Abstract
BACKGROUND AND PURPOSE:
The natural history of the carotid NO is poorly characterized, and the management of patients remains controversial. We report the results and complications associated with CAS and follow-up.
MATERIALS AND METHODS:
Between March 2000 and March 2009, 116 of 836 CAS procedures were performed in patients with carotid NO (13.9%). A total of 99 men (85.3%) and 17 women (14.7%) with a mean age of 65.8 years were included. Presenting symptoms were TIA in 44 patients (37.9%) and minor stroke or noninvalidating stroke in 61 (52.6%). One hundred five patients (90.5%) were symptomatic.
RESULTS:
A distal filter was used for cerebral protection in 92 patients (79.3%). Transient hemodynamic alterations were frequent during balloon inflation: hypotension (37.1%), bradycardia (48.3%), and asystole in 24.1%. Four patients (3.4%) developed a TIA after CAS. Stroke in progression was arrested in the 1 patient (0.9%). The median follow-up period for patients was 36 months. Asymptomatic restenosis >70% occurred in 5 patients (4.3%); asymptomatic occlusion occurred in 3 patients (2.6%). During follow-up, 3 patients (2.6%) experienced a stroke, 1 ipsilateral (at 19 months) and 2 contralateral (at 6 and 30 months, respectively). Thirteen patients (11.2%) died, 7 from vascular causes.
CONCLUSIONS:
Our study showed that carotid NO is an under-recognized condition, and CAS would seem to be beneficial when performed by an experienced neurointerventional team.
“Atheromatous NO of the ICA” is defined as the presence in the ICA of an atheromatous plaque causing severe stenosis, a drop in perfusion pressure distal to the stenosis, and diminished or absent perfusion of the ipsilateral intracranial carotid flow. Collapse of the ICA distal to the stenosis, frequently revealing itself as a string sign, can be seen on angiography.1 Hemispheric circulation is supplied through inverted ophthalmic artery blood flow, leptomeningeal collaterals, and both communicating arteries.
Management of patients with NO remains controversial. The low risk of stroke in medically treated patients with NO reported by the NASCET2 and the results published by the ECST3 for patients with poststenotic narrowing of the ICA could lead to the clinical impression that patients with recently symptomatic NO are not at high risk of stroke, making any interventional approach to treatment unnecessary. However, an 11.1%–33% risk of ipsilateral stroke within the first year has also been reported in medically treated patients.2,4 Furthermore, other authors have reported a significantly lower risk of future stroke after surgery in these patients.5–7 Seeking safe interventional procedures could, therefore, be worthwhile.
CAS, mainly with distal protection, has been shown not to be inferior to CEA in treating patients with severe stenosis of the ICA.8 CAS is currently recommended in specific patients, chiefly those with high-risk surgical conditions .9 So far, only 2 small series of patients with NO treated by CAS have been published.10,11 Here, we report our experience with 116 patients treated by CAS and followed up prospectively. The issue of the suitability of this procedure in managing patients with NO was addressed by considering intraprocedural complications and 30-day and long-term morbidity and mortality.
Materials and Methods
Patients
We began collecting interventional data in 1991, prospectively following all patients who underwent angioplasty for stenosis of >70%. Of 1033 cases on record as a first procedure, 836 were performed in 9 years (from March 2000 to March 2009). In March 2000, our department decided to perform CAS in patients with NO according to the protocol that had been approved by our institutional review board. Since then, 116 patients (13.9%) have been classified as having ICA NO. The initial diagnosis of ICA NO was tentatively made by continuous Doppler sonography in patients with bidirectional waves or flow inversion in the ophthalmic artery, a high resistive index in the common carotid artery, and rhythmic pulsatile spikes with extremely low peak-systolic and end-diastolic velocities along the cervical segment of the ICA. When NO was suspected and B-mode sonography was unable to demonstrate patency of the ICA, contrast medium, color power angiography, and pulsed Doppler sonographic imaging with the lowest wall filter setting and a low pulse-repetition frequency were very helpful.
