Abstract
BACKGROUND AND PURPOSE:
Arterial transit time is the time needed for blood to travel from large arteries to capillaries, as estimated from arterial spin-labeling MR imaging. The purpose of this study was to determine whether vascular risk factors and cognitive performance are related to regional differences in cerebral arterial transit time in patients with coronary artery disease who are at risk for cognitive decline.
MATERIALS AND METHODS:
Arterial transit time was estimated from multiple postlabel delay pseudocontinuous arterial spin-labeling images obtained from 29 men with coronary artery disease. Tests of memory, attention, processing speed, and executive function were administered. Principal component analysis was used to create separate models of cognition and vascular risk, which were related to brain regions through voxelwise analyses of arterial transit time maps.
RESULTS:
Principal component analysis identified 2 components of vascular risk: 1) “pressor” (age, systolic blood pressure, and pulse pressure) and 2) “obesity” (body fat percentage and body mass index). Obesity was inversely related to arterial transit time in the posterior cingulate, precuneus, lateral occipital cortices, middle temporal gyrus, and frontal pole (P corrected < .05), whereas pressor was not significant. Cognitive scores were factored into a single component. Poor performance was inversely related to precuneus arterial transit time (P corrected < .05). The average arterial transit time in regions identified by obesity was associated with poorer cognitive function (r2 = 0.21, t = −2.65, P = .01).
CONCLUSIONS:
Altered cerebral hemodynamics, notably in nodal structures of the default mode network, may be one way that vascular risk factors impact cognition in patients with coronary artery disease.
Obesity and hypertension are prevalent vascular risk factors among older adults, known to impact brain structure and function. Elevated body mass index (BMI), for example, has been associated with reduced gray matter volume.1,2 Furthermore, overweight or obese classifications in midlife have been linked to decline in cognitive performance, involving memory, processing speed, verbal fluency, and visuospatial domains3 and an increased risk of Alzheimer disease and vascular dementia.4 Likewise, elevated systolic blood pressure (SBP) has been associated with reduced cerebral tissue, which appears to be more specific to men than women.5 Elevated SBP also has been associated with cognitive impairment, namely executive function, among older adults.6 Taking these findings into account, researchers have suggested that vascular risk factors contribute to cognitive impairment through cerebrovascular dysfunction itself.7,8
In animal studies, hypertension and obesity lead to cerebrovascular remodeling, including thickened arteriolar walls, reduced lumen area, and rarefaction of the microcirculation.9 Arterial spin-labeling (ASL) is typically used to measure CBF as a single measure of perfusion; acquiring multiple postlabel delays permits more comprehensive hemodynamic estimates, including arterial transit time (ATT). Although ASL is a low SNR technique, considerable research has gone into modeling approaches that incorporate spatial and temporal information to produce robust voxel-level estimates.10–13 Mapping the ASL arterial transit time, for instance, is of interest because it reflects the time for labeled blood to travel from the labeling plane to the microvascular perfusion site. Previously, ATT has been used as an adjunct perfusion measure to study minor stroke and transient ischemic attack,14 large artery steno-occlusive disease,15,16 multiple sclerosis,17 and Alzheimer disease,18,19 but to date, it has not been used to examine the potential effects of vascular risk factors on cognition, to our knowledge.
We have recently reported gray matter perfusion findings in coronary artery disease (CAD) in the context of cerebrovascular health.20 This population is relevant to the study of neurodegenerative disease risk because of the following: 1) They present with multiple vascular risk factors that impact both the heart and the brain, 2) have a propensity for small-vessel disease affecting the white matter, and 3) have a higher susceptibility to cognitive decline.21,22 In older adults, obesity was recently reported to affect the functional connectivity of the default mode network.23 We hypothesized that regional ATT would be associated with aggregate measures of both vascular risk and cognitive function in men with CAD.
Materials and Methods
Participants
Twenty-nine male patients with a history of CAD provided written informed consent and were recruited for this study. Sunnybrook Research Institute and University Health Network research ethics boards approved this study. After providing a detailed clinical history, patients underwent MR imaging and performed a battery of cognitive tests. Inclusion criteria were male sex, 55–80 years of age, and a documented history of CAD characterized by myocardial infarction, narrowing of at least 1 major coronary artery on angiography, prior percutaneous coronary intervention, or coronary artery bypass graft surgery. This study excluded women due to the low referral rate of women to the cardiac rehabilitation program24 and reported differences in ATT between men and women,25 which would have necessitated a larger sample to include sex as a covariate. Patients were excluded on the basis of a history of any neurodegenerative disorder. Demographic information, concomitant medications, anthropometrics, resting blood pressure, and history of hyperlipidemia, diabetes mellitus, hypertension, and smoking were ascertained, in addition to cardiac history.
