disease severity. This finding is independent of the presence of LV systolic and diastolic dysfunction. How clinicians should respond to this finding is less clear, and this study is unable to provide any useful inferences on whether improving RV function will improve clinical outcomes. We need additional prospective translational and clinical studies to understand the impact of RV dysfunction and targeted interventions on survival in patients with sepsis and septic shock.
Acknowledgments
Author contributions: M. J. L., T. D. O., E. L. W., E. L. H., S. M. B., and C. K. conceived of and designed the study. M. L., T. O., and C. K. performed image analysis. M. L. and E. W. performed data abstraction. M. L. and E. W. performed statistical analyses. M. L. and M. C. drafted the manuscript. All authors revised the manuscript for important intellectual contributions. All authors have reviewed and approve the final manuscript. M. L. takes responsibility for the integrity of the work as a whole, from inception to published article.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: This study was approved by the Intermountain Institutional Review Board (#1009957) with a waiver of informed consent.
Additional information: The e-Figure and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was supported by grants from the Easton Family Fund and the Intermountain Research and Medical Foundation. Drs. Brown and Lanspa are supported by NHLBI [grant R01HL144624]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Easton Family, or the Intermountain Research and Medical Foundation.
Supplementary Data
References
- 1.Hotchkiss R.S., Moldawer L.L., Opal S.M., Reinhart K., Turnbull I.R., Vincent J.L. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vieillard-Baron A. Septic cardiomyopathy. Ann Intensive Care. 2011;1(1):6. doi: 10.1186/2110-5820-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landesberg G., Jaffe A.S., Gilon D. Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular dilatation. Crit Care Med. 2014;42(4):790–800. doi: 10.1097/CCM.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 4.Lanspa M.J., Gutsche A.R., Wilson E.L. Application of a simplified definition of diastolic function in severe sepsis and septic shock. Crit Care. 2016;20(1):243. doi: 10.1186/s13054-016-1421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orde S.R., Pulido J.N., Masaki M. Outcome prediction in sepsis: speckle tracking echocardiography based assessment of myocardial function. Crit Care. 2014;18(4):R149. doi: 10.1186/cc13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanspa M.J., Pittman J.E., Hirshberg E.L. Association of left ventricular longitudinal strain with central venous oxygen saturation and serum lactate in patients with early severe sepsis and septic shock. Crit Care. 2015;19:304. doi: 10.1186/s13054-015-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beesley S.J., Weber G., Sarge T. Septic cardiomyopathy. Crit Care Med. 2018;46(4):625–634. doi: 10.1097/CCM.0000000000002851. [DOI] [PubMed] [Google Scholar]
- 8.Chan C.M., Klinger J.R. The right ventricle in sepsis. Clin Chest Med. 2008;29(4):661–676. doi: 10.1016/j.ccm.2008.07.002. ix. [DOI] [PubMed] [Google Scholar]
- 9.Naeije R., Badagliacca R. The overloaded right heart and ventricular interdependence. Cardiovasc Res. 2017;113(12):1474–1485. doi: 10.1093/cvr/cvx160. [DOI] [PubMed] [Google Scholar]
- 10.Mitsuo T., Shimazaki S., Matsuda H. Right ventricular dysfunction in septic patients. Crit Care Med. 1992;20(5):630–634. doi: 10.1097/00003246-199205000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Dhainaut J.F., Lanore J.J., de Gournay J.M. Right ventricular dysfunction in patients with septic shock. Intensive Care Med. 1988;14(Suppl 2):488–491. doi: 10.1007/BF00256967. [DOI] [PubMed] [Google Scholar]
- 12.Vincent J.L., Reuse C., Frank N., Contempre B., Kahn R.J. Right ventricular dysfunction in septic shock: assessment by measurements of right ventricular ejection fraction using the thermodilution technique. Acta Anaesthesiol Scand. 1989;33(1):34–38. doi: 10.1111/j.1399-6576.1989.tb02856.x. [DOI] [PubMed] [Google Scholar]
- 13.Redl G., Germann P., Plattner H., Hammerle A. Right ventricular function in early septic shock states. Intensive Care Med. 1993;19(1):3–7. doi: 10.1007/BF01709270. [DOI] [PubMed] [Google Scholar]