The patient group comprised 99 men (85.3%) and 17 women (14.7%) with a mean age of 65.8 years (range, 40–82). Presenting symptoms were TIA in 44 patients (37.9%) and minor stroke or nondisabling stroke (mRS, ≤2) in 61 (52.6%). Eleven patients (9.5%) were asymptomatic. These patients were treated when progression to NO was clearly present as well as contralateral stenosis or occlusion and/or ipsilateral diminished/exhausted cerebrovascular reserve. Associated risk factors included hypertension (72.4%), hyperlipidemia (56%), diabetes (42.2%), smoking (48.3%), coronary artery disease (30.2%), and peripheral vascular disease (23.3%) (Table 1). In our series, 44 patients (37.9%) would have been excluded from the NASCET (35 with severe coronary artery disease, 5 with renal insufficiency, 2 with postradiotherapy stenosis, and 2 with chronic obstructive pulmonary disease). Three or more vascular risk factors were present in 60 patients (51.7%). All patients were informed about the experimental nature of CAS and signed an informed consent accepting the procedure.
Table 1:
Patient baseline characteristics
| Characteristic | No. (%) |
|---|---|
| Patients | 116 (100.0) |
| Men | 99 (85.3) |
| Women | 17 (14.7) |
| Mean age (range) (yr) | 65.8 (40–82) |
| Symptomatic patients | 105 (90.5) |
| TIA/amaurosis fugax | 44 (37.9) |
| Minor stroke/stroke (mRS, <3) | 61 (52.6) |
| Asymptomatic patients | 11 (9.5) |
| Vascular risk factors | |
| Diabetes | 49 (42.2) |
| Hypertension | 84 (72.4) |
| Hyperlipidemia | 65 (56) |
| Cigarette smoking | 56 (48.3) |
| Coronary artery disease | 35 (30.2) |
| Peripheral vascular disease | 27 (23.3) |
On admission to our hospital, all patients underwent a comprehensive interview and neurologic and vascular examinations. Routine noninvasive studies included periorbital Doppler sonographic examination, continuous-wave Doppler sonography with spectral analysis, duplex evaluation, and transcranial Doppler insonation. A breath-holding test showed diminished vasoreactivity in response to apnea in 11 patients (12.8%) and exhausted vasoreactivity in 19 patients (22.1%). In symptomatic patients, these figures were 9 (11.4%) and 18 (22.8%), respectively, and in asymptomatic patients, 28.6% and 14.3% respectively.
