Somewhere between 30–50% of patients with middle cerebral artery aneurysms will have intracerebral hematomas while others have intra-Sylvian hematomas (that is, in the subarachnoid space). Apparently the prognosis is different for each type of hematoma, and in patients with ICH it depends on the initial brain damage whereas in those with intra-Sylvian hematoma it is related to secondary ischemia. Thus, initial treatment may be different for each type of presentation. Here, the authors used CT angiography to differentiate between these hematomas and found that enhancing blood vessels clearly identified the hematoma as being intra-Sylvian, and absence of enhancing blood vessels placed it intra-axially.
Abstract
BACKGROUND AND PURPOSE:
ISHs and ICHs from ruptured MCA aneurysms can be difficult to distinguish on NCE-CT but may have a different impact on admission status and outcome. The presence of IHCEV on CTA may differentiate ISHs and ICHs.
MATERIALS AND METHODS:
Two observers independently reviewed non-contrast-enhanced CT scans and CTAs of 71 patients with MCA aneurysm hematomas for the site of the hematoma, according to predefined characteristics, and for the presence of IHCEV. We compared CTAs with NCE-CT scans in which both observers were confident about hematoma localization. We calculated κ statistics for interobserver agreement, and RRs for poor clinical condition and poor outcome.
RESULTS:
Agreement for IHCEV was almost perfect (κ, 0.87; 95% CI, 0.74–0.99). After consensus reading, 30 of 71 patients had IHCEV. In 28 of the 71 NCE-CT scans, both observers were confident as to the the site of the hematoma (κ, 0.55; 95% CI, 37%–73%). IHCEV were present in 10 of these 28 patients, of whom 9 had an ISH based on NCE-CT (positive predictive value, 90%; 95% CI, 55%–100%). In all 18 of 28 patients without IHCEV, the hematoma was not intra-Sylvian (negative predictive value, 100%; 95% CI, 82%–100%). Poor admission status occurred in 50% of patients with IHCEV and in 60% without IHCEV (RR, 1.2; 95% CI, 0.8–1.9). Poor outcome occurred in 63% of patients with IHCEV and in 65% without IHCEV (RR, 1.0; 95% CI, 0.7–1.5).
CONCLUSIONS:
Although CTA could reliably and accurately differentiate the hematoma types, admission status and outcome were similar for both groups.
Hematomas occur in 35%–55% of patients with SAH from a ruptured aneurysm of the MCA and are associated with poor outcome.1,2 These hematomas can be intracerebral or intra-Sylvian, and from published series, it is not always clear whether only genuine ICHs are included or also ISHs. Accordingly, it is unknown whether the relation between the presence of a hematoma and poor prognosis holds true only for ICHs or also for ISHs. Some studies that distinguished ICH and ISH, based on NCE-CT, indeed found a different clinical course. Thus, differentiation of these 2 types of hematomas may help in guiding management strategies for the patients.3–5 However, on NCE-CT scans, ICH and ISH can be difficult to distinguish.6 In a subset of patients, differentiation proved to be possible on the basis of the shape of the hematoma, the presence of edema, and the presence of a small amount of blood surrounding the hematoma, corresponding to the compressed Sylvian fissure.3 CTA may be a more useful tool to discriminate ICH and ISH because CTA can depict vessels in the subarachnoid space. Vessels visible within the hematoma could indicate intra-Sylvian bleeding, and no vessels seen within the hematoma could indicate an ICH.
The purpose of our study was to assess the diagnostic characteristics of IHCEV on CTA and to assess the relation between the presence of IHCEV and the patients' clinical condition and functional outcome.
Materials and Methods
Patients
From a prospectively collected data base of patients with SAH, we retrieved patients admitted between January 2003 and May 2009 with SAHs from ruptured MCA aneurysms.
