Abstract
BACKGROUND AND PURPOSE:
The assessment of motor function is an essential component of neurologic examinations, which imaging studies have extended to the fetus. US assessment is hampered by a limited FOV, whereas MR imaging has the potential to be an alternative. Our objectives were to optimize a cine MR imaging sequence for capturing fetal movements and to perform a pilot analysis of the relationship between the frequency of movements and uterine spatial constrictions in healthy fetuses.
MATERIALS AND METHODS:
Initially, a bSSFP cine sequence was selected for optimization, and various compromises were explored in all acquisition parameters to achieve an effective balance between anatomic coverage of the fetus and the temporal resolution of cine data, with the aim of maximizing both. Subsequently, cross-sectional qualitative and quantitative analyses of fetal movements were performed prospectively by using a cohort of 37 healthy fetuses (median GA, 29 weeks; range, 20–37 weeks) with the optimized cine protocol. Two smaller subgroups were selected for representative sampling of overall behavior patterns by using cine data of longer duration and for volumetric quantification of free intrauterine space.
RESULTS:
The optimized cine sequence, with TR/TE of 3.21/1.59 ms, coupled with parallel imaging and partial-Fourier imaging, resulted in a section-acquisition time of 0.303 seconds. Anatomic coverage was enhanced by using a combination of thick sagittal sections (30–40 mm) and multisection acquisitions to display movements in all fetal limbs, head, and trunk simultaneously. All expected motor patterns were observed throughout this gestational period, and a significant decreasing trend in overall movement frequency with age was demonstrated (r = −0.514, P = .0011). Also a significant negative correlation was found between overall movement frequency and the total intrauterine free space (r = −0.703, P = .0001). Furthermore, a significant decrease in the frequency of leg movements was shown in fetuses older then 30 weeks' GA compared with those younger than that (P = .015).
CONCLUSIONS:
Cine MR imaging is effective for observing fetal movements from midgestation with near full-body coverage. Also, reductions in free space with increasing GA appear to be a factor in the gradual reductions in overall levels of fetal activity as well as in restrictions in movement within specific regions of the fetal anatomy.
The assessment of the pattern and quality of movements is an important component of the neurologic examination in infants with congenital or acquired brain abnormalities who are at risk of later neurodevelopmental anomalies.1 A number of approaches have been devised and used in early infancy2; however, a fetus-specific approach has not yet been formulated. Two methods that have been used to assess the preterm infant are the Test of Infant Motor Performance3 and GM analysis.4 A number of studies have demonstrated a role for GM analysis in detecting neurologic abnormalities in preterm infants born from a GA of 24 weeks until approximately 16 weeks' postterm4,5; the theory is that neurologic deficits manifest as early disturbances in the character of spontaneously generated movements of the whole body.1 However, it remains unclear whether movement patterns in the fetus are altered by gestation or neurologic compromise, and it has been proposed that the early motor repertoire is stable from a GA of 12 weeks until 3 months' postterm.1,5 However, attempts to study fetal movements by using US during the second and third trimesters are hampered by the limited FOV afforded by standard transducers, which is insufficient to observe the complete fetus.6–8
The fetus is surrounded by the physical barrier of the uterus, thus an issue arises as to whether its immediate environment plays a role in the development of the motor system. Classic and more recent studies have shown that the neural infrastructure for sensory and proprioceptive feedback is present early in gestation.9,10 Furthermore, animal models of fetal development have shown differences in overall levels of activity and in the relative rates of individual motor patterns in subjects that have been exteriorized compared with those remaining in utero.11
MR imaging is a valuable technique for assessing the fetus. Several dynamic MR imaging sequences have the potential to capture fetal movement patterns.12 Studies have suggested that bSSFP cine is the most suitable sequence for imaging fetal movements,13,14 and it has been shown to have several advantages: lower SAR values compared with alternatives under equivalent imaging conditions12,15 and high SNR and image contrast properties that show fluid/tissue boundaries unambiguously.16,17
The objectives of our study were the following: first, to optimize a cine MR imaging sequence (bSSFP) and to evaluate it as a method for capturing fetal movement; second, to test the hypothesis that changes in spatial restrictions with increasing GA contribute to quantitative reductions in the frequency of fetal movements.
Materials and Methods
Ethics permission for MR imaging examinations was granted by the Hammersmith Hospital Research Ethics Committee, and written consent was obtained from all patients. Images were obtained at the Robert Steiner MR Imaging Unit, Hammersmith Hospital, between October 2007 and October 2009. Patients were recruited as healthy volunteers or were referred to the unit because of suspicious or abnormal cranial US findings. Patients with multiple pregnancies were excluded. All MR imaging examinations were performed on a 1.5T Achieva scanner (Philips Healthcare, Best, the Netherlands) by using a 5-channel phased array cardiac coil, with patients placed in a lateral tilt position to prevent vena cava compression. No sedative was administered.
bSSFP Optimization
The objective was to create a cine imaging protocol for monitoring movement of the whole fetus across gestation. Twenty-three fetuses were imaged for bSSFP optimization (GA range, 20–35 weeks). An adult cardiac bSSFP protocol was used as a starting point, and trade-offs between spatial and temporal resolutions were explored, while other factors, such as voxel size, flip angle, and receiver bandwidth, were adjusted to minimize the TR and TE times, which impact banding artifacts and SNR. We explored the effect of varying the FOV to accommodate mothers and fetuses of varying sizes. Parallel imaging (SENSE)18 and partial-Fourier imaging15 were also explored to reduce section-acquisition time. SAR was limited to 2 W/kg by using “low SAR mode”19 on the scanner.