When the diagnosis was uncertain, MR angiography with a paramagnetic contrast agent was performed. All patients were prescribed intravenous heparin, clopidogrel (75 mg/day), and aspirin (125 mg/day) for at least 3 days before CAS. In emergency cases, higher doses of clopidogrel (300 mg) and aspirin (500 mg) were indicated at the outset.
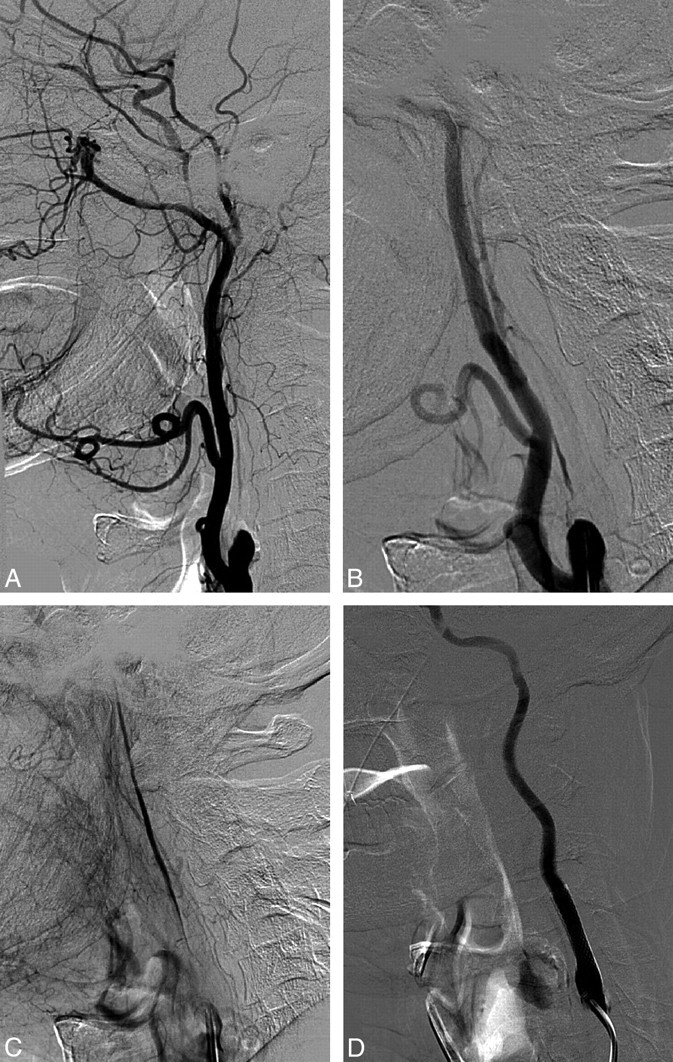
Four-vessel angiography was performed in all patients before CAS. A diagnosis of ICA NO was made when progression of the contrast medium distal to the point of extreme stenosis was markedly delayed and later films showed no discernible washout. Progression of the contrast medium following injection was usually so slow that in most cases, the contrast column failed to reach the base of the skull on 4-second films. When no string sign was present, a diagnosis of NO was made when there was a similar significant delay in antegrade blood flow and an obvious diameter reduction of the distal cervical ICA in comparison with the opposite ICA or the ipsilateral external carotid artery beyond the facial and occipital artery origins at a level similar that to observed in the ICA (Fig 1). In cases initially thought to show occlusion of the proximal ICA, selective stump ICA injection was performed (Fig 2). A thorough examination of the ophthalmic artery, both communicating arteries, and the leptomeningeal circulation was performed to assess the collateral vascular supply (Fig 3).
Fig 1.
NO of the left ICA. A, Reduced ICA diameter compared with the external carotid artery. Retrograde filling of the supraclinoid segment through the ophthalmic artery. B, Multichanneled NO of the ICA. C, ICA angiogram after stent placement.
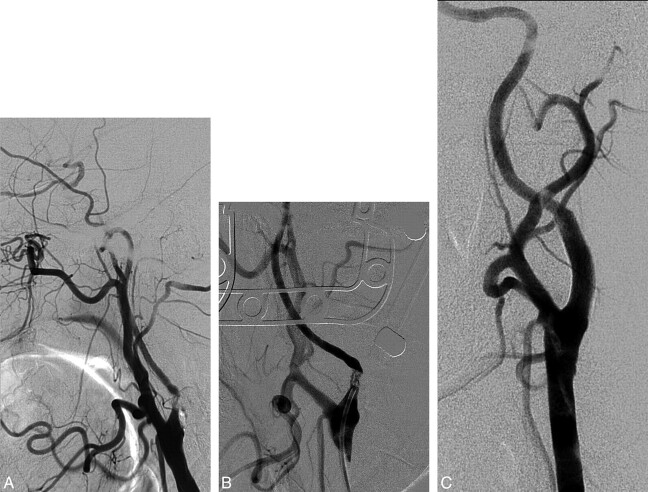
Fig 2.
Right ICA NO with a string sign. A, Lateral angiogram shows occlusion of the proximal ICA. B, Selective stump ICA injection shows the collapsed lumen of the ICA. C, Narrow stream of contrast progressing through the ICA in the late arterial phase. D, ICA angiogram after stent placement.
Fig 3.
