Abstract
Background
Little is known about adherence to asthma biologics.
Research Question
Is adherence to inhaled corticosteroid (ICS) associated with subsequent asthma biologic adherence?
Study Design and Methods
We analyzed individuals with asthma who started asthma biologics in the OptumLab Data Warehouse and used that data until October 2019. We calculated proportion days covered (PDC) for ICS ± long-acting β-agonists in the 6 months before and after asthma biologics were started and asthma biologic PDC for the first 6 months of use. We performed a multivariable analysis to identify factors associated with asthma biologic PDC ≥0.75, ICS PDC ≥0.75 during the 6-month period after asthma biologic were started, and achievement of a ≥50% reduction in asthma exacerbations during the first 6 months of asthma biologic use.
Results
We identified 5,319 people who started asthma biologics. The mean PDC for asthma biologics was 0.76 (95% CI, 0.75-0.77) in the first 6 months after starting, higher than the mean PDCs for ICS in the 6 months before (0.44 [95% CI, 0.43-0.45]) and after (0.40 [95% CI, 0.39-0.40]) starting the asthma biologic. PDC ≥0.75 for ICS 6 months before index biologic use is associated with PDC for asthma biologics ≥0.75 (OR, 1.25; 95% CI, 1.10-1.43) and for ICS during the first 6 months of biologic use (OR, 9.93; 95% CI, 8.55-11.53). Neither ICS PDC ≥0.75 (OR, 0.92; 95% CI, 0.74-1.14) nor asthma biologic PDC ≥0.75 (OR, 1.15; 95% CI, 0.97-1.36) is associated with a statistically significant reduction in asthma exacerbations during the first 6 months of asthma biologic use among people with any exacerbation in the 6 months before first use.
Interpretation
Adherence to asthma biologic is higher than to ICS and is associated with different factors.
Key Words: adherence, asthma, biologic, inhaled corticosteroid
Abbreviations: GERD, gastroesophageal reflux disease; ICS, inhaled corticosteroid; PDC, proportion days covered
FOR EDITORIAL COMMENT, SEE PAGE 891
Many people with asthma can achieve control of the disease by using inhaled corticosteroid (ICS).1 However, adherence to ICS and other asthma controller therapies is variable, with most studies reporting adherence below levels recommended by providers.2 Low adherence to ICS is associated with poorer outcomes.3 Thus, assessing adherence to ICS is a cornerstone of asthma management.
When asthma is not controlled with ICS, current guidelines suggest an option to add therapy with asthma biologics.1 Asthma biologics include monoclonal antibodies directed at Ig E, IL-5, and IL 4/13; each of these pathways is involved in the airway inflammation seen in asthma. The five currently US Food and Drug Administration-approved asthma biologics (omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab) reduce asthma exacerbation rates by roughly 50% compared with placebo as reported in multiple randomized trials.4 Very little is known about adherence to asthma biologics and how adherence may influence asthma outcomes. There is reason to suspect that adherence to asthma biologics may be different than for ICS, because biologics are administered with injections given every 2 to 8 weeks and are often administered under direct supervision in a health-care setting as compared with ICS, which are daily self-administered medications that require attention to inhaler technique.
Our hypothesis was that adherence to ICS prior to starting asthma biologics is not associated with adherence to asthma biologics.
Methods
We conducted an analysis using data from OptumLabs Data Warehouse, a database of >200 million privately insured and Medicare Advantage enrollees.5 This study was exempt from review by the Mayo Clinic Institutional Review Board because all of the data were preexisting and deidentified.
We built our cohort by starting with all beneficiaries who met a modified version of the Healthcare Effectiveness Dataset Information Set criteria for persistent asthma who had at least 6 months of continuous enrollment in both medical and pharmacy coverage between 2003 and 2019 prior to the index biologic use. We modified OptumLabs Data Warehouse criteria by using a rolling year rather than calendar year, and we did not exclude people with other lung comorbidities, such as COPD, in an effort to reflect real-world clinical practice.5 Next, we selected people who had an associated claim for asthma biologic and who did not have a diagnosis of chronic idiopathic urticaria (an indication for omalizumab) or atopic dermatitis (an indication for dupilumab). Finally, to identify incident use of asthma biologic more confidently, we required continuous medical and pharmacy coverage for at least 6 months before the first claim for asthma biologic. To ensure an appropriate evaluation period after asthma biologic incident use, we selected people with at least 6 months of continuous coverage after first biologic use and people who were still reporting any use of asthma biologic 6 months after index use. If a biologic dose was missed for ≥120 days, it was considered stopped.