To accurately display fetal movements, we experimented with section orientation and thickness, as well as with the use of multisection imaging to increase coverage of the fetus in the through-plane direction. We explored sagittal, coronal, and transverse planes through the fetus, variations in section thickness from 3 to 150 mm, and multisection imaging between 1 and 7 sections. Piloting strategies were also explored to develop efficient means of defining scanning planes while avoiding aliasing and other FOV-related artifacts.
A previous study recommended three 30-second cine clips as sufficient to analyze fetal movements.20 We explored a range of clip lengths ≤12 minutes; the number of clips varied depending on the available scanning time. The effect of temporal resolution on the accurate representation of movements was tested by using video recordings of 2 term-age neonates lasting 60 seconds each and shot at 25 fps. Frames were removed by using Premiere Pro, Version 2.0, (Adobe Systems, McLean, Virginia) to simulate the frame rate of the MR imaging cine acquisition. The total movements were first quantified in the edited clip, to avoid prior knowledge of movements that might be less clear, and subsequently in the original clip by 1 observer (T.H.); the resulting data were analyzed by using the ICC.
Qualitative Observations of the Healthy Fetal Motor Repertoire from GA 18 Weeks to Term
This was a cross-sectional study that used the optimized cine MR imaging protocol prospectively on 37 neurologically healthy fetuses (median GA, 29 weeks; range, 20–37 weeks). Analysis of cine data was performed off-line, by using ImageJ, Version 1.43, (National Institutes of Health, Bethesda, Maryland) by 1 rater (T.T.A.H.). The objective was to provide a detailed observational record of the natural history of motor behavior during the second half of gestation. All fetal motor patterns previously described were identified, and any qualitative changes to their execution as a result of the intrauterine environment were noted.
Quantitative Analysis of Fetal Movements and Their Relationship to Intrauterine Constraints
This study was performed by using the same fetal cohort as described in the qualitative substudy above. Fetal movements were recorded for each anatomic region (arm, leg, head, and trunk); intrarater variability was calculated by using the ICC by requantifying the total movements made by 20 fetuses. An operational definition for a “movement” was either a pause followed by displacement followed by pause, or in “repetitive movements,” a displacement followed by return to origin, with “displacements” consisting of flexions, extensions, or rotations. The frequency and duration of each movement were quantified and recorded manually on actograms.21 Within the cohort, 3 fetuses (GA 22, 28, and 36 weeks) were selected for performing a continuous and longer cine acquisition of at least 20 minutes' duration to provide reference data for motor behavior for each GA group.
A subgroup of 24 fetuses (median GA, 30 weeks; range, 21–37 weeks) had volumetric quantification of uterine compartments performed. A T2-weighted single-shot fast spin-echo sequence was used to image the whole uterus with contiguous sections: TR/TE, 1000/98 ms; FOV, 430 × 350 mm; voxel size, 0.84 × 0.84 × 4 mm. Images were segmented manually by using MRIcro, Version 1.40 (http://www.sph.sc.edu/comd/rorden/mricro.html)22 by 2 raters (T.T.A.H. and A.N.). The total unoccupied intrauterine volume available to the fetus was calculated by subtracting the volume of the fetus from that of the uterine cavity (excluding the placenta). Volume calculations were performed by using Matlab, Version 7.9 (MathWorks, Natick, Massachusetts).
Statistical Analysis
Statistical analyses were performed by using StatsDirect, Version 2.7.3 (StatsDirect, Chesire, United Kingdom). The relative frequency of movements in each anatomic region was calculated as a proportion of total movements for each fetus, and a median was obtained for each age group. Differences between groups were tested by using analysis of variance (Kruskal-Wallis) and the Mann-Whitney U test. A simple linear regression analysis and the Pearson correlation coefficient were calculated for changes in activity and uterine volume with GA. Rater variability was calculated by using the ICC for the quantification of fetal movements (repeated measurements on 20 subjects) and of intrauterine compartments (20 repetitions on a single section). Two-sided P values and a significance level of 5% were used.
Results
bSSFP Optimization
We found that both midsagittal and coronal sections through the fetus were the most informative for observing movements throughout the body. A section thickness from 30 to 40 mm was the best compromise between maximizing the anatomic coverage and preventing uterine tissue from encroaching on the image in the through-plane direction (Fig 1 A–C).
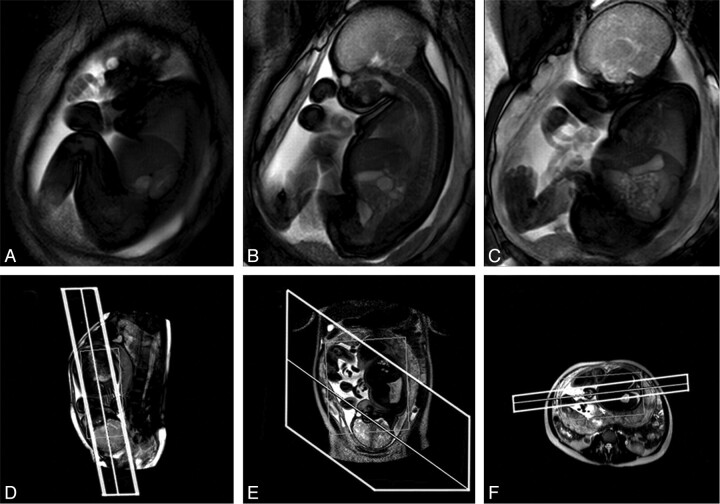
Fig 1.