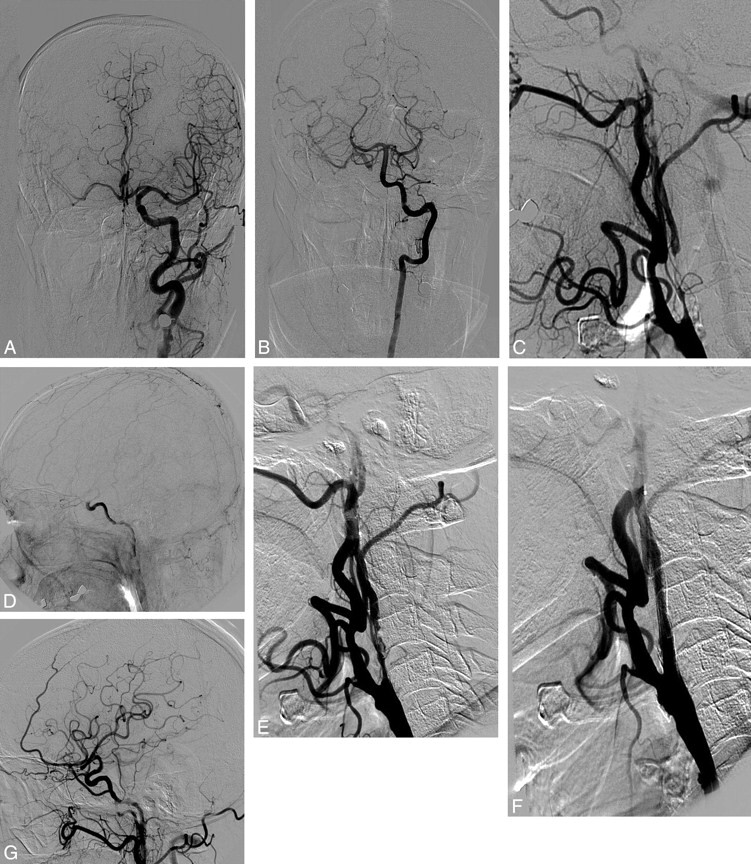
NO of the right ICA. A, Anteroposterior left carotid angiogram. Cross-filling of the contralateral anterior circulation via the AcomA. B, Anteroposterior left vertebral angiogram. Cross-filling of the right anterior circulation via the PcomA. C, Lateral right common carotid angiogram with NO. D, Lateral right carotid angiogram shows a filling delay of the intracranial ICA. Abrupt dilution of contrast in the supraclinoid ICA. E, Severe intimal dissection after predilation. F, Lateral angiogram after stent placement. G, Recovery of normal flow to the middle cerebral artery after stent placement in the NO.
Intervention Protocol
The procedure was performed according to our protocol,12 with the patient under local anesthesia and continuous neurologic, blood pressure, and electrocardiographic monitoring. Activated clotting time during the procedure was kept between 250 and 300 seconds. All procedures were carried out with rapid exchange (monorail) systems for stent placement and distal protection. When the filter could not be advanced through a tight stenosis, an exchange-length 0.014-inch microguidewire with a 120-cm 5F vertebral catheter was used. Dilation with a 2-mm balloon was then performed to allow the filter to cross the stricture. Once open, dilation of the stenosis was performed to make room for stent delivery. After stent deployment, complete dilation of the device was performed with a 5- or 6-mm balloon catheter. No attempt to completely dilate the stent was made when vasoreactivity was exhausted.
Intravenous atropine was administered when severe hemodynamic effects (bradycardia, asystole, or hypotension) appeared secondary to balloon inflation.
Following the procedure, patients who underwent CAS were monitored in the stroke unit for 24 hours. Intravenous heparin was started 3 hours after CAS and maintained for 24 hours. Patients were discharged on clopidogrel, 75 mg/day, for a month and aspirin,125 mg/day, or triflusal, 600 mg/day, indefinitely. Neurologic examination and duplex sonographic evaluation were performed the day after the procedure and at 1, 3, 6, and 12 months for the first year and then annually to determine clinical outcome and vessel patency or possible restenosis.
Several variables were recorded for analysis and included demographics, vascular risk factors, angiographic features of the NO, intraprocedural and 30-day clinical morbidity and mortality, long-term clinical outcome, hemodynamic changes, and restenosis. Descriptive statistical analysis was carried out by using the Statistical Package for the Social Sciences for Windows, Version 15.0 (SPSS, Chicago, Illinois).