We defined adherence by calculating a proportion days covered (PDC) by dividing the days covered by the number of days in the period. PDC for ICS included ICS ± long-acting β-agonists, allowing for ICS monotherapy or ICS/long-acting β-agonists combination therapy to count towards ICS PDC. PDC for asthma biologic was calculated for each claim dose and the associated dosing interval, recognizing that omalizumab can be dosed every 2 or 4 weeks and that benralizumab is dosed every 4 weeks when first started then every 8 weeks after the first three doses (e-Table 1). PDC was considered as a medication class, rather than for individual medications. We used a PDC cut off of ≥0.75 to indicate the likelihood of a high level of adherence.
The primary asthma outcome used for this study is asthma exacerbation, which is consistent with outcomes selected in most clinical trials of asthma biologics.4 Asthma exacerbations were defined as any of the following occurrences: hospitalization with a primary diagnosis of asthma or in a secondary position with a respiratory diagnosis in the first position, ED visit with a primary diagnosis of asthma, or a pharmacy fill for a systemic corticosteroid within 1 month of an outpatient visit. We allowed each patient to be credited with no more than one exacerbation in a 30-day rolling period to avoid double-counting of a single episode that may span multiple ED visits, hospitalizations, or outpatient visits and corticosteroid fills. We performed a sensitivity analysis using asthma exacerbation defined by only ED or hospitalization (ie, excluding systemic corticosteroid as a criterion). In this study, we defined a positive outcome as a ≥50% reduction in asthma exacerbations in the first 6 months of biologic use, which was the average reduction for patients participating in clinical trials. For analyses that involved asthma exacerbation outcomes, people with no asthma exacerbations in the 6 months prior to starting asthma biologics were excluded, because they had no opportunity to achieve this outcome.
Independent variables include age, sex, race/ethnicity, region, annual household income, insurance type (divided into commercial and Medicare Advantage), Charlson condition group (divided into 0-1, 2-3, and 4+), specialist access as a visit with pulmonology or allergy-immunology during the study period, gastroesophageal reflux disease (GERD), rhinitis, sinusitis, COPD, and depression. The presence of these diseases was defined by having one or more diagnosis codes for these diseases prior to their index biologic claim.
Statistical Analysis
ICS adherence was analyzed by treating PDC not only as a continuous but also a binary variable. ICS PDC was described separately for the 6 months before and after the asthma biologic started. Next, we conducted a multivariable analysis using age, sex, race/ethnicity, insurance, region, income, specialist access, Charlson condition group, GERD, rhinitis, sinusitis, COPD, depression, and pre-asthma biologic ICS PDC ≥0.75 as independent variables and asthma biologic PDC ≥0.75 as the dependent variable. Each of these variables was selected for their potential clinical relevance to medication adherence, and each variable coefficient was displayed for transparency so that readers can judge the model’s appropriateness. A multivariable analysis that used the same independent variables and included ICS PDC ≥0.75 as the dependent variable during the 6-month period of asthma biologic use was also conducted, and the results were qualitatively compared with the asthma biologic PDC analysis. The purpose of this multivariable analysis was to determine whether similar or different factors were important for biologic and ICS adherence. Finally, a multivariable analysis was performed with the use of the same independent variables plus ICS PDC (both before and after starting asthma biologics) and asthma biologic PDC, with ≥50% reduction of asthma exacerbation as the dependent variable. The purpose of this multivariable analysis was to test whether biologic adherence was associated with an important asthma outcome. Statistical analysis was performed using Statistical Analysis System (version 9.4; SAS Institute, Cary NC) and R (version 3.6.2; The R Foundation for Statistical Computing Platform).
Results
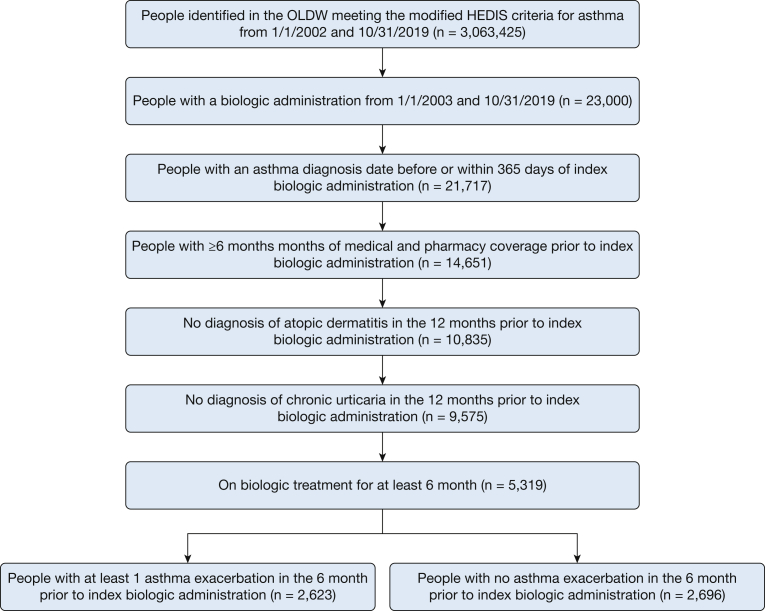
We identified 3,063,425 people with asthma from January 1, 2012, through October 31, 2019, in the OptumLabs Data Warehouse that met our modified definition of persistent asthma; 23,000 of these people had an asthma biologic administration. Additional criteria were applied to increase the likelihood of outcome data during biologic use, to help select for incidence rather than prevalent use, and to decrease the likelihood of prescription for a different disease; this reduced the cohort size to 9,575 people. Next, we selected the 5,319 people who used the biologic for at least 6 months and used them for subsequent analysis. Figure 1 is a flow diagram that summarizes cohort selection.