Example of a cine acquisition at GA 36 weeks. A–C, Successive frames from a multisection cine image sequence (section thickness, 30 mm). D–F, Piloting procedure used to set up the scan. Upper and lower limbs, trunk, and head are all visible within the FOV. D, Fetal localizer scan, which is used to generate sagittal-oriented cine data; E and F, Maternal pilots in orthogonal planes used to ensure that the prescribed sections have FOVs that cover the full extent of maternal tissue.
Multisection imaging increased the anatomic coverage with a constant acquisition rate for individual sections; however, with the introduction of multiple images per frame, the time per frame increased commensurately. Use of >3 sections resulted in an inadequate temporal resolution for accurate interpretation of fetal movements. Below GA 30 weeks, most the fetal anatomy was found to fit within a single-section (40-mm-thick) cine image, whereas older fetuses required 2–3 sections for complete coverage (Fig 1A–C).
We acquired multiple consecutive cine clips each of 2–4 minutes' duration. A shorter length increased the possibility of the acquisition ending during a movement sequence, and longer clips do not allow necessary parameter adjustments to multisection and piloting for subsequent acquisitions.
A persistent problem was aliasing of maternal tissue in the phase-encode direction, causing Moiré fringes,23 the result of the small size of the fetus coupled with its plane of interest lying unpredictably and often obliquely relative to the mother. Our solution was a dual-piloting scheme, using a maximal FOV (50 cm2): first, 3 orthogonal sections were acquired in cardinal planes relative to the mother; second, fetal images were piloted by using the last available fetal localizer scan but checked against the maternal pilots to ensure that there were no foldover or flow problems (Fig 1D–F). The use of dual piloting resulted in only 13% of examinations being affected by aliasing artifacts as opposed to 40% when not used.
Final sequence parameters were the following: flip angle, 60°; FOV, 50 cm2; TR/TE, 3.2/1.59 ms; voxel size, 2.2 × 2.2 mm; section thickness, 30–40 mm; partial-Fourier, 62.5%; SENSE factor, 2; SAR, 2 W/kg; section acquisition time, 0.303 seconds.
Twenty-two (of 25) frames per second were removed from the neonatal video clips to reflect the temporal resolution of our single-section cine data. A total of approximately 100 movements were made by both neonates, including isolated and GM. Testing for differences between the original and edited clips resulted in an ICC of 0.959.
Qualitative Observations of the Healthy Fetal Motor Repertoire from GA 18 Weeks to Term
Visual analysis of cine data showed a range of movement patterns occurring at all ages in our cohort; no gestational age−dependent change was found in the fetal motor repertoire in terms of either the cessation or introduction of new movement patterns. Rotations, flexions, and extensions in the main anatomic regions (upper limbs, lower limbs, head, and trunk; Fig 2 ) were observed. Each of these types of movements was found to occur both in isolation and as part of GM. We also noted fine-motor activity in the fingers occurring from GA 18 weeks, as well as grasping and stroking-like movements. Less frequent were yawns and other mouthing movements, including swallowing. Eye and paradoxic breathing movements were also present at all ages. Non-GM movements involving the whole body occurred irregularly at all GAs, these included kicking, brief twitches, and startles. Fetal GM had an observable pattern similar to those in neonates, usually with a brief isolated twitch indicating the start.
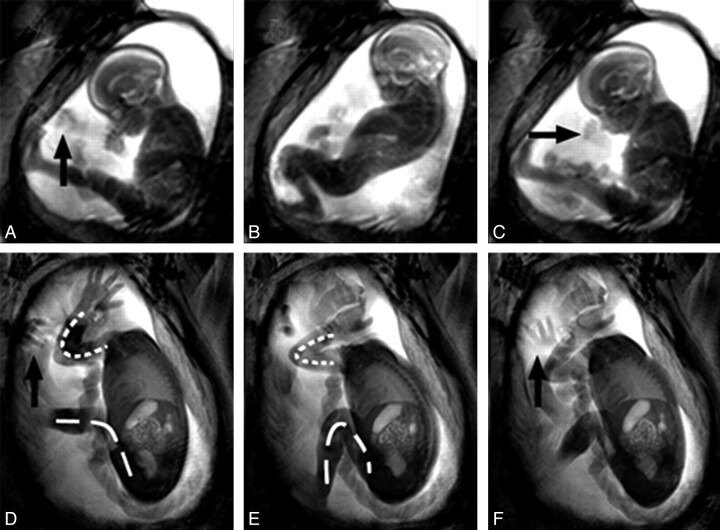
Fig 2.
Examples of the fetal movement repertoire. A–C, Time points 0, 4, and 8 seconds of cine data from a GA 22 weeks' fetus. D–F, A GA 30 weeks' fetus at time points 0, 6, and 10 seconds. The younger fetus is engaging in GM, demonstrating a complete flexion at its knees and extension in its trunk, followed by trunk relaxation and knee extension. D–F, The older fetus also shows GM consisting of flexion at the knee joint (dashed line) as well as at the elbow (dotted line). Arrows indicate hands, 1 of which is rotating in the older fetus. The overall variability in amplitude of movements that older fetuses perform is significantly reduced and appears associated with uterine restrictions giving a cramped appearance to movements. This is in contrast to younger fetuses, that tend to make full use of their surrounding space.
Fetuses younger than approximately GA 32 weeks displayed movements of both small and large amplitudes, making full use of the available space in all directions. As fetuses approached term, movements became noticeably smaller in amplitude and appeared cramped, with few examples of full extension at the knee and elbow joints (Fig 2D−F). Leg movements consisted primarily of rotational movements at the hip. Rotations in the wrist and shoulder and attempts to fully extend the spine were the hallmarks of GM late in gestation. Fetuses early in gestation were observed to make unimpeded extensions as a result of the relatively larger available space, as well as making some movements of small amplitude. Throughout this GA range, there was no evidence of localized distension of the uterus resulting from fetal anatomy impacting the uterine wall during movements.