Results
The angiographic findings concerning the stenosis and the functional status of the circle of Willis are presented in Table 2. Contralateral ICA stenosis ≥70% was found in 23.3% of patients and occlusion in 6.9% of patients.
Table 2:
Angiography findings
| Findings | No. (%) |
|---|---|
| Left ICA NO | 60 (51.7) |
| Right ICA NO | 56 (48.3) |
| String sign | 34 (29.3) |
| Decreased diameter of the ipsilateral ICA | 82 (70.7) |
| Delayed arrival of contrast | 116 (100) |
| Ulceration | 58 (50) |
| Calcification | 69 (59.6) |
| Intracranial lesion | 12 (10.3) |
| Contralateral stenosis (%) | |
| 70–99 | 27 (23.3) |
| 100 | 8 (6.9) |
| Collateral pathways | |
| Patent AcomA | 103 (88.8) |
| Patent PcomA | 64 (55.2) |
| Ophthalmic artery | 44 (37.9) |
| Leptomeningeal artery | 29 (25) |
| Residual stenosis | |
| 0% | 95 (81.9) |
| 1–30% | 14 (12.1) |
| 31–50% | 5 (4.3) |
| 51–70% | 2 (1.7) |
Twelve patients (10.3%) had severe intracranial stenosis undetected before PTA, due to filling defects caused by the extremely low flow. The CAS was performed as an emergency procedure in 21 patients (18.1%) unresponsive to antiplatelet therapy and systemic heparin, 20 (17.6%) with “crescendo” TIA, and 1 (0.9%) with stroke in progress. A distal filter was used for cerebral protection in 92 patients (79.3%): Spider (ev3, Plymouth, Minnesota) in 61 (52.6%), FilterWire EZ (Boston Scientific/Target Therapeutics, Fremont, California) in 26 (22.4%), and EPI FilterWire (Boston Scientific/Target Therapeutics) in 5 (4.3%). In 20.7% of patients, the procedure was performed with rapid exchange (monorail) systems without distal protection. In 5 cases, distal protection was not used because the artery did not recover after predilation a caliber higher than 2 mm, that diameter being the minimum acceptable for display. In the other 19 cases, we were unable to cross the thigh stenosis with the protection system, even after predilation.
A stent was implanted in all patients after angioplasty: carotid Acculink (Abbott Vascular, Santa Clara, California) in 74 (63.8%), carotid WallStent (Boston Scientific/Target Therapeutics) in 39 (33.6%), and Precise (Cordis; Miami Lakes, Florida) in 4 (2.6%). One patient (0.9%) required 2 stents.
Predilation of the stenosis was required in all patients to allow deployment of the stent. Additionally, postdilation after stent placement was performed in 82.8% of patients. Full stent dilation was avoided in most cases with exhausted or diminished vasoreactivity to avert hyperperfusion syndrome. Transient hemodynamic alterations were frequent during balloon inflation: hypotension in 43 patients (37.1%), bradycardia in 56 patients (48.3%), and asystole in 28 patients (24.1%). Transient syncope occurred in 14 patients (12.1%). Postangioplasty intimal dissection occurred in 23 patients (19.8%) and was resolved immediately by stent placement. Occlusion of the filter occurred in 3 patients (2.6%). No major vascular access site complications were recorded. All procedures were successfully completed in a mean time of 30 minutes (range, 8–110 minutes). Residual stenosis postprocedure is shown in Table 2.
Four patients (3.4%) developed a short-lasting ipsilateral TIA <15 minutes immediately after CAS. Stroke in progress was arrested in 1 patient (0.9%), though stroke sequelae remained. No minor or disabling stroke, death, or myocardial infarction occurred in the 30-day period following the procedure.
Clinical follow-up was performed for all patients. The median follow-up period was 36 months (patient 25, 12 months; patient 75, 54 months). Asymptomatic restenosis >70% occurred in 5 patients (4.3%), in all cases before the sixth month. Three of these were successfully retreated with PTA. Asymptomatic occlusion occurred in 3 patients (2.6%). One of these patients had a severe tandem lesion of the cavernous segment of the ICA undetected before PTA. Had this lesion been discovered before PTA, the procedure would not have been undertaken.
