Abstract
BACKGROUND AND PURPOSE:
The use of self-expanding retrievable stents is an emerging and promising treatment strategy for acute stroke treatment. The concept combines the advantages of stent deployment with immediate flow-restoration and of mechanical thrombectomy with definitive thrombus removal. The present study was performed to gain more knowledge about the principle of combined flow restoration and thrombectomy in an established animal model using radiopaque thrombi evaluating efficiency, thrombus-device interaction and possible complications of the first dedicated flow-restoration and mechanical thrombectomy device.
MATERIALS AND METHODS:
The Solitaire FR (4 × 20 mm) was evaluated in 15 vessel occlusions in an established animal model in swine. Flow-restoration effect at T0, T5, and T10; recanalization rate after retrieval; thromboembolic events; and complications were assessed. Radiopaque thrombi (10-mm length) were used for visualization of thrombus-device interaction during application and retrieval.
RESULTS:
Immediate flow restoration was achieved in 80% of occlusions. Mean percentage of recanalization compared with the initial vessel diameter at T0 was 30.8%; at T5, 30.7%; and at T10, 25.4%. Re-occlusion occurred in 20.0% between T0 and T5 and in 13.3% between T5 and T10. Complete recanalization (TICI 3) after retrieval was achieved in 86.7%. In 2 cases (13.3%), partial recanalization was achieved, with the remaining thrombus in a side branch (TICI 2b). No thromboembolic event was observed. The assessment of thrombus-device interaction illustrated the compression of the thrombus against the vessel wall during deployment leading to partial flow restoration. During retrieval, the thrombus was retained by the stent struts even during the passage of vessel curvatures.
CONCLUSIONS:
The Solitaire FR is a safe and effective combined flow-restoration and thrombectomy device in vivo. Partial flow restoration is achieved by thrombus compression immediately after deployment, but flow restoration decreases afterward until final retrieval results in maximal recanalization.
The use of self-expanding intracranial stents is an emerging treatment option for acute stroke.1–7 With the introduction of reconstrainable stent systems, the concept of the temporary endovascular bypass technique emerged.8 Combining the advantages of temporary stent placement with immediate flow restoration without the need for permanent implantation and thrombectomy with definitive thrombus removal is a new promising treatment concept for acute ischemic stroke.8–11
The Solitaire FR Revascularization Device (ev3, Irvine, California) is the first dedicated combined flow-restoration and thrombectomy device for acute stroke treatment. However to date, to our knowledge, no systematic evaluation of this concept and of this novel device has been published.
The present study was performed to gain more knowledge about the usability of the principle of combined flow restoration and thrombectomy for acute stroke treatment in an established animal model by using radiopaque thrombi.12 We evaluated the Solitaire FR regarding the immediate flow-restoration effect, percentage of recanalization compared with the initial vessel diameter with time, thrombectomy efficiency, thrombus-device interaction, and potential complications.
Materials and Methods
Revascularization Device/Application
The Solitaire FR Revascularization Device (CE marked since July 2009) is a laser-cut self-expanding split-design nitinol stent device with a closed-cell pattern that is firmly molded to a nitinol pusher wire (Fig 1). It is available with a diameter of 4 mm (length, 15 or 20 mm) or 6 mm (length, 20 or 30 mm) and is delivered through a 0.021- or 0.027-inch microcatheter. A working length of at least the length of the thrombus is generally recommended. The use of an 8F-9F balloon-guide catheter for proximal flow arrest during retrieval is recommended. In this study, a 9F balloon-guide catheter (Merci Balloon Guide Catheter; Concentric Medical, Mountain View, California) was used. Equipped with a microwire (SilverSpeed 0.014; ev3), the microcatheter (Rebar-18; ev3) was navigated into the occluded vessel, the thrombus was passed, and the Solitaire FR was inserted. Only the Solitaire FR 4 × 20 mm was used in this study. At any point in the deployment procedure, the device can be re-sheathed by pulling it back into the microcatheter even after complete deployment. Finally, the balloon-guide catheter was inflated to create flow arrest, and both the device and the microcatheter were pulled back simultaneously. During retrieval, continuous aspiration was applied at the balloon-guide catheter by using a 60-mL syringe to avoid embolization of thrombus fragments.