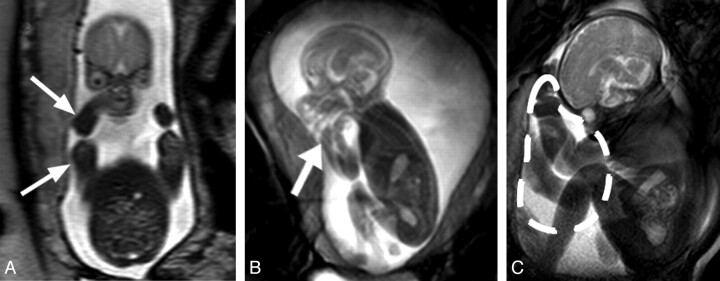
We observed that the distribution of amniotic fluid changed with increasing gestational age, relocating mostly anterior to the fetal thorax near term (Fig 3 C). Furthermore, orthogonal T2-weighted sections through the fetus showed that despite there being relatively large volumes of amniotic fluid in the anteroposterior dimension, the uterine wall abuts the lateral aspects of the fetal body at all gestations, which appears to cause an adducted and flexed posture in the upper limbs, frequently resulting in the fetal hands being located near the head (Fig 3A, -B).
Fig 3.
Facilitation of flexed posture by the uterine wall. A, T2-weighted coronal section through a GA 22 weeks' fetus. The uterine wall is in close proximity to the fetus's lateral aspects (arrows). B, Still image from cine data of the same fetus. The fetal arm is flexed and located adjacent to the fetal head (thick arrow). C, Image of a GA 35 weeks' fetus demonstrates the redistribution of amniotic fluid volume near term, anterior to the thorax of the fetus (dashed line), due to its posture and the uterine limits; this collection of amniotic fluid may facilitate upper limb movements and trunk extensions.
Quantitative Analysis of Fetal Movements and Their Relationship to Intrauterine Constraints
The median cine-acquisition duration for the whole cohort was 7 minutes (range, 4–24 minutes). The cohort was divided into 3 age groups: 18≤GA<25 weeks (median GA, 22 weeks), 25≤GA<31 weeks (median GA, 28 weeks), and 31≤GA≤37 weeks (median GA, 34 weeks). The intrarater variability for the quantification of fetal movements was 0.978 (ICC).
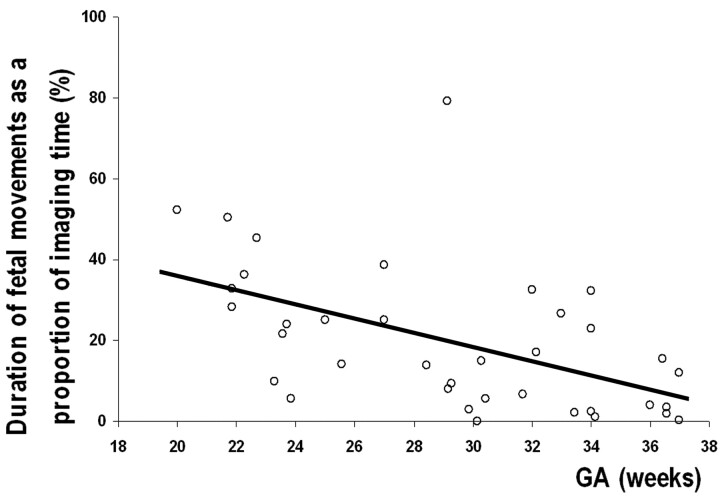
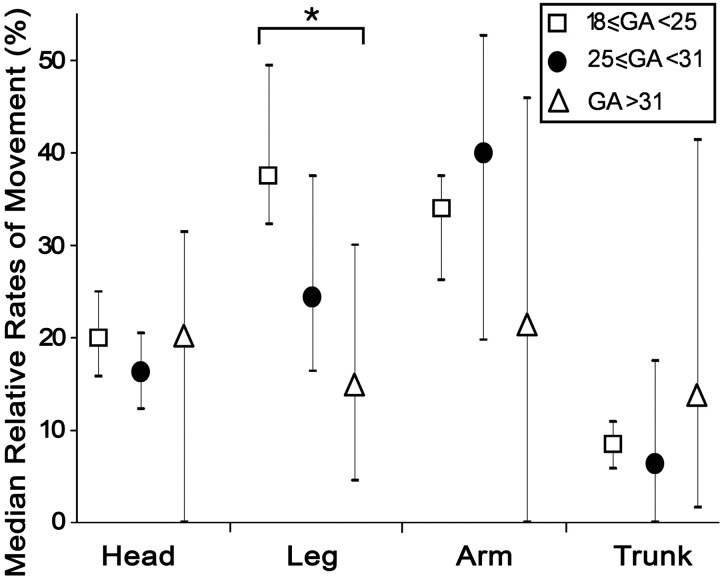
The proportion of imaging time spent moving decreased significantly with increasing GA (r = −0.514, P = .0011; Fig 4). Testing for differences in the relative movement frequency in each anatomic region by age (Fig 5) showed no significant changes with GA in the median proportion of movements in the head, arms, or trunk (P = .746, P = .310, and P = .397, respectively; Kruskal-Wallis). Leg movements showed a significant decrease in fetuses older than GA 30 weeks compared with fetuses younger than GA 30 weeks (P = .015, Kruskal-Wallis).
Fig 4.
Graph shows that the proportion of imaging time spent moving by all fetuses decreases significantly with increasing GA (r = −0.514, P = .001).