MR Imaging
MR imaging was performed on a 3T system (Discovery MR750; GE Healthcare, Milwaukee, Wisconsin) by using a radiofrequency body coil for transmission and an 8-channel phased arrayradiofrequency head coil for signal detection. High-resolution T1-w images were collected by using 3D spoiled gradient-recalled echo (TR/TE/TI = 8.1/3.2/650 ms, flip angle = 8°, acquisition matrix = 256 × 192 × 186, nominal spatial resolution = 0.9 × 0.9 × 1 mm). Standard T2-weighted FLAIR images were acquired to characterize white matter hyperintensities (2D axial images, TR/TE/TI = 9700/140/2200 ms, voxel dimensions = 0.9 × 0.9 × 3 mm, sections = 48). A semiautomated procedure was used to quantify the volume of white matter lesion burden (milliliter).26 Pseudocontinuous ASL was performed with a label duration of 1.5 seconds, and the labeling plane was prescribed above the carotid bifurcation, aided by time-of-flight angiography images. Axial single-shot EPI was used to collect 25 sequential control and tag images. Seventeen slices were collected with a gap of 1.4 mm, section thickness of 4.2 mm, and nominal voxel dimensions of 3.4 × 3.4 × 5.6 mm3. The acquisition was repeated at 6 different postlabel delays (ie, 100, 500, 900, 1300, 1700, and 2100 ms) to produce an ASL kinetic time-series (On-line Figure), as reported previously.27 In keeping with the recommended guidelines for clinical ASL, we did not use bipolar gradients to suppress large-artery flow signal.28 Instead macrovascular signals were isolated, when appropriate, in the ASL model (see below).
Postprocessing
ASL images were processed by using the fMRI of the Brain Software Library tools (FSL; http://www.fmrib.ox.ac.uk/fsl). Postprocessing of ASL data included perfusion-weighted difference images, motion correction, and spatial smoothing by a Gaussian kernel of 5-mm full width at half maximum. Hemodynamic parameter estimates were generated by using the Bayesian Inference for Arterial Spin Labeling tool of FSL. ATT images were coregistered to the T1-w images by using transformation matrices established by CBF images, because both T1-w and CBF images contain good gray-to-white differentiation for registration purposes. T1-w images was subsequently aligned to a standard space template with 12 degrees of freedom.
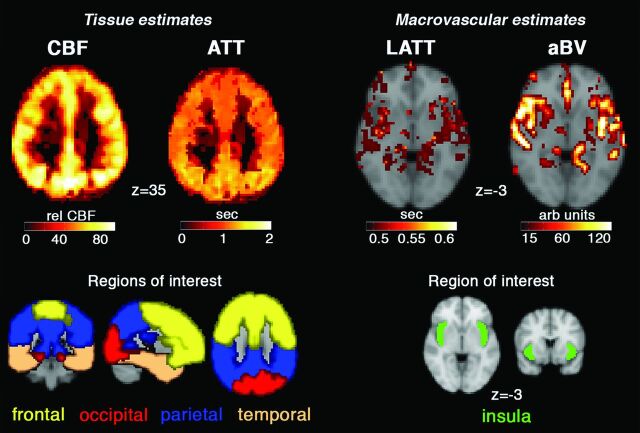
ATT (seconds) was the primary cerebral gray matter hemodynamic outcome measure of interest in this study. ATT was estimated on the basis of the standard ASL model29 and by using a fixed-label duration of 1.5 seconds. A 1-compartment model (tissue only) and a 2-compartment model (tissue and macrovascular compartments) were considered. The former produces ATT and CBF estimates, while the latter produces ATT, CBF, large artery transit time (LATT, seconds), and arterial blood volume estimates.12 Most important, the 2-compartment model takes effect only in cases in which the voxel signal is deemed to be mixed between tissue and macrovascular compartments. This procedure is performed by using an automatic relevancy determination, whereby LATT and arterial blood volume macrovascular estimates are generated in addition to ATT and CBF.12 The macrovascular estimates typically occur in voxels near large intracranial arteries, like the circle of Willis (LATT and arterial blood volume maps are shown in Fig 1). CBF and LATT data were included in secondary analyses.