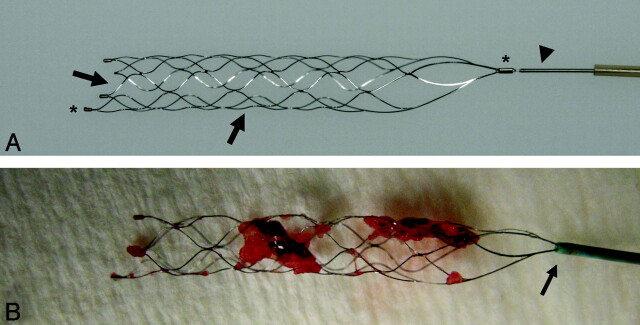
Fig 1.
A, Photograph of the Solitaire FR Revascularization Device after complete deployment. The stent device (arrows) is firmly molded to a nitinol pusher wire (arrowhead). B, Radiopaque markers at the proximal and distal end (asterisk). The Solitaire FR after successful retrieval. Note the encasement of the thrombus between the stent struts and the partial re-sheathing of the stent by advancing the microcatheter just over the proximal marker before retrieval (arrow).
Animal Care
All procedures were conducted according to international guidelines and were approved by the responsible local authorities. Three swine with a weight of 45 kg each were used in this study. With the swine under general anesthesia, we introduced a 45-cm-long 10F catheter sheath (Super Arrow-Flex PSI Set; Arrow International, Reading, Pennsylvania) in the common femoral artery and continuously flushed it with heparinized saline (10 U/mL) without any further heparin bolus. At the end of this surgical procedure, 500-mg acetylsalicylic acid was administered intravenously. Further details were described previously.12–14
Thrombus Preparation
Thrombus preparation and application were described in previous studies on this animal model.12,13,15,16 In brief, thrombi were created by mixing 10-mL autologous blood of the animal with 1-g barium sulfate and 0.25-mL bovine thrombin solution (Dade Behring, Newark, New Jersey). The material was incubated for 1 hour at room temperature and cut into thrombi of 10-mm lengths, which were injected into the target vessel. Due to the radiopacity gained by the added barium sulfate, the thrombus was visualized during angiography.
Experimental Design and Angiographic Evaluation
The study was performed on a biplane high-resolution angiography system (Axiom Artis zee; Siemens, Erlangen, Germany). Selective occlusion of the IMA and LA was performed, which reproduced the anatomic setting of an occlusion of the middle cerebral artery and the basilar artery in the human circulation.12 For selective thromboembolization, the preformed thrombus was injected into a 7F guide catheter, Guider Softip (Boston Scientific, Fremont, California), positioned in the target vessel. After thrombus application, the guide catheter was removed for 10 minutes to restore arterial flow and to allow thrombus embedding.
After deployment of the device, we used immediate flow-restoration effect and percentage of recanalization was compared with the initial vessel diameter by repeated control angiographies: T0, T5, and T10. Finally, the device was retrieved to evaluate mechanical thrombectomy efficiency. The application time for each attempt was recorded, including thrombus passage, device deployment, placement of the balloon catheter, and retrieval. We documented all maneuvers digitally, assessing thrombus movement, fragmentation, and possible distal dislocation during the passing procedure and during deployment. The control angiographies between T0 and T10 were evaluated regarding possible thrombus embolization or fragmentation with time with the device in place. During retrieval, thrombus fragmentation, peripheral embolization, loss of thrombotic material at the tip of the balloon guide catheter, feasibility, and technical failure were assessed. Follow-up angiographies were performed immediately and 1 hour after retrieval to evaluate recanalization rate, vasospasm, and angiographic signs of vessel dissection or perforation.