Fig 5.
Graph shows the median frequency of movements in fetuses grouped by age (± interquartile range). There is a significant decrease in the rate of leg movements between fetuses younger than GA 25 weeks and those older than GA 31 weeks (P = .015). There are no statistically significant differences in the median rates of head, arm, or trunk movements between age groups (P = .746, P = .310, and P = .397, respectively).
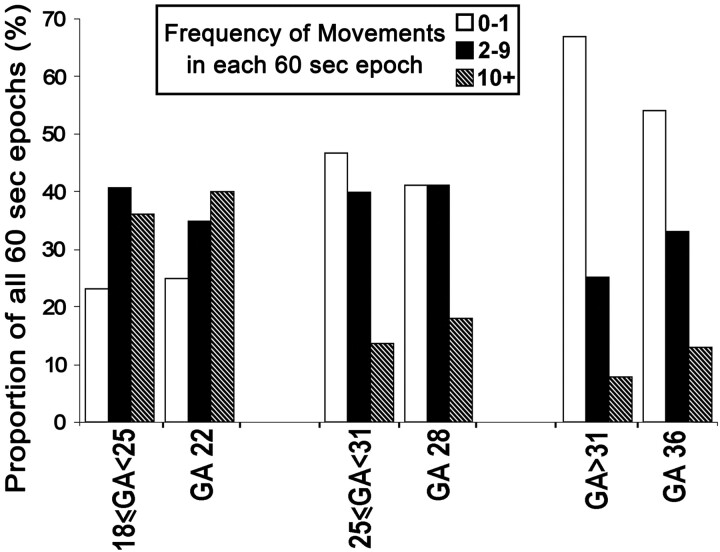
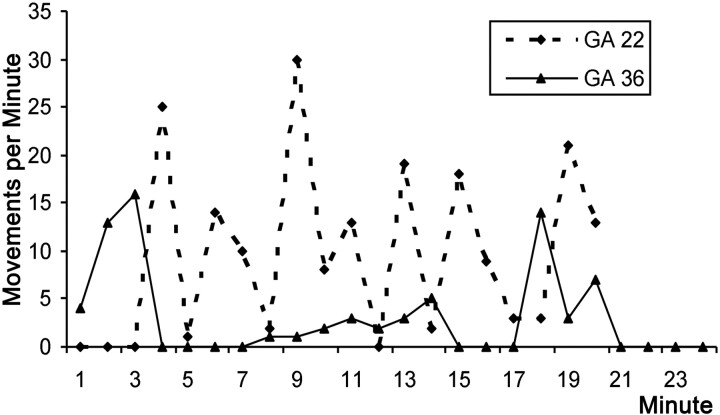
Despite the practical and logistical difficulties involved with acquiring prolonged cine data, we scanned 3 fetuses of GA 22, 28, and 36 weeks for 20, 20, and 24 minutes, respectively. Each fetus showed evidence of being in an active awake state during the observational period,24 based on the presence of gross body and eye movements, with each demonstrating 3 levels of activity: 1) vigorous movements involving the whole body, 2) low intensity/single-limb movements, and 3) complete inactivity (Figs 6 and 7). For each fetus, we calculated the fraction of 60-second epochs that contained each of these activity levels and compared these to the fraction of each activity level in 60-second blocks observed in the cine clips for all other fetuses of corresponding ages (Fig 6). The results showed that the proportions of each activity level for the 3 long acquisitions were similar to those found in their respective age groups. The overall behavior pattern was notably different for the youngest fetuses (reference age, GA 22 weeks), which showed almost continuous but variable levels of activity, whereas at GA 36 weeks, motility appears structured in bursts of activity and inactivity lasting 3–4 minutes (Fig 7).
Fig 6.
Graph shows overall activity levels in long-duration cine acquisitions compared with pooled shorter duration data from the corresponding age groups. The results show that there are similar proportions of 60-second epochs that contain each level of motility between each age group and the corresponding long acquisition.
Fig 7.
Graph shows activity levels during 20 minutes. Data from the long cine acquisitions from the fetuses at GA 22 (dashed line) and GA 36 (solid line) are shown. There are notable differences in the maximum frequency of movements. Also the behavior of the GA 36 weeks' fetus appears more ordered with more clearly defined periods of activity and inactivity than the GA 22 weeks' fetus.
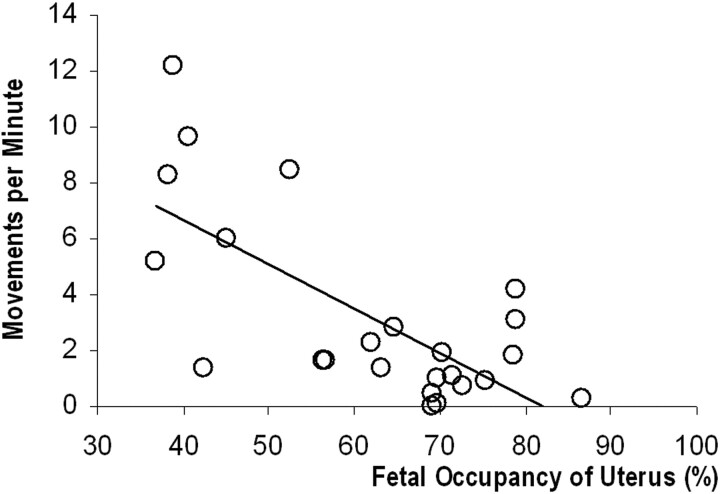
The inter-rater variability for quantification of intrauterine compartments was 0.987 (ICC). The data showed a linear trend for both uterine (r = 0.8596, P < .0001) and fetal (r = 0.907, P < .0001) growth during the latter half of gestation. The amniotic fluid volume, however, showed no significant change across this period (r = 0.312, P = .138). In a subset of 24 neurologically healthy fetuses, we found a statistically significant decrease in movement frequency with increased volumetric occupancy of the uterus (r = −0.703, P = .0001; Fig 8).