Figure 1.
Cohort creation flow. HEDIS = Healthcare Effectiveness Dataset Information Set; OLDW = OptumLabs Data Warehouse.
The majority of the included cohort were adults between 18 and 64 years old (77%), female (61%), and white (74%) with commercial insurance (79%). Comorbid conditions were common, including rhinitis (81%), sinusitis (64%), GERD (47%), COPD (38%), and depression (27%). The mean number of asthma exacerbations in the 6 months before asthma biologic was started was 0.8 (SD, 1.0; median, 0). The mean days on biologic treatment were 642 (SD, 580; median, 447); people filled an average of 2.6 rescue inhalers and 44% of their ICS (81 days covered) in the 6 months leading up to the index use of the biologic. Table 1 summarizes the cohort characteristics.
Table 1.
Description of People With Index Asthma Biologic Use
| Characteristic | Total (N = 5,319) |
|---|---|
| Age group, No. (%), y | |
| 0-17 | 301 (5.66) |
| 18-64 | 4,101 (77.10) |
| ≥65 | 917 (17.24) |
| Insurance, No. (%) | |
| Commercial | 4,182 (78.62) |
| Medicare Advantage | 1,137 (21.38) |
| Sex, No. (%) | |
| Female | 3,264 (61.36) |
| Male | 2,055 (38.64) |
| Race, No. (%) | |
| Missing | 271 (5.09) |
| Asian | 137 (2.58) |
| Black | 515 (9.68) |
| Hispanic | 455 (8.55) |
| White | 3,941 (74.09) |
| Region, No. (%) | |
| Midwest | 1,364 (25.64) |
| Northeast | 599 (11.26) |
| South | 2,520 (47.38) |
| Unknown | 12 (0.23) |
| West | 824 (15.49) |
| Income, No. (%), $ | |
| Missing | 843 (15.85) |
| <40,000 | 814 (15.30) |
| 40,000-74,999 | 1,092 (20.53) |
| 75,000-124,999 | 1,264 (23.76) |
| 125,000-199,999 | 726 (13.65) |
| ≥200,000 | 580 (10.90) |
| Specialist access, No. (%) | 5,105 (95.98) |
| Charlson condition group, No. (%) | |
| 0-1 | 3,650 (68.62) |
| 2-3 | 1,159 (21.79) |
| ≥4 | 510 (9.59) |
| Gastroesophageal reflux disease, No. (%) | 2,477 (46.57) |
| Rhinitis, No. (%) | 4,320 (81.22) |
| Sinusitis, No. (%) | 3,418 (64.26) |
| COPD, No. (%) | 2,004 (37.68) |
| Depression, No. (%) | 1,422 (26.73) |
| Treatment with biologic, mean (SD), d | 641.50 (579.69) |
| Pre-index exacerbation count, mean (SD) | 0.81 (1.02) |
| Reliever fills in 6 mo before index, mean (SD), No. | 2.59 (3.07) |
| Inhaled corticosteroid proportion days covered in 6 mo before index, Mean (SD) | 0.44 (0.36) |
The mean PDC for asthma biologics in the first 6 months of use was 0.76 (95% CI, 0.75-0.77); for ICS, it was 0.44 (95% CI, 0.43-0.45) and 0.40 (95% CI, 0.39-0.40) in the 6 months before and after biologic initiation, respectively. Sixty-one percent of people achieved a biologic PDC ≥0.75. The following statistics show the PDC for each biologic: benralizumab: n = 141; mean, 80.79 (SD, 17.9); dupilumab: n = 251; mean, 98.15 (SD, 6.83); mepolizumab: n = 763; mean, 88.49 (SD, 14.19); omalizumab: n = 4,100; mean, 71.89 (SD, 22.73); and reslizumab: n = 64; mean, 92.04 (SD, 9.79).