Assessment of Vessel Morphology
The recanalization rate of the target vessel by using the TICI grading scale (0–3) was evaluated.17 Grading of the vasospasm was as follows18: 0 = no narrowing; 1 = slight narrowing (<25% reduction in lumen diameter), 2 = moderate narrowing (25% to 50% stenosis), and 3 = severe narrowing (50% to 75% stenosis affecting a long segment of vessel or any stenosis >75%).
Results
A total of 15 recanalization attempts were performed on 15 vessel occlusions (IMA = 8, LA = 7). Mean vessel diameter was 2.1 ± 0.3 mm (range, 1.7–2.8 mm; median, 2.1 mm).
Immediate Flow Restoration
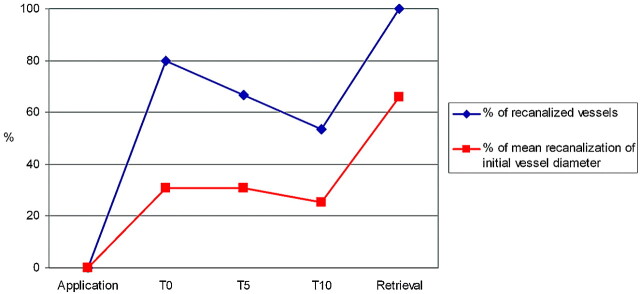
Immediate flow restoration after deployment was achieved in 80.0% (12/15) of vessel occlusions. The mean percentage of recanalization compared with the initial vessel diameter was 30.8% (range, 0%–53.7%) for T0, 30.7% (range, 0%–65.5%) for T5, and 25.4% (range, 0%–66.4%) for T10. The percentage of recanalization increased between T0 and T5 in 40.0% (6/15) and between T5 and T10 in 13.3% (2/15) of the attempts. Re-occlusion occurred in 20.0% (3/15) between T0 and T5 and in 13.3% (2/15) between T5 and T10. In all cases of re-occlusions between T0 and T5, the initial percentage of recanalization had been <30%. On the other hand, when the percentage of recanalization increased between T0 and T5, the initial percentage of recanalization had been >30% of the initial vessel diameter. No flow restoration between T0 and T10 occurred in 13.3% (2/15). The percentage of recanalized vessels and the percentage of recanalization compared with the initial vessel diameter at different time points of the study interval are summarized in the Table and Fig 2.
Percentage of recanalization compared with the initial vessel diameter per attempt at different time points of the study interval
| Attempt No. | Percentage of Recanalization Compared with Initial Vessel Diameter (%) |
||
|---|---|---|---|
| T0 | T5 | T10 | |
| 1 | 53.7 | 65.5 | 61.1 |
| 2 | 26.9 | 0.00 | 0.00 |
| 3 | 0.00 | 0.00 | 0.00 |
| 4 | 0.00 | 0.00 | 0.00 |
| 5 | 21.8 | 0.00 | 0.00 |
| 6 | 24.5 | 0.00 | 0.00 |
| 7 | 37.3 | 34.8 | 26.5 |
| 8 | 46.5 | 32.2 | 0.00 |
| 9 | 33.8 | 57.2 | 54.7 |
| 10 | 0.00 | 16.4 | 66.4 |
| 11 | 35.9 | 42.1 | 42.1 |
| 12 | 44.6 | 59.6 | 0.00 |
| 13 | 34.3 | 45.9 | 31.4 |
| 14 | 49.1 | 47.9 | 49.1 |
| 15 | 53.6 | 58.7 | 48.9 |
| Mean recanalization rate (%) | 30.8 ± 18.0 | 30.7 ± 24.7 | 25.4 ± 25.5 |
Fig 2.
Graph showing percentages of recanalized vessels (blue) and the mean percentage of recanalization compared with the initial vessel diameter (red) at different time points of the study interval.
