Abstract
BACKGROUND AND PURPOSE:
CTA is considered the imaging modality of choice in evaluating the supraaortic vessels in many institutions, but radiation exposure remains a matter of concern. The objective of the study was to evaluate a fully automated, attenuation-based kilovolt selection algorithm in carotid CTA in respect to radiation dose and image quality compared with a standard 120-kV protocol.
MATERIALS AND METHODS:
Ninety-eight patients were included: 53 examinations (patient age, 66 ± 12 years) were performed by use of automated adaption of tube potential (80–140 kV) on the basis of the attenuation profile of the scout scan (study group), and 45 examinations (patient age, 67 ± 11 years) were performed by use of a standard 120-kV protocol (control group). CT dose index volume and dose-length product were recorded from the examination protocol. Image quality was assessed by ROI measurements and calculations of SNR and contrast-to-noise ratio. Subjective image quality was evaluated by 2 observers with the use of a 4-point scale (3, excellent; 0, not diagnostic).
RESULTS:
Subjective image quality was rated as “excellent” or “good” in all examinations (study group, 2.8; control group, 2.8). The algorithm automatically selected 100 kV in 47% and 80 kV in 34%; 120 kV was retained in 19%. An elevation to 140 kV did not occur. Compared with the control group, overall CT dose index volume reduction was 33.7%; overall dose-length product reduction was 31.5%. In the low-kilovolt scans, image noise and mean attenuation of ROIs inside the carotid arteries were significantly higher than in 120-kV scans, resulting in a constant or increased (80-kV group) contrast-to-noise ratio.
CONCLUSIONS:
The attenuation-based, kilovolt selection algorithm enables a dose reduction of >30% in carotid artery CTA while maintaining contrast-to-noise ratio and subjective image quality at adequate levels.
CT angiography is recommended as a second-line imaging technique in patients with extracranial carotid artery disease after screening with Doppler sonography.1 As the result of rapid technological evolution, the number of CT examinations is still rising,2 even with the significant medical x-ray exposure2,3 and the increasing awareness of the potential risks of even relatively low radiation doses2,4—especially in usage as a screening tool5 or in serial use.6 Biologic experiments have demonstrated that the number of DNA double-strand breaks is closely related to the applied dose.3,7
Several techniques for CT dose reduction, maintaining image quality on a diagnostic level, became a hot topic in clinical research.8–11 Automated attenuation-based tube current modulation or so-called automatic exposure control techniques are widely used.12,13 All major manufacturers provide iterative reconstruction algorithms, which are fast enough to be used in clinical practice and aim to compensate the increased image noise of low-dose CTA scans.14,15
Besides minimizing tube current, the reduction of the tube voltage is a potent option to reduce radiation dose. Such low-kilovolt protocols are used in cerebral perfusion CT in the evaluation of patients with stroke16 and in pediatric CT.17 In phantom18 as well as in clinical studies, it has been demonstrated that this technique can also be used in chest and abdominal imaging in adults.19–24
Lower tube voltage in CTA results in higher noise levels on one hand, but the attenuation of iodine is increased25,26 on the other hand. This relationship must be considered when trying to reduce radiation exposure by use of low-kilovolt scanning and to maintain image quality.24,27 The higher noise level in low-kilovolt scans will not be completely compensated by the higher iodine attenuation; therefore, additional adjustments of the tube current (milliampere setting) are necessary. Optimal manual adjustment of these parameters is complex, which prevented low-kilovolt scanning from general use until now. New software solutions that automatically adjust the kilovolt and milliampere setting to the individual patient anatomy by the attenuation profile of the scout scan may overcome this limitation.20
In a previous study, significant dose reduction and higher attenuation profiles of the carotid arteries were reported but were limited in image quality at the level of the common carotid artery.28
In the present study, we aimed to evaluate the effects on radiation exposure and image quality in CTA of the carotid arteries by use of a commercially available automated tube voltage and tube current adaption software tool.
Materials and Methods
Ninety-eight patients were prospectively enrolled in this institutional review board–approved study and scheduled for CTA of the carotid arteries. All examinations were performed on a 128-section CT scanner (Somatom Definition AS+, Siemens, Erlangen, Germany) equipped with an automated attenuation-based kilovolt selection and milliampere adaption algorithm (CAREkV, Siemens). Fifty-three examinations (mean patient age, 66 ± 12 years; range, 25–83 years; 36 male and 17 female patients) were performed by use of automated kilovolt and milliampere adaption (study group); 45 examinations (mean patient age, 67 ± 11; range, 40–88 years; 30 male and 15 female patients) were performed by use of a standard 120-kV protocol (control group). Automated exposure control was used in both groups.
The scan range included the lateral ventricles to the aortic arch. Collimation was 128 × 0.6 mm and pitch, 0.6. The image quality level of the attenuation-based tube current modulation algorithm (CAREdose 4D) corresponded to 140 Ref. mA at 120 kV for all scans.
After the lateral scout scan, the individual kilovolt setting was calculated on the basis of the attenuation profile. Different settings of the algorithm can be selected; we chose a setting that aims to maintain the SNR constant and allows more aggressive dose reduction.