The following independent variables were associated with biologic PDC ≥0.75: older age, male sex, Medicare Advantage insurance type, no specialist care, no COPD, depression, and pre-biologic ICS PDC ≥0.75 (Table 2). The following independent variables were associated with ICS PDC ≥0.75 during biologic treatment period: older age, commercial insurance type, specialist care, sinusitis, and pre-biologic use ICS (Table 3). Pre-biologic use ICS was associated with a very strong odds of post-biologic ICS use (OR, 9.93; 95% CI, 8.55-11.53). We tested whether exacerbation reduction was associated with reduced ICS PDC after biologic initiation by comparing the mean change in ICS PDC in those patients with and without a reduction in asthma exacerbations (among those who had at least one asthma exacerbation before starting), finding no significant association (mean [SD]: 4.27 [29.7], 6.11 [29.6], respectively; OR, 0.81: 95% CI, 0.62-1.06). Therefore, we think it is less likely that asthma exacerbation reduction is associated with decreased ICS use compared with baseline. The only independent variable associated with a ≥50% reduction in asthma exacerbation was not having COPD (Table 4). Biologic PDC was associated with an increased odds or a ≥50% reduction in asthma exacerbation that was not statistically significant (OR, 1.15; 95% CI, 0.97-1.36). Older age, higher income, having rhinitis, and not having depression were all associated with experiencing a ≥50% reduction in asthma exacerbation when asthma exacerbation was defined only as asthma exacerbations associated with ED or hospitalization (Table 5). We used three or more rescue inhaler fills as an additional outcome variable, finding that post-index ICS PDC, COPD, and high number of comorbidities all had higher odds of three or more rescue inhaler fills and that age, higher income, and rhinitis had lower odds of three or more rescue inhaler fills (Table 6). Finally, to test ICS PDC ≥0.5 as another level of potentially meaningful ICS adherence, we repeated the analyses for the data presented in Table 2, Table 3, Table 4, Table 5, Table 6 using PDC ≥0.5 and found no major differences compared with the analysis presented with PDC ≥0.75 (data not shown).
Table 2.
Multivariable Analysis for Biologic Adherence During First 6 Months of Biologic Use
| Effect | OR | 95% CI |
|---|---|---|
| Age | 1.008 | 1.004-1.013 |
| Sex: Female vs Male | 0.866 | 0.769-0.976 |
| Race | ||
| Asian vs white | 1.209 | 0.838-1.744 |
| Black vs white | 0.988 | 0.803-1.216 |
| Hispanic vs white | 0.953 | 0.773-1.175 |
| Missing vs white | 0.809 | 0.617-1.062 |
| Region | ||
| Midwest vs Northeast | 1.083 | 0.879-1.333 |
| South vs Northeast | 0.887 | 0.732-1.075 |
| West vs Northeast | 0.858 | 0.686-1.073 |
| Income | ||
| $125,000-$199,999 vs <$40,000 | 0.726 | 0.578-0.911 |
| ≥$200,000 vs <$40,000 | 0.807 | 0.633-1.029 |
| $40,000-$74,999 vs <$40,000 | 0.842 | 0.687-1.031 |
| $75,000-124,999 vs <$40,000 | 0.844 | 0.69-1.033 |
| Missing vs <$40,000 | 1.013 | 0.81-1.266 |
| Insurance: Medicare Advantage vs commercial | 2.823 | 2.316-3.442 |
| Specialist: yes vs no | 0.586 | 0.429-0.801 |
| Charlson conditions | ||
| 2-3 vs 0-1 | 0.994 | 0.855-1.156 |
| ≥4 vs 0-1 | 0.761 | 0.605-0.955 |
| Gastroesophageal reflux: yes vs no | 1.066 | 0.943-1.205 |
| Rhinitis: yes vs no | 0.917 | 0.778-1.081 |
| Sinusitis: yes vs no | 0.998 | 0.876-1.138 |
| COPD: yes vs no | 0.842 | 0.739-0.96 |
| Depression: yes vs no | 1.145 | 1-1.31 |
| Before index inhaled corticosteroid proportion days covered in 6 mo before index: ≥75% vs 0-74.9% | 1.253 | 1.096-1.432 |
| C statistic | 0.635 |
Table 3.