Thrombus Retrieval
Complete recanalization (TICI 3) after retrieval was achieved in 86.7% (13/15). In 2 cases (13.3%), partial recanalization was achieved, with the remaining thrombus located in a side branch (TICI 2b). The mean application time was 15 minutes (range, 9–27 minutes). Vasospasm rate was grade 0 in 6.7% (1/15), grade 1 in 40.0% (6/15), grade 2 in 13.3% (2/15), and grade 3 in 40.0% (6/15; mean, 1.9). At follow-up angiography after 1 hour, vasospasms usually had resolved, allowing assessment of the final recanalization result.
Complications
No distal thromboembolic events into the target vessel or previously unaffected vessel territories occurred during passing of the thrombus, deployment, and retrieval of the device. Evaluation of the control angiographies between T0 and T10 showed no signs of thrombus embolization or fragmentation with time, with the device remaining in place. Signs of neither vessel perforation nor vessel dissection were found at control angiography. No device fracture occurred during the study.
Thrombus-Device Interaction
Passing of the thrombus was possible in all cases, did not lead to dislocation or fragmentation of the thrombus, and always occurred between the vessel wall and the thrombus, without penetration of the clot itself.
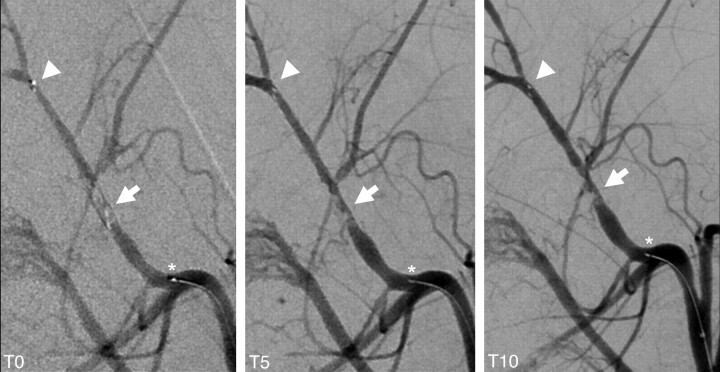
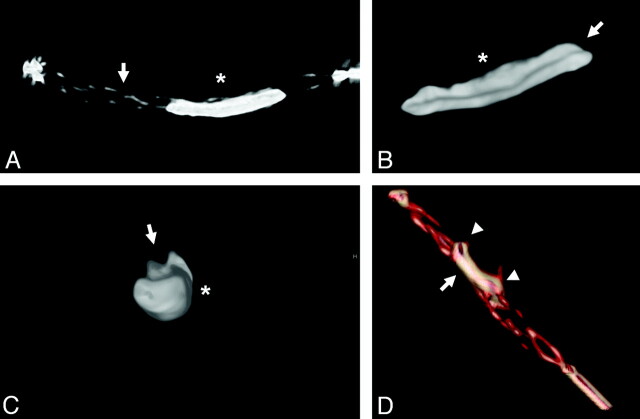
Assessment of thrombus-device interaction during deployment revealed immediate expansion of the device, resulting in compression of the thrombus against the contralateral vessel wall, leading to immediate partial flow restoration in most cases. Indentation of the thrombus by the stent struts was typically seen as a tubercular or bumpy thrombus surface reflecting the honeycomb-like stent pattern on conventional angiography (Fig 3). 3D reconstructions of a flat panel CT angiogram (DynaCT; Siemens) depicted the compression effect of the stent as a tubular channel in the surface of the opacified thrombus (Fig 4A-C). In case of progressive recanalization with time, increasing expansion of the stent could be visualized not only by the increasing diameter of the reperfused target vessel but also by progressive distension of the distal stent markers during control angiographies (Fig 3).