During long-term precautionary follow-up, 3 (2.6%) patients experienced a stroke, 1 ipsilateral to the NO (at 19 months) and 2 contralateral to it (at 6 and 30 months, respectively). Thirteen patients (11.2%) died, 7 from vascular causes (5 myocardial infarction and 2 pulmonary embolism) and 6 from nonvascular causes. The overall survival rate in the mean follow-up period was 85.4%.
Discussion
The natural history of ICA NO is poorly understood, and management of these patients remains controversial. The actual frequency of this entity in patients with stroke is also unknown. Absent an awareness of this condition, NO can easily be misdiagnosed as occlusion, not only in routine sonography studies but even in MR angiography or angiography. More frequently, it can be overlooked in cases in which there is a proximal ICA stump, extremely low filling, or a string sign in the distal portion of the ICA. Clinicians should keep NO in mind whenever an apparently occluded ICA continues to be symptomatic. In our prospective hospital-based series, 13.9% of patients undergoing CAS in a 10-year period had NO. This percentage seems high enough to encourage pursuit of a more thorough knowledge of the natural history of NO and the most appropriate treatment options.
The finding of a low risk of stroke in medically treated patients with NO in the NASCET2 and ECST3 could lead to the clinical impression that patients with recently discovered symptomatic ICA NO are at low risk of stroke, making any interventional approach unnecessary. However, in some series4 ≤33% of patients in the medically treated group had a new stroke in the course of 30 months of follow-up. In contrast, only 8.7% of patients who underwent CEA had another episode of stroke.
In medically treated patients with ICA NO in the NASCET,2 7.1% with a string sign on angiography and 15.9% of patients without a string sign presented with a second stroke by the end of 1 year. Fox et al13 recently compiled NASCET and ECST data and found 262 patients (21.5%) with NO, 6.1% with a string sign. After a follow-up of 3 years, 17.9% of patients in the NASCET and 11.1% of those in the ECST in the medically treated groups had another ipsilateral stroke. These percentages could be related to a natural evolution of NO to total occlusion. Actually, while anecdotal reports have described NO arteries remaining patent for years,14 in the O'Leary et al series,4 100% of the NO arteries followed for 30 months evolved to total occlusion. In the NASCET,15 occlusion occurred more frequently with higher baseline levels of stenosis. In fact, in 26% of patients assigned to medical therapy, the stenotic artery progressed to total occlusion during a mean follow-up period of 2 years. However, in the O'Leary et al series, 83% of the ICA NOs were patent 30 months after CEA. If interventions had been performed on the patients in NASCET and ECST, arterial patency rates would have been higher and long-term morbidity might have been lower.
CEA results in patients with NO have been considered satisfactory. According to a review of the literature before the 1990s, there were no reported complications of CEA in small series, suggesting that aggressive management subjected patients with ICA NO to no particular risk.16–18 Morbidity-mortality rates of 3%–6% have more recently been reported in larger series.4,6 In the NASCET,19 a 6.5% perioperative risk of stroke was not appreciably greater than the risk in patients with 70%–90% ICA stenosis. Morgenstern et al2 reported 1-year morbidity-mortality rates of 6.7% and 9.1%, respectively, in patients with and without a string sign on arteriography. In the group without a string sign, absolute risk was reduced by 6.8% compared with the patients in the medically treated group (NNT = 15). By contrast, in the patients with a string sign, the absolute risk was reduced by just 0.4% (NNT = 250) due the low morbidity rate under medical treatment regimens. Patient selection is probably of great relevance because in patients with a string sign, especially in those with good collaterals, the risk of brain embolization is probably minimal because the low blood flow should not be able to dislodge emboli from the surface of the plaque.