Multivariable Analysis for Inhaled Corticosteroid Adherence During First 6 Months of Biologic Use
| Effect | OR | 95% CI |
|---|---|---|
| Age | 1.018 | 1.012-1.024 |
| Sex: female vs male | 1.005 | 0.86-1.174 |
| Race | ||
| Asian vs white | 1.114 | 0.699-1.777 |
| Black vs white | 0.854 | 0.648-1.126 |
| Hispanic vs white | 0.965 | 0.726-1.284 |
| Missing vs white | 1.387 | 0.984-1.956 |
| Region | ||
| Midwest vs Northeast | 0.964 | 0.741-1.253 |
| South vs Northeast | 0.869 | 0.681-1.11 |
| West vs Northeast | 0.86 | 0.646-1.147 |
| Income | ||
| $125,000-$199,999 vs <$40,000 | 1.014 | 0.757-1.358 |
| ≥$200,000 vs <$40,000 | 1.157 | 0.85-1.576 |
| $40,000-$74,999 vs <$40,000 | 0.881 | 0.679-1.144 |
| $75,000-124,999 vs <$40,000 | 1.115 | 0.864-1.439 |
| Missing vs <$40,000 | 0.738 | 0.546-0.999 |
| Insurance: Medicare Advantage vs commercial | 0.753 | 0.597-0.95 |
| Specialist: yes vs no | 1.955 | 1.202-3.178 |
| Charlson conditions | ||
| 2-3 vs 0-1 | 0.948 | 0.784-1.147 |
| 4+ vs 0-1 | 0.917 | 0.695-1.211 |
| Gastroesophageal reflux: yes vs no | 0.908 | 0.774-1.064 |
| Rhinitis: yes vs no | 0.908 | 0.736-1.119 |
| Sinusitis: yes vs no | 1.233 | 1.038-1.466 |
| COPD: yes vs no | 1.058 | 0.896-1.25 |
| Depression: yes vs no | 1.007 | 0.846-1.2 |
| Before index inhaled corticosteroid proportion days covered in 6 mo before index: ≥75% vs 0-74.9% | 9.929 | 8.549-11.532 |
| C Statistic | 0.789 |
Table 4.
Multivariable Analysis for ≥50% Reduction in Asthma Exacerbations During First 6 Months of Asthma Biologic Use
| Effect | OR | 95% CI |
|---|---|---|
| Age | 0.999 | 0.993-1.005 |
| Sex: female vs male | 1.103 | 0.929-1.309 |
| Race | ||
| Asian vs white | 0.842 | 0.496-1.43 |
| Black vs white | 0.978 | 0.751-1.274 |
| Hispanic vs white | 1.214 | 0.905-1.629 |
| Missing vs white | 1.377 | 0.92-2.059 |
| Region | ||
| Midwest vs Northeast | 0.981 | 0.735-1.311 |
| South vs Northeast | 1.012 | 0.773-1.326 |
| West vs Northeast | 0.982 | 0.71-1.358 |
| Income | ||
| $125,000-$199,999 vs <$40,000 | 1.02 | 0.736-1.414 |
| ≥$200,000 vs <$40,000 | 1.122 | 0.782-1.61 |
| $40,000-$74,999 vs <$40,000 | 0.977 | 0.746-1.28 |
| $75,000-124,999 vs <$40,000 | 1.136 | 0.863-1.496 |
| Missing vs <$40,000 | 0.79 | 0.583-1.071 |
| Insurance: Medicare Advantage vs commercial | 1.026 | 0.797-1.322 |
| Specialist: yes vs no | 0.839 | 0.476-1.477 |
| Charlson conditions | ||
| 2-3 vs 0-1 | 0.943 | 0.77-1.155 |
| ≥4 vs 0-1 | 0.822 | 0.612-1.105 |
| Gastroesophageal reflux disease: yes vs no | 1.032 | 0.869-1.227 |
| Rhinitis: yes vs no | 1.231 | 0.98-1.548 |
| Sinusitis: yes vs no | 0.986 | 0.816-1.192 |
| COPD: yes vs no | 0.784 | 0.655-0.939 |
| Depression: yes vs no | 0.956 | 0.792-1.154 |
| Before index inhaled corticosteroid proportion days covered in 6 mo before index: ≥75% vs 0-74.9% | 0.92 | 0.75-1.128 |
| After index inhaled corticosteroid proportion days covered in 6 mo before index: ≥75% vs 0-74.9% | 0.917 | 0.74-1.136 |
| Biologic proportion days covered: ≥75% vs 0-74.9% | 1.146 | 0.968-1.356 |
| C Statistic | 0.56 |
Table 5.