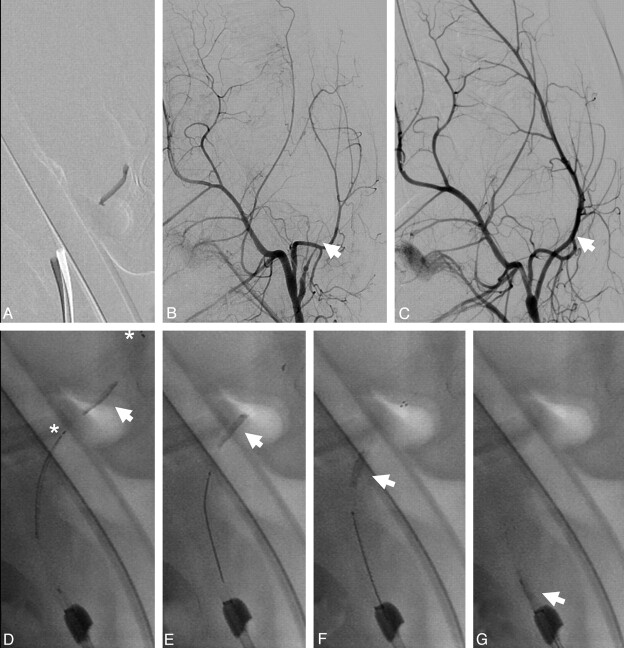
Fig 3.
Control angiogram at T0, T5, and T10 showing the immediate flow-restoration effect at T0 with a progressive percentage of recanalization with time between T0 and T10 (arrow). Increasing expansion of the stent is further depicted by the distension of the distal stent markers (arrowhead; asterisk indicates the proximal stent marker). Note thrombus compression to the contralateral vessel wall of the initial passing and the typically tubercular and bumpy surface pattern of the thrombus caused by indentation of the stent struts.
Fig 4.
A, 3D reconstructions of the flat panel CT angiogram illustrating thrombus-device interaction. Solitaire FR (arrow) and opacified thrombus (asterisk) immediately after deployment. B and C, Depiction of the compression effect of the stent on the opacified thrombus (asterisk) in the longitudinal (B) and axial (C) planes by creating a tubular channel (arrows). D, Note embedding of the thrombus within the stent struts, with parts of the thrombus inside (arrow) and outside of the stent device (arrowheads).
During retrieval of the device, the thrombus remained encased and embedded by the stent struts, with parts of the thrombus located inside and outside the stent (Fig 1B and 4D). During mobilization of the thrombus under proximal vessel occlusion and additional aspiration, the thrombus remained in a stretched position, avoiding significant compression and compaction (Figs 5 and 6).
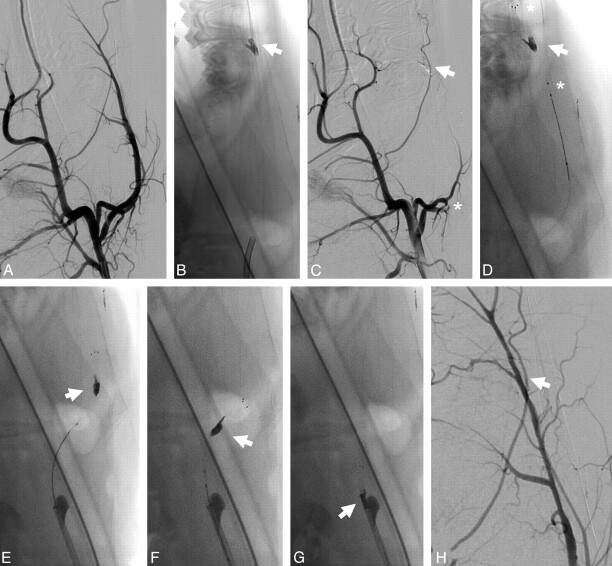
Fig 5.
A, Unsubtracted image of the thrombus application in the LA. B and C, Angiogram depicts complete occlusion of the LA before treatment (B, arrow) and complete recanalization after treatment (C, arrow). D−G, Retrieval procedure of the device from the LA. Note the stretched position of the thrombus during mobilization and retrieval, without significant thrombus compaction or compression (arrow; asterisk indicates the proximal and distal stent markers).