Imaging Protocol
Imaging studies were performed on a 16- or 64-section spiral CT scanner (Mx8000 LDT; Philips Healthcare, Best, the Netherlands). For the CTA scan, 70 mL of nonionic contrast agent was injected into the cubital vein: 50 mL at a rate of 5 mL/s followed by a 40 mL saline flush at a rate of 4 mL/s.
Image Evaluation
NCE-CT scans were reviewed for hematomas, defined as a blood collection with a diameter ≥1 cm. The hematoma was measured in 3 directions to calculate the volume, by using the formula 4/ 3πabc.7 The NCE-CT scans were independently evaluated by 2 authors to determine hematoma localization on the basis of previously defined criteria.3 Intra-Sylvian localization was based on a bleeding pattern in accordance with the Sylvian fissure. A different bleeding pattern or the presence of a small amount of blood in the compressed Sylvian fissure next to the hematoma was indicative of an ICH. The presence of edema suggested intracerebral localization, though this criterion alone was not conclusive. For each hematoma, the observers reported whether they were confident in determining hematoma type on the basis of non-contrast-enhanced CT. Only NCE-CT scans in which both observers were confident and in consensus about localization were used as reference tests for CTA. Both observers also independently reviewed the CTAs for contrast-enhanced vessels within the hematoma. In case of disagreement, the observers reviewed the scans together to reach con-sensus. If vessels were not visible in a substantial part of the hematoma, the patient was included in the group without IHCEV, irrespective of vessels elsewhere in the hematoma.
Assessment of Baseline Characteristics, Clinical Course, and Outcome
We retrieved data on age, sex, date of hemorrhage, date of admission, neurologic examination, treatment, and discharge time and destination. Neurologic condition on admission was assessed, with poor clinical condition defined as a WFNS score of ≥IV8 and poor outcome at 3 months defined as a GOS score of ≤3.9
Data Analysis
To calculate the level of interobserver agreement, we used κ statistics.10 κ values were categorized as follows: 0, no agreement; 0–0.2, slight agreement; 0.2–0.4, fair agreement; 0.4–0.6, moderate agreement; 0.6–0.8, substantial agreement; 0.8–1.0, almost perfect agreement; and 1, excellent agreement.11 To calculate positive and negative predictive values for vessel enhancement on CTA, we compared CTA with NCE-CT in the subset of patients in whom the observers were confident and agreed about hematoma localization.
For the patient groups with and without contrast-enhanced vessels, we calculated RRs for poor clinical status and focal deficits at admission and for poor outcome at 3 months, with 95% CIs. To assess the influence of hematomas with both an intra-Sylvian and intracerebral component (“combined hematomas”), we performed a sensitivity analysis without these patients.
Results
In total, 148 patients were admitted with ruptured MCA aneurysms during the study period; 80 (54%) had a hematoma on the admission CT, and in 71, a CTA was available for review.
After independent assessment of the non-contrast-enhanced CT scans for hematoma localization, the observers agreed in 54 of 71 patients (κ, 0.55; 95% CI, 37%–73%). The observers were confident and agreed on the site of the hematoma in 28 of the 71 patients. In this subset, in 9 of 10 patients with IHCEV on CTA, the hematoma was indeed localized in the Sylvian fissure on the basis of NCE-CT scans (positive predictive value, 90%; 95% CI, 55%–100%). In 18 of the 18 patients without vessel enhancement, the hematoma was not localized in the Sylvian fissure (NPV, 100%; 95% CI, 82%–100%) but was intracerebral, in agreement with the results of the NCE-CT (Table 1).