Fig 8.
Graph of movement frequency in a subset of 24 healthy fetuses shows a significant reduction as the proportion of uterine volume that the fetus occupies increases (r = −0.703, P = .0001).
Discussion
The assessment of fetal motor behavior may play an important role in the fetal neurologic examination; however, it has been difficult to assess movements during the third trimester by using US because of inadequate spatial coverage.25–27 We optimized an MR imaging sequence to capture fetal movements throughout the whole body from GA 18 weeks to term. We observed a consistent repertoire of movements throughout this GA range. The frequency of all movement patterns decreased with GA; quantification of the frequency of individual movement patterns suggests that the age-related decrease was associated with fewer lower limb movements. Quantification of the intrauterine volume shows that the fetus/uterus ratio doubles across this GA range, and this may, in part, explain the difference in the quantity and quality of movements.
Implication of Findings
Three issues must be addressed to achieve an effective MR imaging capability to study fetal movements: controlling image artifacts, maintaining an appropriate temporal resolution, and capturing the whole anatomy at all GAs. We will deal with each of these in turn: First, planning acquisitions solely from the fetus frequently wraps maternal anatomy over the region of interest, highlighting the need to consider the surrounding tissue during scan piloting. With the fetus often positioned obliquely and the mother in a lateral tilt, it is difficult to conceptualize the relationship between their respective orientations. We found that unambiguous orthogonal maternal pilots referenced against the fetal pilot were effective in ensuring that the FOV borders were clear of anatomy, with each cine acquisition preventing phase-wrap artifacts (Fig 1D–F). Second, prior MR imaging studies13,14 have used similar section-acquisition times, which have been criticized for the low frame rates. However, after comparing with edited video simulating cine data, we are confident that 3 fps are sufficient for capturing fetal movement patterns because there was no loss of detectable neonatal movements compared with a 25-fps video. Third, we found that 30- to 40-mm sections coupled with multisection imaging (Fig 1A–C) allowed sufficient spatial coverage to capture all of the fetal limbs together with the head and trunk while also providing a detailed view of fine movements in the extremities at all GAs and helped to unambiguously track limbs as they moved in 3D space. This is 3–4 times thicker than the 10-mm sections used by Guo et al,13 which can result in restricted views of the fetus and make movements in the through-plane direction difficult to observe.
Fetal US studies have shown a reduction in movement frequency with gestation in both healthy and unhealthy fetuses.21,27 The exact values differ between studies but range from 16% to 17% of imaging time at GA 24 weeks and 7%–11% near term; however, a detailed comparison is hampered by differences in the observational methodology. Our results showed the same trend, but the movement frequency was 30% at GA 24 weeks and 4% at GA 36 weeks (Fig 4). It was hypothesized that the decrease in movement frequency could be, in part, attributable to decreased intrauterine space,25 with the establishment of behavior states after GA 30 weeks being a separate contributory factor.28
We found a statistically significant decrease in movement frequency with increased volumetric occupancy of the uterus (Fig 8), such that near term, the fetus occupied approximately 90% of the intrauterine volume, double that at GA 20 weeks. In addition, the small variations in amniotic fluid volume present across our cohort correspond to normograms published previously.29 With cine MR imaging, we observed that fetuses are unable to easily change from a flexed to an extended posture or vice versa from approximately GA 28 weeks, likely due to global constraints imposed by the uterus (Fig 2D−F). Also, we did not observe the uterus stretching under the pressure of fetal kicking and so conclude that near-term fetuses are not as free to move as is observed earlier in gestation.
There are several published studies that imply that movements near term are performed as freely as in the first trimester with no difference compared with ex utero infants.1,6,30 However, we have found that the quality of movements is affected by spatial constraints, as can be observed in near-term fetuses that perform movements with a reduced amplitude. Furthermore, the intrauterine environment may be affecting movement quantity. Our data show a significant reduction in lower limb movement from GA 30 weeks (Fig 5), which is associated with a high degree of flexion at the hip and knee joints (Figs 1D–F and 3C). Interestingly, neonatal studies have found that kick frequency in full-term infants is half that of preterm infants for the first 3 months post-term-corrected age,31 after which it equalizes. Furthermore, Heriza32 found that full-term infants are more flexed in their lower limbs than preterm infants at term-equivalent age, indicated by smaller joint angles in the knees and ankles. This author further suggested that a quantitative approach to motion highlights differences in motor development that are not identified by the method of Einspieler and Prechtl,1 and that environmental influences are likely to play a role.33 It may be that the differences in amplitude and frequency of leg movements found in preterm infants are the result of exposure to a different set of constraints, whereas their in utero counterparts are experiencing specific restrictions as shown by the reduction we found in fetal leg movements (Fig 5).