Fig 6.
A, Angiography of the LA before treatment. B, Subtracted image after thrombus application illustrates the position of the thrombus in a bifurcation of the LA. C, Pretreatment angiogram shows complete occlusion of the LA (asterisk) and the position of the thrombus (arrow). D−G, Successful retrieval of the thrombus (arrows) from the bifurcation (asterisk indicates the proximal and distal stent markers). H, Magnified posttreatment angiogram shows complete revascularization and slight residual vasospasm (arrow).
Discussion
Immediate recanalization without repetitive recanalization attempts can be achieved by using self-expanding intracranial stents, with a high technical success rate.1–7 However, permanent stent implantation is associated with major disadvantages, including the need for multiple antiplatelet therapy, which might cause hemorrhagic complications,3,4 and the risk of delayed in-stent stenosis or thrombosis.19
In theory, the retrievable stent combines the advantages of immediate flow restoration and a high recanalization rate achievable by stent application and only temporary use, with subsequent thrombus retrieval achievable by mechanical thrombectomy devices.8–11 Furthermore, final complete removal of the temporary device eliminates the need for aggressive antiplatelet therapy and its potential complications.
However to date, to our knowledge, no systematic evaluation of this treatment principle for acute stroke has been reported.
In the present study, the Solitaire FR was found to be effective for immediate flow restoration and for mechanical thrombectomy, with an overall complete recanalization rate (TICI 3) of 86.7%. Moreover, partial recanalization was achieved in the remaining occlusions (13.3%), and vessel recanalization was achieved in all occlusions with the first attempt. The time to recanalization was short. Besides residual occlusion of local side branches in 2 cases, no complications were noted.
The radial force of the device is sufficient to compress the thrombus against the vessel wall and to restore blood flow immediately after deployment. Consistent with previous experimental findings14 and clinical experience, the thrombus is compressed against the vessel wall opposite the initial passing site. No thrombus fragmentation or dislocation during deployment or expansion of the stent during the observation period with the device in place was observed, indicating sufficient stabilization and coverage of the thrombus by the stent struts. Therefore, the risk of thrombembolism due to thrombus fragmentation seems to be low. However, thrombus formation within the stent has not been observed, but minor thrombus formation may have contributed to the progressive in-stent reocclusion.
Typically, indentation of the thrombus by the stent struts as a tubercular and bumpy thrombus surface could be visualized on conventional angiography (Fig 3). In combination with partial flow restoration, an increasingly accessible thrombus surface is created that might facilitate the thrombolytic effect in case of additional local or systemic thrombolysis.
In this study, immediate flow restoration was achieved in a high number of vessel occlusions. A percentage of recanalization of 30% compared with the initial vessel diameter appeared to be the cutoff of sufficient flow: In cases with an initial percentage of recanalization of at least 30%, no reocclusion occurred within the first 5 minutes. Most interesting, the probability of progressive recanalization decreased with time, especially after 5 minutes, indicating no necessity to leave the stent in place for longer than 5 minutes. Complete recanalization generally resulted after final retrieval. The decrease of the perfused lumen with time might be due to insufficient radial force of the stent. Another explanation might be migration of the thrombus through the stent struts with time. This theory is supported by the indentation of the thrombus by the stent struts that could typically be visualized during the control angiographies. The thrombus propagation into the stent struts might, furthermore, have a positive influence on the friction between device and thrombus and, therefore, on recanalization success.
Remarkably, the Solitaire FR showed a high efficiency as a mechanical thrombectomy device with complete recanalization (TICI 3) in 86.7% of vessel occlusions after retrieval. In 2 cases (13.3%), complete recanalization of the target vessel was achieved, but thrombus remained in a minor side branches (TICI 2b). Previous in vivo studies on distal mechanical thrombectomy have shown a compression of the thrombus during retrieval as a typical finding, leading to an increase of thrombus diameter. This increased diameter causes a higher friction between the thrombus and vessel wall and complicates the retrieval procedures.13,15,16,20 The retention force between the thrombus and the device is applied by the stent struts embedding and encasing the whole length of the thrombus. This application leads to a more distributed transmission of the retention force between thrombus and stent, allowing mobilization of the thrombus-device complex in a rather stable and stretched position without obvious compression (Figs 4–6). Consequently, high recanalization and low complication rates could be achieved.