Fig 1.
The top row of images shows cerebral blood flow (intensity-normalized units), arterial transit time, large-artery transit time, and arterial blood volume (arbitrary units) from a representative participant. In the ATT image, the posterior circulation tends to have a longer ATT compared with the middle cerebral artery vascular territory. LATT and arterial blood volume are macrovascular measures that are estimated from voxels containing large-artery/intravascular ASL signal. The bottom row shows 4 lobe-based regions of interest chosen for ATT (and CBF) analysis, while a middle cerebral artery/insula region of interest was chosen the LATT analysis.
Neuropsychological Testing
Cognitive assessments were chosen on the basis of the recommendations of the National Institute of Neurological Disorders and Stroke and Canadian Stroke Network harmonized standards.30 The Trail-Making Test B (connecting a sequence of alternating letters and numbers) and the Victoria version of the Stroop interference task (color-word) were chosen for their sensitivity to executive function (eg, set shifting and overcoming interference between conflicting visual and lexical cognitive sets). These are timed tasks, with greater time to completion indicating poorer performance. The digit symbol-coding task from the Wechsler Adult Intelligence Scale, 3rd Edition, was chosen as a highly sensitive measure of complex attention and psychomotor processing speed. Digit symbol-coding performance is summarized by the number of symbols matched to digits based on a provided key within 2 minutes. Memory was assessed by the California Verbal Learning Test (CVLT) word list after a 20-minute delay (long-delay free recall [LDFR]). For the latter 2 tasks, greater scores indicate better performance. All cognitive testing was performed at 9:30 am (±30 minutes) after an 8-hour fast.
Statistics
Analyses of the 2-compartment ATT data were performed in standard space at 3-mm isotropic voxel dimensions (Montreal Neurological Institute standard atlas). ATT estimates from the 1-compartment model were also analyzed to assess the effect of the ASL model on the results. ATT was characterized by using the 4 bilateral lobes of the standard atlas brain (3-mm isotropic) as gray matter ROIs (frontal, occipital, parietal, temporal; Fig 1) and by using voxelwise approaches. LATT was analyzed from a single region of interest in the bilateral insula.12 Two separate models were constructed to explain between-subject variance in ATT, CBF, and LATT data: 1) a vascular risk factor model, and 2) a cognitive model. The vascular risk model consisted of the following variables: age, BMI, percentage body fat, SBP, and pulse pressure (ie, the difference between systolic and diastolic pressures). The cognitive model consisted of age and raw scores on the CVLT-LDFR, digit symbol-coding test, Stroop, and Trail-Making Test B. Principal component analysis was performed in R statistical computing software (http://www.r-project.org/) to extract uncorrelated vascular risk and cognitive predictors of ATT. Principal components (PCs) were considered in the model if individually they accounted for at least 10% of the total variance and collectively, >80% of the total explained variance.
General linear models were conducted by using R for the region-of-interest analyses and FSL for voxelwise group analyses. Explanatory variables in the general linear models were the demeaned vascular risk or cognitive PCs, and the 2 predictors were treated separately. For the region-of-interest analyses, a Bonferroni correction was performed to compute adjusted P values. For the voxelwise analyses, statistical maps were calculated by using permutation testing (ie, the FSL Randomise tool).31 Five thousand permutations were performed followed by threshold-free cluster enhancement32 to correct for multiple comparisons. Significant voxels were reported at a corrected P value of .05 (pcorrected = .05).
Results
Patient Characteristics
Patient demographics and characteristics are reported in Table 1. Evidence of severe symptomatic CAD was demonstrated by histories of myocardial infarction (41.4%), coronary artery bypass graft surgery (48.3%), and percutaneous coronary intervention (44.8%). All patients had a history of hyperlipidemia. Histories of diabetes mellitus (13.8%), hypertension (41.4%), and smoking (55.2%) were also common. All patients were using acetylsalicylic acid. The most common concomitant medications included cholesterol-lowering medication (96.6%), β-blockers (69.0%), antihypertensive agents (55.2%), and antidiabetic agents (13.8%). FLAIR white matter hyperintensities amounted to a median lesion burden of 2.63 mL (interquartile range = 1.54–6.76 mL).