The risk of ipsilateral stroke at 2 years was between 10.4% and 11.1% in the patients with ICA NO in the NASCET,2 considerably higher than that in patients with 70%–95% stenosis. This means that even though the NASCET group concluded that CEA could be performed safely in patients with ICA NO, seeking other interventional procedures with better outcomes would be worthwhile.
We prospectively followed all patients who underwent angioplasty or CAS since 1991. Since the introduction of stents and filter systems for distal cerebral protection 10 years ago, 116 patients (13.9% of the series) were deemed to have ICA NO. Most (90%) were symptomatic. Sixty patients (51.7%) had ≥3 vascular risk factors, and 44 patients (37.9%) would not have been included in the NASCET. Twenty-seven (23%) had contralateral stenosis >70%, and 8 (7%), had occlusion, both important markers of high risk on CEA.20 Despite frequent transient hemodynamic changes during balloon inflation, only 4 patients (3.4%) had a TIA, and 1 patient with progressive stroke did not recover to a normal condition, even though progression was arrested. No TIA, minor or disabling stroke, death, or myocardial infarction occurred in the 30-day period that followed the procedure.
One third of the patients in our series displayed exhausted (22%) or diminished (13%) vasoreactivity in response to apnea in the transcranial Doppler sonography study. However, no cases of hyperperfusion occurred in the 30-day period after CAS. Strict control of blood pressure and avoidance of full stent dilation in these patients, particularly when collaterals were deemed rather insufficient, probably contributed to these results.
The mean follow-up of patients was 3 years. During follow-up, 3 patients (2.6%) had a stroke, 2 contralateral, and 13 patients (11.2%) died for reasons other than stroke, for an overall morbidity-mortality rate of 14.6%. Asymptomatic restenosis >70% occurred in 5 patients (4.3%).
Apart from a small series we published previously,10 only 1 other small series of ICA NO has been published.11 Causes of the dearth of publications in this area are difficult to identify, though difficulties in achieving a correct diagnosis, the previously mentioned NASCET results for medically treated patients, technical difficulties, and limited experience with CAS may be some of the reasons. In the Terada et al series,11 only 1 patient (5%) died after a successful procedure. The event occurred following hemodialysis the day after CAS, and the cause was not clearly elucidated. Another patient died of myocardial infarction 5 months after CAS, yielding an overall complication rate of 10% during a mean follow-up period of 24.8 months. Even though they reported that maneuvering the microguidewire past the lesion was not difficult, in our experience, some cases may represent a serious challenge calling for considerable interventional expertise.
Conclusions
Although the natural history of ICA NO is not well-known, this condition may well be more frequent than is currently thought. Efforts to achieve a correct diagnosis are essential, particularly in patients with new ipsilateral symptoms after a diagnosis of total ICA occlusion. Once the diagnosis has been made, CAS would seem to be beneficial when performed by a skilled and experienced neurointerventional team that has attained low complication rates, and initial indications suggest that it may outperform more conservative medical treatment. Because of the numerous complex clinical and hemodynamic factors posed by every patient with ICA NO, larger series are needed to establish which groups of patients are likely to benefit the most.