Table 1:
CT and CTA findings
| Observer 1 | Observer 2 | Agreement (κ) | PPV/NPV of CTA | |
|---|---|---|---|---|
| NCE-CT (n = 71) | ||||
| Intracerebral hematoma | 46 | 38 | 31 | |
| Inta-Sylvian hematoma | 25 | 33 | 23 | |
| Total | 54 (0.55) | |||
| Confident about localization | 28 (0.85) | |||
| CTA (n = 71) | ||||
| Vessel enhancement | 30 | 32 | 28 | |
| No vessel enhancement | 34 | 32 | 32 | |
| Combined hematoma | 7 | 7 | 7 | |
| Total | 67 (0.87) | |||
| CTA compared with noncontrast CT in subset of “certain” NCE-CT (n = 28) | PPV = 90% NPV = 100% |
After independent reviewing of CTAs for the presence of contrast-enhanced vessels, the observers agreed in 67 of the 71 patients (κ, 0.87; 95% CI, 0.74–0.99). Consensus was reached after the second review in the remaining 4 patients. IHCEV were present in 30 patients, and absent in 34 (Figs 1–4). For 7 patients, the hematoma consisted of both a component with and a component without contrast-enhanced vessels. Hematoma volume was larger in patients without vessel enhancement; otherwise baseline characteristics were comparable (Table 2). Overall, patients with a hematoma of ≥50 mL had a higher risk of death (RR, 2.0; 95% CI, 1.2–3.4) and poor outcome (RR, 1.6; 95% CI, 1.1–2.2). There were no statistically significant differences between patients with and without IHCEV for any of the outcome measures (Table 3). After exclusion of the 7 patients with a combined hematoma, the results were essentially the same.
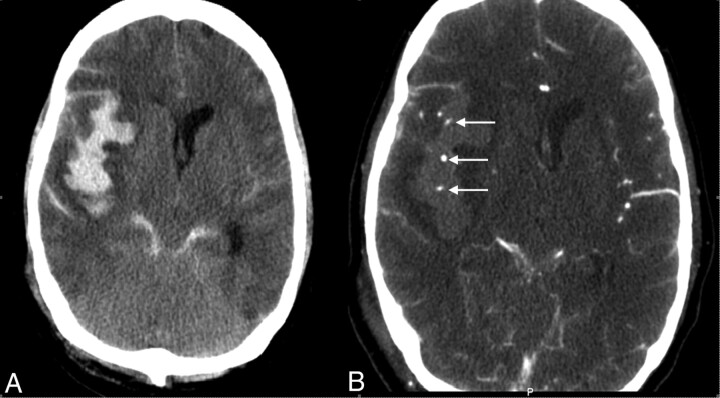
Fig 1.
CTA shows contrast-enhanced vessels in the hematoma, indicated by arrows (right).
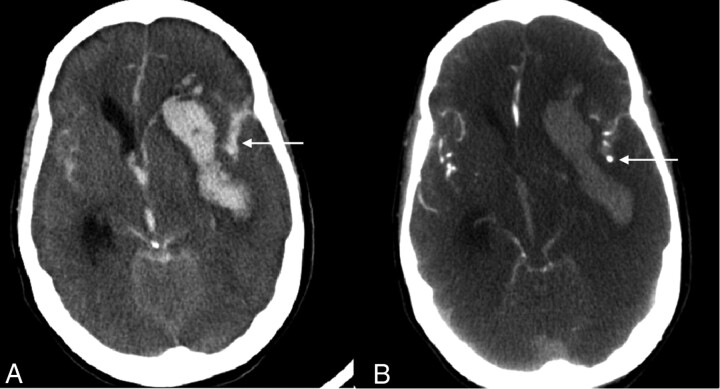
Fig 4.
Comparing CTAs of 2 different patients. CTA shows vessels within the hematoma (arrows, left) and no vessels within the hematoma (right).