A number of studies have examined preference postures for different sections of the fetal anatomy, particularly with regard to the location of the fetal hands, and it is believed that frequent movement toward the face is indicative of a neurodevelopmental process near term and may have significance as a clinical marker for normal development.30,34 With MR imaging providing a fuller appreciation of the intrauterine environment, we observed that the fetal posture appears to facilitate the adduction of the arms toward the midline and the flexion of the hands toward the face across the age range studied (Fig 3A,-B). It is difficult to quantify the effect that the shape of the uterus has on determining this posture, though coronal sections through the fetal body show that from GA 20 weeks, there is negligible amniotic fluid on its lateral aspects (Fig 3A), with collections located mostly anterior and posterior to the fetus (Fig 3B). Furthermore, studies have shown that placing preterm infants in a nest facilitates midline-directed movements by the hand, which allow higher rates of hand-to-hand and hand-to-face contact.35
Limitations
It is still not possible to image the fetal anatomy in its absolute entirety at each time point. This is directly related to the section-acquisition rate, which currently permits a practical maximum multisection factor of 3, above which movement-containing frames are missed, as seen in both cine data and the edited neonatal videos. Our compromise inclines toward maintaining a high temporal resolution in preference to more complete anatomic coverage, because this allows more accurate assessment of each movement visualized and is important for preserving the capability to assess movement fluidity.
The maximum duration of cine acquisitions was limited by departmental protocol, which allows 60 minutes total scanning time, within which other sequences must be performed. Thus the zero-valued data points on Fig 4 are a reflection of the limited observational window because periods of inactivity are part of normal fetal behavior at all GAs. Our total imaging duration per fetus is shorter than that used to observe neonates4 and in fetal US studies.5,6 We used clips of 2–4 minutes, which allowed time to repilot in case of changes in fetal orientation. Our aim was to acquire 10 minutes of cine data per fetus, though actual median scanning duration was 7 minutes (range, 2–13 minutes). We have attempted to compensate for this by performing group analyses for the rate of fetal movements and acquiring significantly longer and continuous cine acquisitions in a representative subject from each age group to provide a reference for overall trends in behavior. A comparison in overall levels of activity between the longer acquisitions and the corresponding age group shows high concordance, suggesting that our grouped data are representative of true behavior patterns (Fig 6), with the caveat that data from a single subject are not necessarily typical of that fetus's full movement repertoire in terms of the range of movements performed and the proportion of imaging time spent in each activity state.
Conclusions
In summary, we have developed and validated a fetal MR imaging protocol and used it to observe the full range of fetal movements. We have shown that cine MR imaging is an effective method for monitoring and quantifying movements in late gestation with near full-body coverage. A comparison with criterion standard neonatal video data has illustrated that the temporal resolution of cine MR imaging data is sufficient to accurately analyze fetal movements. The nature of the intrauterine environment is likely to be an important factor, which imposes both global and localized spatial restrictions on fetal movements. This again leads to the question of how early neuromotor development may be modulated by sensory and proprioceptive feedback mechanisms. Our observations may contribute to informing future studies of the fetus that take this restrictive environment into account and suggest that added insight may be gained from studying the fetus within its own paradigm as distinct from that of the preterm infant.
Abbreviations
- bSSFP
balanced steady-state free precession
- fps
frames per second
- GA
gestational age
- GM
general movements
- ICC
intraclass correlation coefficient
- SAR
specific absorption rate
- SENSE
sensitivity encoding
- SNR
signal-to-noise ratio
- US
ultrasound
- W
watts
Footnotes
Funding was provided by Medical Research Council, United Kingdom.
Paper previously presented by T. Hayat, J.M. Allsop, A. McGuinness, F. Ferrari, M.A. Rutherford, J.V. Hajnal at: Proceedings of the International Society for Magnetic Resonance in Medicine, April 17, 2009; Honolulu, Hawaii; and by T. Hayat, J.M. Allsop, M.A. Rutherford, J.V. Hajnal at: Second International Congress on Fetal MRI, May 15–18, 2008; Vienna, Austria.
References
- 1. Einspieler C, Prechtl HF. Prechtl's assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment Retard Dev Disabil Res Rev 2005;11:61–67 [DOI] [PubMed] [Google Scholar]
- 2. Spittle AJ, Doyle LW, Boyd RN. A systematic review of the clinimetric properties of neuromotor assessments for preterm infants during the first year of life. Dev Med Child Neurol 2008;50:254–66. Epub 2008 Jan 7 [DOI] [PubMed] [Google Scholar]
- 3. Murney ME, Campbell SK. The ecological relevance of the Test of Infant Motor Performance elicited scale items. Phys Ther 1998;78:479–89 [DOI] [PubMed] [Google Scholar]
- 4. Cioni G, Ferrari F, Einspieler C, et al. Comparison between observation of spontaneous movements and neurologic examination in preterm infants. J Pediatr 1997;130:704–11 [DOI] [PubMed] [Google Scholar]