Table 1:
Patient demographics (N = 29)
| Demographic | Mean (SD) | Minimum | Maximum |
|---|---|---|---|
| Age (yr) | 65 (7) | 55 | 78 |
| BMI (kg/m2) | 28.2 (3.9) | 20.5 | 35.3 |
| Body fat (%) | 26.0 (5.9) | 13.3 | 38.7 |
| SBP (mm Hg) | 124 (15.4) | 94.0 | 152.0 |
| PP (mm Hg) | 51 (13.0) | 24.0 | 80.0 |
Note:—PP indicates pulse pressure.
Vascular Risk Factors versus Regional Hemodynamics
The first 3 PCs explained >80% of the vascular risk data and were subsequently used in this model. PC1, PC2, and PC3 explained 45%, 36%, and 13% of the variance, respectively (Table 2). PC1 was viewed as a “pressor” variable because SBP and pulse pressure contributed to this component with a correlation coefficient of r < −0.9. PC2 was viewed as an “obesity” variable because BMI and body fat contributed to this component with r > 0.9. PC3 was influenced by age to a lesser extent (r = 0.69).
Table 2:
Vascular risk and cognitive model details
| Correlation Coefficient (r) |
|||
|---|---|---|---|
| Component 1 | Component 2 | Component 3 | |
| Vascular model | |||
| Age (yr) | −0.65 | 0.32 | 0.69 |
| BMI (kg/m2) | 0.23 | 0.91 | −0.06 |
| Body fat (%) | 0.24 | 0.91 | −0.10 |
| SBP (mm Hg) | −0.90 | 0.18 | −0.32 |
| PP (mm Hg) | −0.94 | 0.06 | −0.21 |
| Percentage of variance (%) | |||
| Explained variance | 0.45 | 0.36 | 0.13 |
| Cumulative variance | 0.45 | 0.81 | 0.94 |
| Cognitive model | |||
| Age (yr) | 0.48 | 0.76 | −0.39 |
| CVLT-LDFR | −0.73 | −0.37 | −0.50 |
| Digit symbol-coding | −0.70 | 0.53 | 0.30 |
| Stroop | 0.85 | −0.06 | −0.16 |
| TMT B | 0.82 | −0.26 | 0.21 |
| Percentage of variance (%) | |||
| Explained variance | 0.53 | 0.21 | 0.11 |
Note:—TMT indicates Trail-Making Test; PP, pulse pressure.
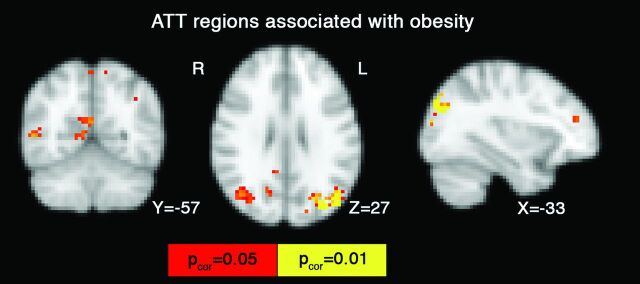
The lobe region-of-interest analyses revealed that PC2 was significantly associated with ATT, while PC1 and PC3 were not. The obesity component (PC2) was inversely related to ATT—that is, higher body fat was associated with shorter ATT in the occipital lobe (t = −3.2, P = .004, Bonferroni-corrected P = .015), but none of the other lobes were significant (Bonferroni-corrected P > .10). Voxelwise ATT analysis revealed significant findings for the obesity component in the posterior cingulate, precuneus, lateral occipital cortices, right middle temporal gyrus, and the left frontal pole (pcorrected < .05, Fig 2 and Table 3). The insula region of interest had a LATT that was inversely associated with the pressor component (ie, LATT versus PC1, t = −2.2, P = .034), but not the obesity component, PC2, or PC3 (P > .27). The inverse association here suggests that higher pulse pressure and SBP were associated with prolonged ATT. Use of the more parsimonious ASL model (ie, with only a tissue compartment) did not influence the voxelwise associations between the vascular risk model and ATT.
Fig 2.
Voxelwise results from the regression analyses in which the vascular model (“pressor” and “obesity” factor) was used as an independent factor that influences ATT. The obesity factor produced a negative association with ATT, in the sense that the higher the BMI and/or body fat, the shorter the ATT. Significant voxels in red and yellow are corrected for multiple comparison (pcorrected = .05).