Multivariable Analysis for ≥50% Reduction in Asthma Exacerbations (Defined by ED Or Hospitalization Only) During First 6 Months of Asthma Biologic Use
| Effect | OR | 95% CI |
|---|---|---|
| Age | 1.016 | 1.003-1.029 |
| Sex: female vs male | 0.836 | 0.559-1.251 |
| Race | ||
| Asian vs white | 0.876 | 0.269-2.847 |
| Black vs white | 1.053 | 0.64-1.732 |
| Hispanic vs white | 1.138 | 0.638-2.031 |
| Missing vs white | 0.907 | 0.409-2.011 |
| Region | ||
| Midwest vs Northeast | 1.245 | 0.664-2.335 |
| South vs Northeast | 0.805 | 0.456-1.418 |
| West vs Northeast | 1.214 | 0.564-2.613 |
| Income | ||
| $125,000-$199,999 vs <$40,000 | 1.982 | 0.968-4.061 |
| ≥$200,000 vs <$40,000 | 2.793 | 1.062-7.349 |
| $40,000-$74,999 vs <$40,000 | 2.164 | 1.264-3.703 |
| $75,000-124,999 vs <$40,000 | 1.827 | 1.064-3.137 |
| Missing vs <$40,000 | 0.996 | 0.583-1.703 |
| Insurance: Medicare Advantage vs commercial | 0.773 | 0.464-1.286 |
| Charlson conditions | ||
| 2-3 vs 0-1 | 1.024 | 0.665-1.579 |
| 4+ vs 0-1 | 0.651 | 0.375-1.131 |
| Gastroesophageal reflux disease: yes vs no | 0.962 | 0.659-1.403 |
| Rhinitis: yes vs no | 1.605 | 1.015-2.539 |
| Sinusitis: yes vs no | 1.134 | 0.764-1.684 |
| COPD: yes vs no | 0.947 | 0.635-1.413 |
| Depression: yes vs no | 0.526 | 0.358-0.772 |
| Before index inhaled corticosteroid proportion days covered in 6 mo before index: ≥75% vs 0-74.9% | 1.468 | 0.903-2.386 |
| After index inhaled corticosteroid proportion days covered in 6 mo before index: ≥75% vs 0-74.9% | 0.747 | 0.457-1.222 |
| Biologic proportion days covered: ≥75% vs 0-74.9% | 1.249 | 0.868-1.798 |
| C Statistic | 0.67 |
Table 6.
Multivariate Analysis for ≥3 Rescue Inhaler Fills During First 6 Months of Asthma Biologic Use
| Effect | OR | 95% CI |
|---|---|---|
| Age | 0.984 | 0.978-0.99 |
| Sex: female vs male | 0.883 | 0.739-1.056 |
| Race | ||
| Asian vs white | 0.507 | 0.259-0.993 |
| Black vs white | 1.213 | 0.926-1.588 |
| Hispanic vs white | 1.334 | 0.999-1.783 |
| Missing vs white | 0.624 | 0.406-0.959 |
| Region | ||
| Midwest vs Northeast | 1.338 | 0.987-1.813 |
| South vs Northeast | 1.053 | 0.791-1.401 |
| West vs Northeast | 1.2 | 0.852-1.689 |
| Income | ||
| $125,000-$199,999 vs <$40,000 | 0.706 | 0.5-0.999 |
| ≥$200,000 vs <$40,000 | 0.664 | 0.453-0.973 |
| $40,000-$74,999 vs <$40,000 | 0.951 | 0.722-1.253 |
| $75,000-124,999 vs <$40,000 | 0.866 | 0.654-1.148 |
| Missing vs <$40,000 | 1.315 | 0.965-1.792 |
| Insurance: Medicare Advantage vs commercial | 0.866 | 0.664-1.129 |
| Charlson conditions | ||
| 2-3 vs 0-1 | 1.179 | 0.955-1.456 |
| ≥4 vs 0-1 | 1.39 | 1.023-1.888 |
| Gastroesophageal reflux disease: yes vs no | 1.034 | 0.864-1.238 |
| Rhinitis: yes vs no | 0.73 | 0.577-0.924 |
| Sinusitis: yes vs no | 0.846 | 0.696-1.029 |
| COPD: yes vs no | 1.712 | 1.419-2.064 |
| Depression: yes vs no | 1.19 | 0.981-1.444 |
| Before index inhaled corticosteroid proportion days covered in 6 mo before index: ≥75% vs 0-74.9% | 0.96 | 0.775-1.19 |
| After index inhaled corticosteroid proportion days covered in 6 mo before index: ≥75% vs 0-74.9% | 1.617 | 1.295-2.019 |
| Biologic proportion days covered: ≥75% vs 0-74.9% | 0.94 | 0.789-1.119 |
| C Statistic | 0.639 |
Discussion
In a sample of people with commercial or Medicare Advantage insurance, adherence to asthma biologics was observed to be higher than adherence to ICS, both before starting and while using asthma biologics. Nonetheless, adherence to ICS before starting biologics is associated weakly (OR, 1.25) with adherence to a biologic in the first 6 months of use. Although these data suggest that common factors for adherence may exist for ICS and biologics, our multivariable analysis of factors associated with adherence to each medication class yielded results for insurance type and specialist access that were significant and in opposite directions, which may suggest that adherence to ICS and biologics are different in important ways. In our dataset, we found that increased biologic adherence was associated with response to biologic treatment (OR, 1.15; 95% CI, 0.97-1.26), with a similar result in a sensitivity analysis for response as measured by exacerbations defined as ED and hospital only (OR, 1.25; 95% CI, 0.87-1.80), which may suggest a more complicated relationship than our model measured (for example, there may be a cut off level for biologic adherence that is associated with treatment response).