Table 2:
Baseline characteristics
| No Vessel Enhancement within Hematoma, Suspect ICH (n=41) | Vessel Enhancement within Hematoma, Suspect ISH (n=30) | |
|---|---|---|
| Sex | ||
| Female | 25 (61%) | 24 (80%) |
| Age | ||
| Mean (range) | 52 (21–75) | 54 (27–70) |
| Aneurysm treatment | ||
| Clip | 22 (54%) | 17 (58%) |
| Coil | 2 (5%) | 2 (7%) |
| None | 17 (42%) | 11 (37%) |
| Largest diameter hematoma (cm) | ||
| Mean (range) | 5.2 (2.0–9.4) | 4.3 (1.8–6.9) |
| Median | 4.8 | 4.5 |
| Volume hematoma (mL) | ||
| Mean (range) | 57.6 (3–248) | 34.4 (2–123) |
| Median | 44.0 | 27.0 |
Table 3:
Clinical course and outcome
| No Vessel Enhancement within Hematoma, Suspect ICH (n=41) | Vessel Enhancement within Hematoma, Suspect ISH (n=30) | RR, No Vessel Enhancement (Suspect ICH) | |
|---|---|---|---|
| Condition on admission | |||
| Good (WFNS I-III) | 16 (40%) | 14 (50%) | |
| Poor (WFNS IV-V) | 24 (60%) | 14 (50%) | 1.2 (95% CI, 0.8–1.8) |
| Not assessablea | 1 | 2 | |
| Focal deficits | |||
| None | 9 (29%) | 10 (41%) | |
| Present | 22 (71%) | 14 (58%) | 1.2 (95% CI, 0.8–1.9) |
| Hemiparesis | 9 | 5 | |
| Dysphasia | 3 | 3 | |
| Other | 10 | 6 | |
| Not assessableb | 10 | 6 | |
| Day of discharge | |||
| Mean (range) | 18 (0–92) | 26 (0–149) | |
| Median | 17 | 19 | |
| Discharge destination | |||
| Nursing home | 2 (4.9%) | 3 (10.0%) | |
| Rehabilitation center | 4 (9.8%) | 4 (13.3%) | |
| Other hospital | 10 (24.4%) | 6 (20.0%) | |
| Home | 6 (14.6%) | 5 (16.7%) | |
| Death | 19 (46.3%) | 12 (40.0%) | 1.2 (95% CI, 0.7–2.0) |
| Outcome, 3 months | |||
| Poor (GOS ≤3) | 26 (65%) | 17 (63%) | 1.0 (95% CI, 0.7–1.5) |
| Good (GOS >3) | 14 (35%) | 7 (37%) | |
| Unknown | 1 | 3 |
Evaluation of coma score not possible as a result of sedation.
Evaluation of focal deficits not possible as a result of poor clinical status.
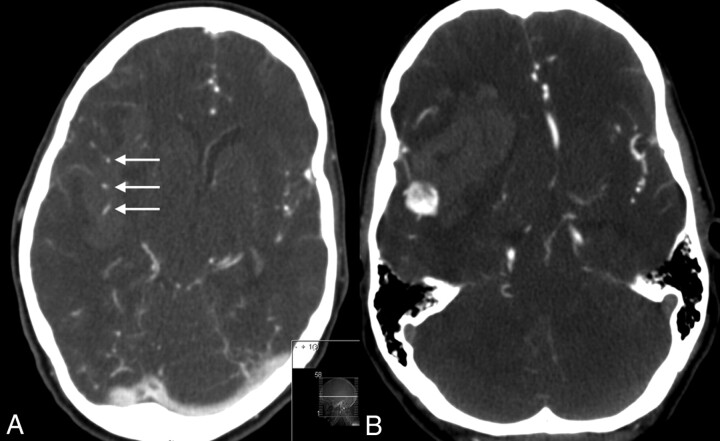
Fig 2.
NCE-CT (left) and CTA (right) of patient B. CTA shows contrast-enhanced vessels in the hematoma (right).