Table 3:
Summary of voxelwise findings
| No. of Voxels | MNI Coordinates |
|||
|---|---|---|---|---|
| X | Y | Z | ||
| Vascular model | ||||
| 1) Lateral occipital, R | 540 | −33 | −78 | 30 |
| 2) Lateral occipital, L | 38 | 39 | −69 | 27 |
| 3) Middle temporal gyrus, R | 36 | 54 | −57 | 6 |
| 4) Precuneus | 13 | 0 | −60 | 60 |
| 5) Frontal pole, L | 11 | −36 | 45 | 18 |
| 6) Angular gyrus, L | 11 | −39 | −51 | 42 |
| 7) Lateral occipital, L | 4 | −27 | −63 | 48 |
| Cognitive model | ||||
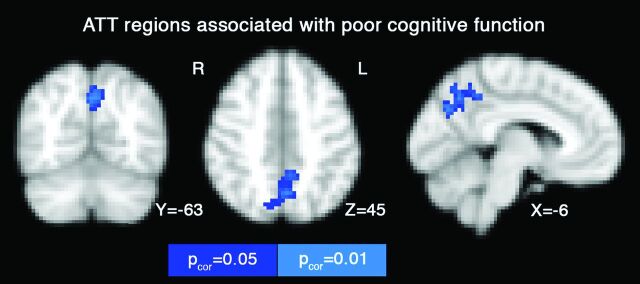
| 1) Precuneus | 189 | 0 | −66 | 42 |
Note:—MNI indicates Montreal Neurological Institute; R, right; L, left.
Cognition versus Regional Hemodynamics
The first 3 cognitive PCs (cog-PCs) explained >80% of the cognitive data, and they were subsequently used in the cognitive model. Cog-PC1, cog-PC2, and cog-PC3 explained 53%, 21%, and 11% of the variance, respectively (Table 2). Cog-PC1 can be viewed as an indicator of cognitive dysfunction because the CVLT-LDFR and digit symbol-coding scores were negative associations, while the timed Stroop and Trail-Making Test B measures were positive associations. Each of the 4 cognitive measures contributed significantly to PC1 (r > .82 or r < −0.70). Less relevant cog-PCs were cog-PC2, which was influenced by aging, and cog-PC3, which did not contribute a meaningful cognitive effect.
Region-of-interest analyses revealed that cog-PC1 showed negative associations with ATT, indicating that individuals with poorer cognitive scores had shorter ATT, but these associations did not survive false discovery rate correction for multiple comparison (ie, 4 brain lobes, adjusted P > .054). Voxelwise analysis was significant, however, with an association between cognitive decline and shorter ATT in the precuneus region (pcorrected < .05, Fig 3 and Table 3). The cog-PC2 and cog-PC3 did not show any significant associations. The insula LATT region of interest was not significant with any of the cognitive model parameters (P > .086). The use of the more parsimonious ASL model did not influence the voxelwise associations between the cognitive model and ATT.
Fig 3.
Voxelwise results from the regression analyses, in which the cognitive model was used as an independent factor that influences ATT. The first cognitive factor (ie, characterized by poor cognitive performance) was positively associated with ATT, in the sense that a lower cognitive score was associated with a shorter ATT. Significant voxels in blue and cyan are corrected for multiple comparison (pcorrected = .05).
Post Hoc Tests
Brain regions that were associated with the obesity component of the vascular model were used as a mask, and the average ATT in these regions was calculated for each participant. ATT in this obesity mask was significantly correlated with cognitive function (r2 = 0.21, t = −2.65, P = .013).
CBF data produced no significant voxels related to components of vascular risk or cognition (pcorrected > .05) by using the same voxelwise analyses as used in the ATT data. However, within the ATT mask circumscribed by the vascular risk model results (PC2, Fig 2), CBF was negatively associated with obesity (t = −2.43, P = .022), and within the ATT mask circumscribed by the cognitive model results (PC1, Fig 3), CBF was negatively associated with cognitive PC1 (t = −2.53, P = .018).
The widely used BMI classifications (ie, healthy, overweight, obese)4 produced results similar to those of the obesity component (PC2) in region-of-interest analyses, albeit with slightly reduced statistical significance (data not shown).
Discussion
The current study demonstrates relationships among cerebral hemodynamics, obesity, and cognition. In older men with CAD, microvascular ATT decreased in association with obesity and poorer cognitive function. The implicated regions included areas of the lateral occipital, precuneus, angular gyrus, middle temporal, and frontal pole. In addition, the macrovascular LATT was significantly associated with hypertension, but not with obesity.