Abbreviations
- AcomA
anterior communicating artery
- CAS
carotid angioplasty and stent placement
- CEA
carotid endarterectomy
- ECST
European Carotid Surgery Trial
- ICA
internal carotid artery
- mRS
modified Rankin Scale
- NASCET
North American Symptomatic Carotid Endarterectomy Trial
- NNT
number needed to treat
- NO
near occlusion
- PcomA
posterior communicating artery
- PTA
percutaneous transluminal angioplasty
- TIA
transient ischemic attack
References
- 1. Mehigan JT, Olcott C. The carotid “string sign”: differential diagnosis and management. Am J Surg 1980;140:137–43 [DOI] [PubMed] [Google Scholar]
- 2. Morgenstern LB, Fox AJ, Sharpe BL, et al. The risks and benefits of carotid endarterectomy in patients with near occlusion of the carotid artery: North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Neurology 1997;48:911–15 [DOI] [PubMed] [Google Scholar]
- 3. Rothwell PM, Warlow CP. Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists' Collaborative Group. Stroke 2000;31:622–30 [DOI] [PubMed] [Google Scholar]
- 4. O'Leary DH, Mattle H, Potter JE. Atheromatous pseudo-occlusion of the internal carotid artery. Stroke 1989;20:1168–73 [DOI] [PubMed] [Google Scholar]
- 5. Ringelstein EB, Berg-Dammer E, Zeumer H. The so-called atheromatous pseudoocclusion of internal carotid artery: a diagnostic and therapeutical challenge. Neuroradiology 1983;25:147–55 [DOI] [PubMed] [Google Scholar]
- 6. Fredericks RK, Thomas TD, Lefkowitz DS, et al. Implications of the angiographic string sign in carotid atherosclerosis. Stroke 1990;21:476–79 [DOI] [PubMed] [Google Scholar]
- 7. Kniemeyer HW, Aulich A, Schlachetzki F, et al. Pseudo- and segmental occlusion of the internal carotid artery: a new classification, surgical treatment and results. Eur J Vasc Endovasc Surg 1996;12:310–20 [DOI] [PubMed] [Google Scholar]
- 8. CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Lancet 2001;357:1729–37 [PubMed] [Google Scholar]
- 9. Yadav JS, Wholey MH, Kuntz RE, et al. , for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–501 [DOI] [PubMed] [Google Scholar]
- 10. Gil-Peralta A, González A, González-Marcos JR, et al. Internal carotid artery stenting in patients with symptomatic atheromatous pseudo-occlusion. Cerebrovasc Dis 2004;17(suppl 1):105–12 [DOI] [PubMed] [Google Scholar]
- 11. Terada T, Tsuura M, Matsumoto H, et al. Endovascular treatment for pseudo-occlusion of the internal carotid artery. Neurosurgery 2006;59:301–09 [DOI] [PubMed] [Google Scholar]
- 12. Piñero P, González A, Martínez E, et al. Silent ischemia after neuroprotected percutaneous carotid stenting: a diffusion-weighted MRI study. AJNR Am J Neuroradiol 2006;27:1338–45 [PMC free article] [PubMed] [Google Scholar]
- 13. Fox AJ, Eliasziw M, Rothwell PM, et al. Identification, prognosis, and management of patients with carotid artery near occlusion. AJNR Am J Neuroradiol 2005;26:2086–94 [PMC free article] [PubMed] [Google Scholar]
- 14. Gabrielsen TO, Seeger JF, Knake JE, et al. The nearly occluded internal carotid artery: a diagnostic trap. Radiology 1981;138:611–18 [DOI] [PubMed] [Google Scholar]
- 15. Paciaroni M, Eliasziw M, Sharpe BL, et al. Long-term clinical and angiographic outcomes in symptomatic patients with 70% to 99% carotid artery stenosis. Stroke 2000;31:2037–42 [DOI] [PubMed] [Google Scholar]
- 16. Perler BA, Burdick JF, Williams GM. Progression to total occlusion is an underrecognized complication of medical management of carotid disease. J Vasc Surg 1991;14:821–28 [DOI] [PubMed] [Google Scholar]
- 17. Sekhar LN, Heros RC, Lotz PR, et al. Atheromatous pseudo-occlusion of the internal carotid artery. J Neurosurg 1980;52:782–89 [DOI] [PubMed] [Google Scholar]
- 18. Berman SS, Bernhard VM, Erly WK, et al. Critical carotid artery stenosis: diagnosis, timing of surgery and outcome. J Vasc Surg 1994;20:499–510 [DOI] [PubMed] [Google Scholar]
- 19. Henderson RD, Eliasziw M, Fox AJ, et al. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis: North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke 2000;31:128–32 [DOI] [PubMed] [Google Scholar]
- 20. Gasecki AP, Eliasziw M, Fergurson GG, et al. Long-term prognosis and effect of endarterectomy in patients with symptomatic severe carotid stenosis and contralateral carotid stenosis or occlusion: results from NASCET. J Neurosurg 1995;83:778–82 [DOI] [PubMed] [Google Scholar]