Apart from a moderate degree of transitory vasospasm, no angiographic signs of severe complications such as vessel dissection and perforation were found. This finding may be due to the limited number of attempts performed in the study or may be biased by the different vessel morphology lacking atherosclerotic changes in the animal model.
Limitations
In the animal model applied in this study, the vessels are less tortuous, are not elongated, have no atherosclerotic changes compared with the human vasculature typically encountered in patients with stroke, and are more prone to vasospasms. Therefore, the navigability and technical feasibility of the procedure might vary in human patients with stroke, and complications such as vessel dissection and perforation might be underestimated.
Although fragmentation or embolization of thrombus material can be directly depicted due to the opacification of the thrombus and the additional postinterventional control angiography indirectly depicts possible distal embolic events, small but potentially significant downstream emboli might not have been visualized and, therefore, could have been missed in this study.
Evaluation of vessel wall injury was restricted to the presence of angiographic signs. Therefore, possible endothelial damage or significant endothelial disruption of the vessel wall that might have occurred during device deployment or retrieval would not have been recognized. Additional histologic analysis of vessel wall injury after the use of stent retrievers would be necessary to evaluate the full extent of possible vessel wall injury.
Different compositions of thrombi are encountered in human patients with stroke. The thrombus used in this animal model is a whole-blood thrombus or “red clot” and conforms to a slightly firm clot. This kind of clot has different mechanical properties compared with harder or softer clots. The generated whole-blood thrombus used in this model might, therefore, not represent the whole spectrum of possible mechanical properties of thrombi found in acute stroke. The composition (eg, fibrin-rich thrombus or “white clot”) might have a significant influence on the compressibility by the stent device and the mechanical stability during the thrombectomy procedure.
Conclusions
In this small animal study of acute arterial occlusion in swine, the Solitaire FR Revascularization Device was effective in providing a means for rapid flow restoration and thrombectomy. Partial flow restoration was achieved by thrombus compression immediately after deployment, with no significant increase with time. Because final retrieval achieves complete or incomplete recanalization, the time interval between deployment and retrieval can be minimized. According to these in vivo findings, the concept of a retrievable stent seems to be a promising straightforward treatment strategy for acute stroke. However, further work is required to establish its safety and effectiveness in humans.
Abbreviations
- FR
Flow-Restoration
- IMA
internal maxillary artery
- LA
lingual artery
- TICI
Thrombolysis in Cerebral Infarction
- T0
flow-restoration effect immediately after deployment
- T5
flow-restoration effect after 5 minutes
- T10
flow-restoration effect after 10 minutes
References
- 1. Sauvageau E, Levy EI. Self-expanding stent-assisted middle cerebral artery recanalization: technical note. Neuroradiology 2006;48:405–08 [DOI] [PubMed] [Google Scholar]
- 2. Levy EI, Ecker RD, Horowitz MB, et al. Stent-assisted intracranial recanalization for acute stroke: early results. Neurosurgery 2006;58:458–63 [DOI] [PubMed] [Google Scholar]
- 3. Levy EI, Mehta R, Gupta R, et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol 2007;28:816–22 [PMC free article] [PubMed] [Google Scholar]