ATT differences associated with obesity occurred primarily in the parietal and occipital lobes. This result is notable for a few reasons. First, the regions identified are consistent with the default mode network, which is a network implicated in cognitive function in later life.33,34 Second, precuneus ATT was also found to be negatively associated with cognitive function. Third, ATT in brain regions identified by obesity explained 21% of the variance in cognitive function across the group.
The pressor component was defined by SBP and pulse pressure; however, it did not significantly correlate with regional ATT at a voxelwise level or in the lobe-based region-of-interest analyses. This finding was unexpected, given previous findings relating blood pressure with cerebral hemodynamics35; however, the literature, to date, has focused primarily on large-artery hemodynamics, such as the middle cerebral artery pulsatility index or blood flow velocity. On the other hand, LATT provides macrovascular information and was significantly associated with the pressor component in the insula region of interest. These results suggest that vascular risk factors may have differential effects on cerebral hemodynamics, which appear to be region-specific.
While the ATT results were significant at a voxelwise level after a conservative correction for multiple comparisons, CBF analyses were not significant in stringent voxelwise analyses. However, post hoc tests suggested that CBF was decreased in proportion to both obesity and cognitive dysfunction in regions identified by ATT; this finding suggests complementary utility between ATT and CBF measures. The focus of the current study was on estimating hemodynamic ATT, which requires sampling multiple-postlabel delay acquisitions at the potential expense of the CBF estimate. Our choice of ASL parameters may therefore have contributed to the reduced sensitivity of CBF analyses.
Ischemic diseases, such as stroke and steno-occlusive large-artery disease, are known to produce prolonged ATT due to the necessity for collateral delivery of blood to tissue or narrowing of the large supplying arteries.15,16 In the current study, prolonged LATT and reduced ATT were associated with vascular risk factors. The reduced ATT may be attributed to altered pulsatile pressures, as reflected in the literature,35,36 and may be an indication of vessel wall hardening and thickening, which are arteriosclerosis processes consistent with higher BMI and body fat.9
This study was designed to detect only within-group differences, lacking comparisons between patients with CAD and age-matched controls without a history of CAD. Such normative data would be beneficial to determine whether similar associations are found in healthy age-related processes. The current study was limited to men, due to their relative over-representation in cardiac rehabilitation and the need to otherwise control for robust differences in ATT between men and women.25 Future work should assess the extent to which both female and male patients with CAD show similar ATT changes in association with vascular and cognitive variables. The absence of uncontrolled diabetic symptoms and current smoking habits within the present cohort prevented these risk factors from being addressed. Furthermore, blood lipid levels were not available for all participants. These additional measures may have helped to explain a greater proportion of the variance and should be explored through future studies. Finally, principal component analysis was used to restrict the number of independent variables, which amounted to 2 vascular risk factors and 1 cognitive factor. This strategy proved useful in identifying voxelwise ATT associations with obesity and cognitive performance. Correlations with additional risk factors (eg, blood lipid levels and smoking habits) and, in particular, a mechanistic understanding of these relationships, including causality, should be addressed by studies that incorporate a longitudinal design.
Conclusions
We found that men with CAD have altered cerebral hemodynamics in proportion to both cardiometabolic risk factors and cognitive function. ATT was used as a microvascular measure, while LATT characterized macrovascular hemodynamics. We observed that ATT in nodes of the default mode network was associated with obesity and cognition, whereas LATT was associated with hypertension. The use of these ASL-derived hemodynamic measures produced novel associations that converge with other studies to implicate changes in the default mode network as markers of cognitive decline.34,37 Future studies might explore mechanisms potentially relating adiposity to cerebral hemodynamics and cognition, such as blood lipid levels associated with cognitive decline.38
Supplementary Material
Acknowledgments
We thank Dr Michael Chappell for useful discussion concerning the ASL model.