There are other studies of adherence to asthma biologics and of adherence to biologics used for diseases other than asthma that can help put our data into context. Adherence to the oldest asthma biologic, omalizumab (US Food and Drug Administration approved in 2003), was reported for 3,058 people with Medicare insurance from 2012 to 2014 and was very similar to rates from our study (percent days covered 74%).6 Good adherence (<10% of doses missed) was reported for 91% of people in a retrospective study from Italy,7 and overall visit adherence for omalizumab was 78% in a separate US cohort.8 Adherence to biologic drugs for dermatology, rheumatology, and gastroenterology conditions are similar to what we found for asthma biologics.9,10 Adherence to IV or subcutaneous biologics may be higher than oral biologics for people with inflammatory bowel disease,10 which may be similar to differences that we see for medications given subcutaneously or intravenously compared with inhaled medications for asthma.
Other studies have identified risk factors for low adherence to biologics. Age, income subsidy, and provider visits were factors associated with adherence to omalizumab in people with Medicare.6 Similarly, we found age, economic indicators, and specialist visits were associated with adherence to asthma biologics. We speculate that there are other important factors for adherence to asthma biologics that are not captured in the claims dataset, such as processes a clinical practice may use to encourage timely, regular treatments, facilitate insurance cost sharing,11 and reduce access barriers (eg, transportation, appointment availability) for people who receive their injections in clinical settings.
There are important weaknesses and strengths to consider when interpreting data from this study. First, people without insurance or some types of governmental insurance are not represented in this study, nor are people living outside of the United States. Second, PDC is a surrogate measure for adherence. It is possible that people will fill their medications and not use them or that others acquire their medications independently of insurance coverage (eg, samples from provider offices or buying inhalers from different countries). For asthma biologics, though, most were administered in a clinic setting; therefore, the data are highly likely to represent actual use. Third, most of the people in this sample had their asthma biologic paid through the medical benefit,5 similar to what was reported by Li et al.6 However, a temporal shift toward use of the pharmacy benefit is noted in a recent study5 and may be further accelerated by coronavirus disease 2019 pandemic conditions where in-home administration is preferred. The influence of economic factors may be different when the pharmacy benefit is used more frequently. Fourth, the differences seen with specialist access were difficult to understand; therefore, we performed additional analyses comparing people with and without specialist access 6 months before index biologic use and found that people who saw specialists had higher asthma exacerbation rates, higher rescue inhaler use, and more comorbidities. This additional analysis does not provide a clear explanation for the opposite differences that were observed for the specialist access variable for ICS and biologic PDC. Fifth, there are important outcome domains that are not captured in this dataset, including day-to-day asthma symptoms, quality of life, and missed school and work days. However, the primary outcome for most asthma biologic trials, asthma exacerbations, is captured in the dataset. The weaknesses are balanced by strengths that include a large sample size that is generally representative of people in the United States with commercial or Medicare Advantage insurance, the availability of several important independent factors included in the multivariable model, and the inclusion of multiple biologic medications.
Current asthma guidelines are structured with the use of step-wise treatment algorithms.1 Many people who are potentially eligible for treatment with asthma biologics do not receive them12,13; the reasons for this are still not entirely clear. Decisions on insurance coverage for asthma biologics, following information from trials of asthma biologics where people were taking ICS, are often contingent on adherent use of ICS.14 A previous argument has been published that questions whether prior ICS adherence should be required for all patients before starting asthma biologics; this argument was based on a case report.15 Furthermore, many interventions to improve ICS adherence may not be effective. Normansell et al2 performed a systematic review and meta-analysis that assessed the efficacy and safety of interventions to improve ICS adherence in people with asthma. They synthesized results from 28 studies and found low-to-moderate quality of evidence that an improvement of 4% to 20% in adherence, on average, could be achieved, depending on the intervention type.2 However, the studies were unable to show that the interventions improved exacerbation rates, control, or quality of life.2 Therefore, insisting on adherence to ICS prior to the use of asthma biologic may not translate to meaningful improvements in asthma outcomes. The findings from our study suggest that adherence to biologics is higher than for ICS, which raises the possibility of achieving potentially positive clinical outcomes with asthma biologics, even in the absence of prior high levels of adherence for ICS. Our data do not suggest that people with low ICS adherence are more likely than people with high ICS adherence to respond to asthma biologic; therefore, electing to start or not start asthma biologics based on prior ICS adherence level may not be helpful. More research into the question about treatment benefit with asthma biologic in the absence of ICS is needed. Further complicating this speculation is that we did not find a statistically significant association between asthma biologic PDC and asthma exacerbation reductions in this study. Patients who did not achieve reductions in exacerbations may have experienced improvements in other outcome domains that were not assessed in this study.
There are a number of next steps that could be considered, based on the information that asthma biologic adherence is higher than ICS adherence: (1) Additional studies with the use of asthma biologics without ICS to test efficacy of biologic monotherapy could be conducted.16 (2) Additional research to understand and test strategies to enhance biologic adherence could build from factors identified in this study. (3) Studies that test outcomes related to different levels of biologic adherence could help understand which adherence goals must be met to achieve patient-important asthma outcomes. (4) Policy efforts to address cost, insurance coverage, and disparities that may prevent asthma biologic use could be initiated that may improve access to these medications, because both insurance and income variables were important in our analysis.