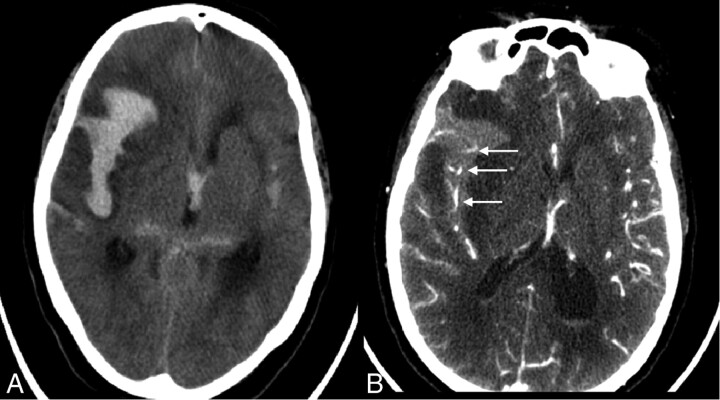
Fig 3.
On noncontrast CT scan of patient C (left), arrow indicates the compressed Sylvian fissure with some subarachnoid blood next to the hematoma (left). CTA of patient C (right) shows no contrast-enhanced vessels in the hematoma, only in the Sylvian fissure as indicated by the arrow (right).
Discussion
In patients with a hematoma from a ruptured MCA aneurysm, CTA can reliably and accurately differentiate ISHs and ICHs. The agreement (κ) for hematoma-location assessment was higher for CTA compared with non-contrast-enhanced CT. However, we found no relation between the presence of intrahematomal vessel enhancement and clinical condition or functional outcome. Despite a larger hematoma volume in the patients without ICHEV, outcome was not better in the group with ICHEV, as we had suspected.
Two explanations are possible for the absence of differences between the 2 groups. First, the presence of contrast-enhancing vessels may be an inadequate tool to discriminate ISH and ICH. In our opinion, several arguments contradict this explanation. Current studies on CTA characteristics of ICH never described obvious contrast-enhanced vessels within the hematoma.12,13 In our 28 patients in whom the NCE-CT scan showed an evident bleeding pattern for either ISH or ICH, vessel enhancement on CTA was nearly always in agreement with this finding. Furthermore, the almost perfect interobserver agreement for CTA assessments suggests a clear radiologic difference. The second explanation would be that ICH and ISH have a similar impact on clinical condition on admission and outcome. ICH in patients with aneurysmal SAH is consistently reported as a poor prognostic factor, though in most series, it is unclear whether ISHs are also included in the ICH group.4,14,15 Two studies that discriminated ICH and ISH by means of NCE-CT found no differences in clinical condition on admission or outcome but did notice differences in clinical course and treatment.3,5 In 1 of these studies, a retrospective study on 92 patients, outcome was determined mostly by initial brain damage in patients with ICH and by secondary ischemia in patients with ISH.3 The second study reported different complication rates after surgery.5
CTA proved to have additional value in discriminating ICH and ISH above assessment on NCE-CT. By means of NCE-CT, we could make a distinction reliably in only one-third of patients, which is in line with previously reported data.3 With CTA, we could differentiate ICH and ISH in almost all patients. We could not find other studies on this distinction based on CTA, thus we cannot compare our results with those from others.
The strength of our study is the availability of systematically and prospectively collected data from 2 equally large groups. The fact that a hematoma volume of ≥50 mL was significantly associated with poor outcome demonstrates that the population is representative and sufficiently large to find associations.
A limitation is the absence of a validated reference test for discrimination between ISH and ICH. In future studies, the current findings should be validated prospectively with intraoperative results. Still, we believe that blood directly surrounding multiple MCAs in the Sylvian fissure is very suggestive and, in some obvious cases, conclusive for the diagnosis of ISH. Contrast extravasation may also cause enhancement in the Sylvian fissure in patients with aneurysms.12,13 Still, we believe that arteries and extravasation of contrast at the Sylvian fissure may be distinguishable on the basis of CTA imaging appearance. On CTA images, the MCA branches in the Sylvian fissure are usually visible as round structures on multiple axial sections. Contrast extravasation usually appears as a blush and not as a small round structure.