ABBREVIATIONS:
- ASL
arterial spin-labeling
- ATT
arterial transit time
- BMI
body mass index
- CAD
coronary artery disease
- cog-PC
cognitive principal component
- CVLT-LDFR
California Verbal Learning Test long-delay free recall
- LATT
large-artery transit time
- PC
principal component
- SBP
systolic blood pressure
Footnotes
Disclosures: Nathan Herrmann—RELATED: Grant: The Drummond Foundation,* Physicians' Services Incorporated Foundation,* Canadian Institutes of Health Research,* Comments: peer-reviewed grants; UNRELATED: Consultancy: Sonexa Therapeutics, Wyeth Pharmaceuticals, Pivina Consulting, Sanofi-Aventis Canada; Grants/Grants Pending: Alzheimer Society of Canada,* Canadian Institute of Health Research,* Heart and Stroke Foundation,* Ontario Ministry of Health and Long-Term Care, Provincial Innovation Fund,* Ontario Brain Institute,* Comments: peer-reviewed grants; Other: Pfizer Canada,* F. Hoffman-La Roche Ltd,* Elan Pharma International Ltd,* Lundbeck Canada,* Comments: research contracts. Krista L. Lanctôt—RELATED: Grant: Canadian Institutes of Health Research,* The Drummond Foundation,* Physicians' Services Incorporated Foundation*; UNRELATED: Consultancy: AbbVie, F. Hoffman-La Roche Ltd; Grants/Grants Pending: Alzheimer's Society Canada),* Alzheimer's Drug Discovery Foundation,* Canadian Institute of Health Research,* Heart and Stroke Foundation,* Consortium of Canadian Centres for Clinical Cognitive Research,* Canadian Consortium on Neurodegeneration in Aging;* Other: AbbVie,* F. Hoffman-La Roche Ltd,* Elan Pharma International Ltd,* Lundbeck Canada Inc,* Comments: research funding. *Money paid to the institution.
This work was funded by The Drummond Foundation (K.L.L., N.H.), Physicians' Services Incorporated Foundation (K.L.L., N.H.), and the Canadian Institutes of Health Research (K.L.L., N.H.: MOP-114913). W.S. was supported by the Canadian Partnership for Stroke Recovery, the Canadian Institutes of Health and Research and by the Toronto Rehabilitation Institute.
REFERENCES
- 1. Kurth F, Levitt JG, Phillips OR, et al. Relationships between gray matter, body mass index, and waist circumference in healthy adults. Hum Brain Mapp 2013;34:1737–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raschpichler M, Straatman K, Schroeter ML, et al. Abdominal fat distribution and its relationship to brain changes: the differential effects of age on cerebellar structure and function—a cross-sectional, exploratory study. BMJ Open 2013;3:pii: e001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hassing LB, Dahl AK, Pedersen NL, et al. Overweight in midlife is related to lower cognitive function 30 years later: a prospective study with longitudinal assessments. Dement Geriatr Cogn Disord 2010;29:543–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu WL, Atti AR, Gatz M, et al. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology 2011;76:1568–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gianaros PJ, Greer PJ, Ryan CM, et al. Higher blood pressure predicts lower regional grey matter volume: consequences on short-term information processing. Neuroimage 2006;31:754–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuo HK, Sorond F, Iloputaife I, et al. Effect of blood pressure on cognitive functions in elderly persons. J Gerontol A Biol Sci Med Sci 2004;59:1191–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke 2002;33:1152–62 [DOI] [PubMed] [Google Scholar]
- 8. Sato N, Morishita R. Roles of vascular and metabolic components in cognitive dysfunction of Alzheimer disease: short- and long-term modification by non-genetic risk factors. Front Aging Neurosci 2013;5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joutel A, Monet-Lepretre M, Gosele C, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest 2010;120:433–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Asllani I, Borogovac A, Brown TR. Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn Reson Med 2008;60:1362–71 [DOI] [PubMed] [Google Scholar]
- 11. Groves AR, Chappell MA, Woolrich MW. Combined spatial and non-spatial prior for inference on MRI time-series. Neuroimage 2009;45:795–809 [DOI] [PubMed] [Google Scholar]
- 12. Chappell MA, MacIntosh BJ, Donahue MJ, et al. Separation of macrovascular signal in multi-inversion time arterial spin labelling MRI. Magn Reson Med 2010;63:1357–65 [DOI] [PubMed] [Google Scholar]
- 13. Francis ST, Bowtell R, Gowland PA. Modeling and optimization of look-locker spin labeling for measuring perfusion and transit time changes in activation studies taking into account arterial blood volume. Magn Reson Med 2008;59:316–25 [DOI] [PubMed] [Google Scholar]