- 4. Zaidat OO, Wolfe T, Hussain SI, et al. Interventional acute ischemic stroke therapy with intracranial self-expanding stent. Stroke 2008;39:2392–95 [DOI] [PubMed] [Google Scholar]
- 5. Chiam PT, Samuelson RM, Mocco J, et al. Navigability trumps all: stenting of acute middle cerebral artery occlusions with a new self-expandable stent. AJNR Am J Neuroradiol 2008;29:1956–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brekenfeld C, Schroth G, Mattle HP, et al. Stent placement in acute cerebral artery occlusion: use of a self-expandable intracranial stent for acute stroke treatment. Stroke 2009;40:847–52 [DOI] [PubMed] [Google Scholar]
- 7. Levy EI, Siddiqui AH, Crumlish A, et al. First Food and Drug Administration-approved prospective trial of primary intracranial stenting for acute stroke: SARIS (stent-assisted recanalization in acute ischemic stroke).Stroke 2009;40:3552–56. Epub 2009 Aug 20 [DOI] [PubMed] [Google Scholar]
- 8. Kelly ME, Furlan AJ, Fiorella D. Recanalization of an acute middle cerebral artery occlusion using a self-expanding, reconstrainable, intracranial microstent as a temporary endovascular bypass. Stroke 2008;39:1770–73 [DOI] [PubMed] [Google Scholar]
- 9. Suh SH, Lee KY, Hong CK, et al. Temporary stenting and retrieval of the self-expandable, intracranial stent in acute middle cerebral artery occlusion. Neuroradiology 2009;51:541–44 [DOI] [PubMed] [Google Scholar]
- 10. Hauck EF, Mocco J, Snyder KV, Levy EI. Temporary endovascular bypass: a novel treatment for acute stroke. AJNR Am J Neuroradiol 2009;30:1532–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castano C, Serena J, Davalos A. Use of the new Solitaire AB Device for mechanical thrombectomy when Merci Clot Retriever has failed to remove the clot: a case report. Interv Neuroradiol 2009;15:209–14. Epub 2009 Sep 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gralla J, Schroth G, Remonda L, et al. A dedicated animal model for mechanical thrombectomy in acute stroke. AJNR Am J Neuroradiol 2006;27:1357–61 [PMC free article] [PubMed] [Google Scholar]
- 13. Brekenfeld C, Schroth G, El Koussy M, et al. Mechanical thromboembolectomy for acute ischemic stroke: comparison of the catch thrombectomy device and the Merci Retriever in vivo. Stroke 2008;39:1213–19 [DOI] [PubMed] [Google Scholar]
- 14. Brekenfeld C, Tinguely P, Schroth G, et al. Percutaneous transluminal angioplasty and stent placement in acute vessel occlusion: evaluation of new methods for interventional stroke treatment. AJNR Am J Neuroradiol 2009;30:1165–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gralla J, Schroth G, Remonda L, et al. Mechanical thrombectomy for acute ischemic stroke: thrombus-device interaction, efficiency, and complications in vivo. Stroke 2006;37:3019–24 [DOI] [PubMed] [Google Scholar]
- 16. Mordasini P, Hiller M, Brekenfeld C, et al. In vivo evaluation of the Phenox CRC Mechanical Thrombectomy device in a swine model of acute vessel occlusion. AJNR Am J Neuroradiol 2010;31:972–78. Epub 2009 Dec 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109–37 [DOI] [PubMed] [Google Scholar]
- 18. Grandin CB, Cosnard G, Hammer F, et al. Vasospasm after subarachnoid hemorrhage: diagnosis with MR angiography. AJNR Am J Neuroradiol 2000;21:1611–17 [PMC free article] [PubMed] [Google Scholar]
- 19. Levy EI, Turk AS, Albuquerque FC, et al. Wingspan in-stent restenosis and thrombosis: incidence, clinical presentation, and management. Neurosurgery 2007;61:644–50 [DOI] [PubMed] [Google Scholar]
- 20. Gralla J, Burkhardt M, Schroth G, et al. Occlusion length is a crucial determinant of efficiency and complication rate in thrombectomy for acute ischemic stroke. AJNR Am J Neuroradiol 2008;29:247–52 [DOI] [PMC free article] [PubMed] [Google Scholar]