Conclusions
The main clinical implication of our study is that CTA can be used to discriminate ISH and ICH from a ruptured MCA hematoma in those patients in whom this difference is not apparent on the NCE-CT scans. In contrast to our hypothesis, this distinction did not affect outcome on a group level, but this result may be influenced by the retrospective design of the study, in which management was not tailored according to the site of the hematoma. Further studies are needed to assess whether indication and timing for surgical treatment should be tailored according to the site of the hematoma.
Abbreviations
- CI
confidence interval
- CTA
CT angiography
- GOS
Glasgow Outcome Scale
- ICH
intracerebral hematoma
- IHCEV
intrahematomal contrast-enhancing vessels
- ISH
intra-Sylvian hematoma
- MCA
middle cerebral artery
- NCE-CT
non-contrast-enhanced CT
- NPV
negative predictive value
- PPV
positive predictive value
- RR
risk ratio
- SAH
subarachnoid hemorrhage
- WFNS
World Federation of Neurological Surgeons grading scale for SAH
References
- 1. Pasqualin A, Bazzan A, Cavazzani P, et al. Intracranial hematomas following aneurysmal rupture: experience with 309 cases. Surg Neurol 1986;25:6–17 [DOI] [PubMed] [Google Scholar]
- 2. Rinne J, Hernesniemi J, Niskanen M, et al. Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery 1996;38:2–11 [DOI] [PubMed] [Google Scholar]
- 3. Yoshimoto Y, Wakai S, Satoh A, et al. Intraparenchymal and intrasylvian haematomas secondary to ruptured middle cerebral artery aneurysms: prognostic factors and therapeutic considerations. Br J Neurosurg 1999;13:18–24 [DOI] [PubMed] [Google Scholar]
- 4. Tokuda Y, Inagawa T, Katoh Y, et al. Intracerebral hematoma in patients with ruptured cerebral aneurysms. Surg Neurol 1995;43:272–77 [DOI] [PubMed] [Google Scholar]
- 5. Shimoda M, Oda S, Mamata Y, et al. Surgical indications in patients with an intracerebral hemorrhage due to ruptured middle cerebral artery aneurysm. J Neurosurg 1997;87:170–75 [DOI] [PubMed] [Google Scholar]
- 6. Silver AJ, Pederson ME, Jr, Ganti SR, et al. CT of subarachnoid hemorrhage due to ruptured aneurysm. AJNR Am J Neuroradiol 1981;2:13–22 [PMC free article] [PubMed] [Google Scholar]
- 7. Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–93 [DOI] [PubMed] [Google Scholar]
- 8. Ogungbo B. The World Federation of Neurological Surgeons scale for subarachnoid haemorrhage. Surg Neurol 2003;59:236–37, discussion 237–38 [DOI] [PubMed] [Google Scholar]
- 9. Jennett B, Snoek J, Bond MR, et al. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry 1981;44:285–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen J. A coefficient of agreement for nominal scales. Education and Psychological Measurement 1960;20:37–46 [Google Scholar]
- 11. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74 [PubMed] [Google Scholar]
- 12. Gazzola S, Aviv RI, Gladstone DJ, et al. Vascular and nonvascular mimics of the CT angiography “spot sign” in patients with secondary intracerebral hemorrhage. Stroke 2008;39:1177–83 [DOI] [PubMed] [Google Scholar]
- 13. Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 2007;38:1257–62 [DOI] [PubMed] [Google Scholar]
- 14. Guresir E, Beck J, Vatter H, et al. Subarachnoid hemorrhage and intracerebral hematoma: incidence, prognostic factors, and outcome. Neurosurgery 2008;63:1088–93, discussion 1093–94 [DOI] [PubMed] [Google Scholar]
- 15. Rosengart AJ, Schultheiss KE, Tolentino J, et al. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007;38:2315–21 [DOI] [PubMed] [Google Scholar]