Interpretation
Adherence to asthma biologics is weakly associated with prior adherence to ICS and is considerably higher than adherence to ICS. Factors associated with adherence to asthma biologics are different than for ICS. Policymakers who determine criteria for asthma biologic use and insurance coverage should consider these data in their decisions. Researchers who study asthma biologics should account for adherence. Providers and patients who make decisions about asthma biologics should consider strategies to measure and promote adherence to asthma biologics.
Acknowledgments
Author contributions: M. A. R. takes responsibility for the contents of the manuscript, including the data and the interpretation. J. T. M., J. W. I., M. M. J., R. W. L., N. D. S., and M. A. R. contributed to the study design. J. T. M., J. W. I., M. M. J., and M. A. R. contributed to the data analysis and data interpretation. J. T. M., J. W. I., M. M. J., R. W. L., N. D. S., and M. A. R. participated in the drafting and revision of the manuscript. J. T. M., J. W. I., M. M. J., and M. A. R. had access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Table 1 can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the NIH/NHLBI grant # HL140287 and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Healthcare Delivery.
Parts of this project were reported in an abstract submitted to the 2020 AcademyHealth annual research meeting (which was postponed).
Supplementary Data
References
- 1.Global Initiative for Asthma Global strategy for asthma management and prevention, 2020. www.ginasthma.org
- 2.Normansell R., Kew K.M., Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev. 2017;4:CD012226. doi: 10.1002/14651858.CD012226.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams L.K., Peterson E.L., Wells K. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid non-adherence. J Allergy Clin Immunol. 2011;128(6):1185–1191. doi: 10.1016/j.jaci.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holguin F., Cardet J.C., Diver S. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1):1900588. doi: 10.1183/13993003.00588-2019. [DOI] [PubMed] [Google Scholar]
- 5.Inselman J.W., Jeffery M.M., Maddux J.T., Shah N.D., Rank M.A. Trends and disparities in asthma biologic use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549–554. doi: 10.1016/j.jaip.2019.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li P., Kavati A., Puckett J.T. Omalizumab treatment patterns among patients with asthma in the US Medicare population. J Allergy Clin Immunol Pract. 2020;8(2):507–515. doi: 10.1016/j.jaip.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Campisi R., Crimi C., Intravaia R. Adherence to omalizumab: a multi-center “real-world” study. World Allergy Organ J. 2020;13(2):100103. doi: 10.1016/j.waojou.2020.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh H., Peters J.I., Kaur Y., Maselli D.J., Diaz J.D. Long-term evaluation of response to omalizumab therapy in real life by a novel multimodular approach: the Real-life Effectiveness of Omalizumab Therapy (REALTY) study. Ann Allergy Asthma Immunol. 2019;123(5):476–482. doi: 10.1016/j.anai.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Murage M.J., Tongbram V., Feldman S.R. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence. 2018;12:1483–1503. doi: 10.2147/PPA.S167508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran K., Null K., Lissoos T., Kane S. Retrospective claims analysis indirectly comparing medication adherence and persistence between intravenous biologics and oral small-molecule therapies in inflammatory bowel diseases. Adv Ther. 2019;36(9):2260–2272. doi: 10.1007/s12325-019-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P., Hu T., Yu X. Impact of cost-sharing increases on continuity of specialty drug use: a quasi-experimental study. Health Serv Res. 2018;53(suppl1):2735–2757. doi: 10.1111/1475-6773.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeffery M.M., Shah N.D., Karaca-Mandic P., Ross J.S., Rank M.A. Trends in omalizumab utilization for asthma: evidence for suboptimal patient selection. J Allergy Clin Immunol Pract. 2018;6(5):1568–1577. doi: 10.1016/j.jaip.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Akenroye A., McCormack M., Keet C. Severe asthma in the US population and eligibility for mAb therapy. J Allergy Clin Immunol. 2020;145(4):1295–1297. doi: 10.1016/j.jaci.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UnitedHealthcare Coverage Medical Benefit Drug Policy, Respiratory Interleukins. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-medical-drug/respiratory-interleukins.pdf Effective May 1, 2020; accessed May 9, 2020.
- 15.Green R.H., Shaw D. Strict adherence rules to obtain monoclonal therapy might cost lives. Lancet Respir Med. 2017;5(9):678–679. doi: 10.1016/S2213-2600(17)30238-2. [DOI] [PubMed] [Google Scholar]
- 16.d’Ancona G., Kavanagh J., Roxas C. Adherence to inhaled corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55(5):1902259. doi: 10.1183/13993003.02259-2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.