- 5. de Vries JI, Visser GH, Prechtl HF. The emergence of fetal behaviour. II. Quantitative aspects. Early Hum Dev 1985;12:99–120 [DOI] [PubMed] [Google Scholar]
- 6. Kurjak A, Miskovic B, Stanojevic M, et al. New scoring system for fetal neurobehavior assessed by three- and four-dimensional sonography. J Perinat Med 2008;36:73–81 [DOI] [PubMed] [Google Scholar]
- 7. Sival DA, Visser GH, Prechtl HF. The effect of intrauterine growth retardation on the quality of general movements in the human fetus. Early Hum Dev 1992;28:119–32 [DOI] [PubMed] [Google Scholar]
- 8. Visser GH, Laurini RN, de Vries JI, et al. Abnormal motor behaviour in anencephalic fetuses. Early Hum Dev 1985;12:173–82 [DOI] [PubMed] [Google Scholar]
- 9. Hooker D. Development reaction to environment. Yale J Biol Med 1960;32:431–40 [PMC free article] [PubMed] [Google Scholar]
- 10. Sarnat HB. Functions of the corticospinal and corticobulbar tracts in the human newborn. J Pediatr Neurol 2003;1:3–8 [DOI] [PubMed] [Google Scholar]
- 11. Smotherman WP, Robinson SR. Environmental determinants of behaviour in the rat fetus. Anim Behav 1986;34:1859–73 [Google Scholar]
- 12. Anquez J, Angelini E, Bloch I, et al. Interest of the steady state free precession (SSFP) sequence for 3D modeling of the whole fetus. Conf Proc IEEE Eng Med Biol Soc 2007;2007:771–74 [DOI] [PubMed] [Google Scholar]
- 13. Guo WY, Ono S, Oi S, et al. Dynamic motion analysis of fetuses with central nervous system disorders by cine magnetic resonance imaging using fast imaging employing steady-state acquisition and parallel imaging: a preliminary result. J Neurosurg 2006;105:94–100 [DOI] [PubMed] [Google Scholar]
- 14. Shen SH, Guo WY, Hung JH. Two-dimensional fast imaging employing steady-state acquisition (FIESTA) cine acquisition of fetal non-central nervous system abnormalities. J Magn Reson Imaging 2007;26:672–77 [DOI] [PubMed] [Google Scholar]
- 15. Chung HW, Chen CY, Zimmerman RA, et al. T2-weighted fast MR imaging with true FISP versus HASTE: comparative efficacy in the evaluation of normal fetal brain maturation. AJR Am J Roentgenol 2000;175:1375–80 [DOI] [PubMed] [Google Scholar]
- 16. Scheffler K, Lehnhardt S. Principles and applications of balanced SSFP techniques. Eur Radiol 2003;13:2409–18 [DOI] [PubMed] [Google Scholar]
- 17. Carr JC, Simonetti O, Bundy J, et al. Cine MR angiography of the heart with segmented true fast imaging with steady-state precession. Radiology 2001;219:828–34 [DOI] [PubMed] [Google Scholar]
- 18. Pruessmann KP, Weiger M, Scheidegger MB, et al. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999;42:952–62 [PubMed] [Google Scholar]
- 19. International Electrotechnical Commission (IEC). 2008. Medical electrical equipment: part 2-33. Particular requirements for the safety of magnetic resonance equipment for medical diagnosis. IEC 60601-2-33 ed 2.2. (Geneva, Switzerland: IEC; ) Consolidated with amendments 1 and 2. [Google Scholar]
- 20. Prayer D, Kasprian G, Krampl E, et al. MRI of normal fetal brain development. Eur J Radiol 2006;57:199–216 [DOI] [PubMed] [Google Scholar]
- 21. Roodenburg PJ, Wladimiroff JW, van Es A, et al. Classification and quantitative aspects of fetal movements during the second half of normal pregnancy. Early Hum Dev 1991;25:19–35 [DOI] [PubMed] [Google Scholar]
- 22. Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol 2000;12:191–200 [DOI] [PubMed] [Google Scholar]
- 23. Stadler A, Schima W, Ba-Ssalamah A, et al. Artifacts in body MR imaging: their appearance and how to eliminate them. Eur Radiol 2007;17:1242–55 [DOI] [PubMed] [Google Scholar]
- 24. Romanini C, Rizzo G. Fetal behaviour in normal and compromised fetuses: an overview. Early Hum Dev 1995;43:117–31 [DOI] [PubMed] [Google Scholar]
- 25. D'Elia A, Pighetti M, Moccia G, et al. Spontaneous motor activity in normal fetuses. Early Hum Dev 2001;65:139–47 [DOI] [PubMed] [Google Scholar]
- 26. Sival DA, Prechtl HF, Sonder GH, et al. The effect of intra-uterine breech position on postnatal motor functions of the lower limbs. Early Hum Dev 1993;32:161–76 [DOI] [PubMed] [Google Scholar]
- 27. Ten Hof J, Nijhuis IJ, Mulder EJ, et al. Longitudinal study of fetal body movements: nomograms, intrafetal consistency, and relationship with episodes of heart rate patterns A and B. Pediatr Res 2002;52:568–75 [DOI] [PubMed] [Google Scholar]
- 28. Nijhuis JG. Fetal behavior. Neurobiol Aging 2003;24(suppl 1):S41–46, discussion S47–49, S51–42 [DOI] [PubMed] [Google Scholar]
- 29. Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol 2005;25:341–48 [DOI] [PubMed] [Google Scholar]
- 30. Kurjak A, Azumendi G, Vecek N, et al. Fetal hand movements and facial expression in normal pregnancy studied by four-dimensional sonography. J Perinat Med 2003;31:496–508 [DOI] [PubMed] [Google Scholar]
- 31. Geerdink JJ, Hopkins B, Beek WJ, et al. The organization of leg movements in preterm and full-term infants after term age. Dev Psychobiol 1996;29:335–51 [DOI] [PubMed] [Google Scholar]
- 32. Heriza CB. Comparison of leg movements in preterm infants at term with healthy full-term infants. Phys Ther 1988;68:1687–93 [DOI] [PubMed] [Google Scholar]
- 33. Piek JP. Is a quantitative approach useful in the comparison of spontaneous movements in fullterm and preterm infants? Hum Mov Sci 2001;20:717–36 [DOI] [PubMed] [Google Scholar]
- 34. Ververs IA, Van Gelder-Hasker MR, De Vries JI, et al. Prenatal development of arm posture. Early Hum Dev 1998;51:61–70 [DOI] [PubMed] [Google Scholar]
- 35. Ferrari F, Bertoncelli N, Gallo C, et al. Posture and movement in healthy preterm infants in supine position in and outside the nest. Arch Dis Child Fetal Neonatal Ed 2007;92:F386–90 [DOI] [PMC free article] [PubMed] [Google Scholar]