- 14. MacIntosh BJ, Lindsay AC, Kylintireas I, et al. Multiple inflow pulsed arterial spin-labeling reveals delays in the arterial arrival time in minor stroke and transient ischemic attack. AJNR Am J Neuroradiol 2010;31:1892–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macintosh BJ, Marquardt L, Schulz UG, et al. Hemodynamic alterations in vertebrobasilar large artery disease assessed by arterial spin-labeling MR imaging. AJNR Am J Neuroradiol 2012;33:1939–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bokkers RP, van Laar PJ, van de Ven KC, et al. Arterial spin-labeling MR imaging measurements of timing parameters in patients with a carotid artery occlusion. AJNR Am J Neuroradiol 2008;29:1698–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paling D, Thade Petersen E, Tozer DJ, et al. Cerebral arterial bolus arrival time is prolonged in multiple sclerosis and associated with disability. J Cereb Blood Flow Metab 2014;34:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mak HK, Chan Q, Zhang Z, et al. Quantitative assessment of cerebral hemodynamic parameters by QUASAR arterial spin labeling in Alzheimer's disease and cognitively normal elderly adults at 3-Tesla. J Alzheimers Dis 2012;31:33–44 [DOI] [PubMed] [Google Scholar]
- 19. Yoshiura T, Hiwatashi A, Yamashita K, et al. Simultaneous measurement of arterial transit time, arterial blood volume, and cerebral blood flow using arterial spin-labeling in patients with Alzheimer disease. AJNR Am J Neuroradiol 2009;30:1388–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MacIntosh BJ, Swardfager W, Crane DE, et al. Cardiopulmonary fitness correlates with regional cerebral grey matter perfusion and density in men with coronary artery disease. PLoS One 2014;9:e91251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Volonghi I, Pendlebury ST, Welch SJ, et al. Cognitive outcomes after acute coronary syndrome: a population based comparison with transient ischaemic attack and minor stroke. Heart 2013;99:1509–14 [DOI] [PubMed] [Google Scholar]
- 22. Zheng L, Mack WJ, Chui HC, et al. Coronary artery disease is associated with cognitive decline independent of changes on magnetic resonance imaging in cognitively normal elderly adults. J Am Geriatr Soc 2012;60:499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kullmann S, Heni M, Veit R, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 2012;33:1052–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swardfager W, Herrmann N, Dowlati Y, et al. Relationship between cardiopulmonary fitness and depressive symptoms in cardiac rehabilitation patients with coronary artery disease. J Rehabil Med 2008;40:213–18 [DOI] [PubMed] [Google Scholar]
- 25. MacIntosh BJ, Filippini N, Chappell MA, et al. Assessment of arterial arrival times derived from multiple inversion time pulsed arterial spin labeling MRI. Magn Reson Med 2010;63:641–47 [DOI] [PubMed] [Google Scholar]
- 26. Gibson E, Gao F, Black SE, et al. Automatic segmentation of white matter hyperintensities in the elderly using FLAIR images at 3T. J Magn Reson Imaging 2010;31:1311–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chaudhary S, Scouten A, Schwindt G, et al. Hemodynamic effects of cholinesterase inhibition in mild Alzheimer's disease. J Magn Reson Imaging 2013;38:26–35 [DOI] [PubMed] [Google Scholar]
- 28. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2014. April 8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buxton RB, Frank LR, Wong EC, et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 1998;40:383–96 [DOI] [PubMed] [Google Scholar]
- 30. Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006;37:2220–41 [DOI] [PubMed] [Google Scholar]
- 31. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002;15:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44:83–98 [DOI] [PubMed] [Google Scholar]
- 33. Greicius MD, Srivastava G, Reiss AL, et al. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 2004;101:4637–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Celone KA, Calhoun VD, Dickerson BC, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci 2006;26:10222–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Webb AJ, Simoni M, Mazzucco S, et al. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke 2012;43:2631–36 [DOI] [PubMed] [Google Scholar]
- 36. Hirata K, Yaginuma T, O'Rourke MF, et al. Age-related changes in carotid artery flow and pressure pulses: possible implications for cerebral microvascular disease. Stroke 2006;37:2552–56 [DOI] [PubMed] [Google Scholar]
- 37. Damoiseaux JS, Beckmann CF, Arigita EJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex 2008;18:1856–64 [DOI] [PubMed] [Google Scholar]
- 38. Saleem M, Ratnam Bandaru VV, Herrmann N, et al. Ceramides predict verbal memory performance in coronary artery disease patients undertaking exercise: a prospective cohort pilot study. BMC Geriatr 2013;13:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.