- 14.Haddad F., Hunt S.A., Rosenthal D.N., Murphy D.J. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117(11):1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 15.Singh R.K., Kumar S., Nadig S. Right heart in septic shock: prospective observational study. J Intensive Care. 2016;4:38. doi: 10.1186/s40560-016-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harmankaya A., Akilli H., Gul M. Assessment of right ventricular functions in patients with sepsis, severe sepsis and septic shock and its prognostic importance: a tissue Doppler study. J Crit Care. 2013;28(6) doi: 10.1016/j.jcrc.2013.07.059. 1111.e7-1111.e11. [DOI] [PubMed] [Google Scholar]
- 17.Vallabhajosyula S., Kumar M., Pandompatam G. Prognostic impact of isolated right ventricular dysfunction in sepsis and septic shock: an 8-year historical cohort study. Ann Intensive Care. 2017;7(1):94. doi: 10.1186/s13613-017-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudski L.G., Lai W.W., Afilalo J. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Voigt J.U., Exner B., Schmiedehausen K. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation. 2003;107(16):2120–2126. doi: 10.1161/01.CIR.0000065249.69988.AA. [DOI] [PubMed] [Google Scholar]
- 20.Longobardo L., Suma V., Jain R. Role of two-dimensional speckle-tracking echocardiography strain in the assessment of right ventricular systolic function and comparison with conventional parameters. J Am Soc Echocardiogr. 2017;30(10):937–946. doi: 10.1016/j.echo.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Mouton S., Ridon H., Fertin M. 2D-speckle tracking right ventricular strain to assess right ventricular systolic function in systolic heart failure: analysis of the right ventricular free and posterolateral walls. Int J Cardiol. 2017;245:190–195. doi: 10.1016/j.ijcard.2017.07.077. [DOI] [PubMed] [Google Scholar]
- 22.Shukla M., Park J.H., Thomas J.D. Prognostic value of right ventricular strain using speckle-tracking echocardiography in pulmonary hypertension: a systematic review and meta-analysis. Can J Cardiol. 2018;34(8):1069–1078. doi: 10.1016/j.cjca.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Bone R.C., Balk R.A., Cerra F.B. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 24.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.H., Park J.H. Strain analysis of the right ventricle using two-dimensional echocardiography. J Cardiovasc Imaging. 2018;26(3):111–124. doi: 10.4250/jcvi.2018.26.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yingchoncharoen T., Agarwal S., Popovic Z.B., Marwick T.H. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26(2):185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Lanspa M.J., Olsen T.D., Wilson E.L. A simplified definition of diastolic function in sepsis, compared against standard definitions. J Intensive Care. 2019;7:14. doi: 10.1186/s40560-019-0367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown S.M., Lanspa M.J., Jones J.P. Survival after shock requiring high-dose vasopressor therapy. Chest. 2013;143(3):664–671. doi: 10.1378/chest.12-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 30.Vincent J.L., Moreno R., Takala J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 31.R: A Language and Environment for Statistical Computing [computer program] R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- 32.Spencer K.T., Kirkpatrick J.N., Mor-Avi V., Decara J.M., Lang R.M. Age dependency of the Tei index of myocardial performance. J Am Soc Echocardiogr. 2004;17(4):350–352. doi: 10.1016/j.echo.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Hamada-Harimura Y., Seo Y., Ishizu T. Incremental prognostic value of right ventricular strain in patients with acute decompensated heart failure. Circ Cardiovasc Imaging. 2018;11(10) doi: 10.1161/CIRCIMAGING.117.007249. [DOI] [PubMed] [Google Scholar]
- 34.Pinsky M.R. The right ventricle: interaction with the pulmonary circulation. Crit Care. 2016;20:266. doi: 10.1186/s13054-016-1440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landesberg G., Gilon D., Meroz Y. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012;33(7):895–903. doi: 10.1093/eurheartj/ehr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pro C.I., Yealy D.M., Kellum J.A. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer M., Deutschman C.S., Seymour C.W. The Third International Consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.