Circulation time was individually calculated by use of a test bolus injection with 10 mL of iodinated contrast medium (Imeron 350; Bracco, Milan, Italy) at a flow rate of 5 mL/s, chased by a 50-mL saline bolus at the same flow rate. The ROI to measure the TTP was placed in the aortic arch. The delay of the diagnostic scan was calculated by use of the formula TTP + 2 seconds. For the diagnostic scan, 50 mL of iodinated contrast medium and a 50-mL saline chaser were used at the same flow rate of 5 mL/s.
Radiation Dose
Values for milliamperes, the CT dose index volume (CTDIvol, milligray), and the dose-length product (DLP, milligray centimeters) were recorded from the patient protocol for the diagnostic scans, allowing a direct comparison of radiation dose exposure in the study group and the control group.
Image Quality
The objective assessment of the image quality was performed on a standard PACS workstation (Syngo Plaza, Siemens) in 3-mm images in the axial plane by use of a soft-tissue kernel. For each examination, 2 ROIs were bilaterally placed in the carotid arteries, in the musculature of the neck, and in the air right and left anterior to the neck. Calcifications of the carotid walls were carefully excluded in the ROI. ROIs were not placed in sections with severe artifacts (ie, caused by dental hardware or motion).
ROI size (cm2), mean attenuation (Hounsfield Unit [HU]), and the standard deviation (HU) were obtained for each measurement. Standard deviation (HU) was considered as a measure of image noise. SNR (mean attenuation [HU] carotid artery/standard deviation [HU]) and contrast-to-noise ratio (CNR) (mean attenuation [HU] carotid artery−mean attenuation [HU] muscle)/standard deviation [HU]) for the carotid artery were calculated.
Subjective image quality assessment of all examinations was evaluated by means of a 4-grade scale (3, excellent; 2, good; 1, moderate; 0, not diagnostic). Artifacts (beam-hardening, windmill artifacts, etc) were grouped into 4 classes (3, no artifacts; 2, mild artifacts not affecting diagnostic value; 1, artifacts affecting diagnostic value; 0, strong artifacts, not diagnostic). Metal artifacts (caused by dental hardware) were not considered. The same evaluation system was used for the control group.
Image quality and the presence of artifacts were independently evaluated by 2 observers (both with more than 4 years of experience in CTA).
Statistical Analyses
Descriptive statistics were performed (SPSS 15.0, IBM, Armonk, New York). A Student t test was performed for dose assessment after testing for normal distribution of the data, and the Mann-Whitney U rank test was performed to evaluate the subjective image quality scores. A P value of <.05 was considered to be statistically significant.
Cohen κ was used to assess interobserver agreement in rating image quality and artifacts.
Results
Automated kilovolt selection was successfully applied in all examinations without any user interaction or delay, thus not interfering with the clinical workflow. In the study group, in 10 (18.8%) patients, 120 kV was maintained; in 25 (47.2%) cases, 100 kV was used; and in 18 (34%) cases, 80 kV was used. No case was elevated to 140 kV.
Overall CTDI reduction was 33.7%: 55.8% in 80-kV and 31.6% in 100-kV examinations. In the 10 examinations of the study group scanned at 120 kV, the CTDI was 2.1% higher than in the control group.
Overall DLP reduction was 31.5%: 54% in the 80-kV group and 29.7% in the 100-kV group. In the 120-kV group, the DLP was 0.6% higher than in the control group.
Dose Evaluation
Dose evaluations specified for the different kilovolt groups are given in Table 1.
Table 1:
Dose parameters
| Group | 80 kV | 100 kV | 120 kV | Study Group | Control Group (120 kV) |
|---|---|---|---|---|---|
| n | 18 | 25 | 10 | 53 | 45 |
| kV | 80 | 100 | 120 | 97 ± 14 | 120 |
| Reference mAs | 210 | 145 | 140 | 166 ± 32 | 140 |
| Effective mAs | 224 ± 14 | 165 ± 22 | 144 ± 11 | 181 ± 37 | 141 ± 9 |
| CTDIvol, mGyb | 4.2 ± 0.3a | 6.5 ± 0.9a | 9.7 ± 0.7 | 6.3 ± 2.1a | 9.5 ± 0.6 |
| DLP, mGy cmb | 134.5 ± 19.6a | 208.7 ± 40a | 299.5 ± 54.9 | 200.6 ± 69.4a | 292.8 ± 46.2 |
Note:—The Reference mAs are automatically adapted to match the image quality level of 140 mA at 120 kV.
P < .05.
CTDIvol and DLP referenced to 32-cm phantom.
All CTA examinations were of high quality and fully diagnostic; no examination of the study group or control group was classified as “moderate” or “not diagnostic.” Mild beam-hardening artifacts, none of which affected diagnostic values, were found in all examinations in some sections (usually at the shoulder level) independent of the kilovolt setting, but all artifacts were classified as mild (grade 2) and were not exaggerated by use of the low-kilovolt technique.
CTA studies performed at 80 kV had quality scores similar to those in studies from the control group (120 kV). Interobserver agreement was substantial in the study group (κ = 0.695) and moderate in the control group (κ = 0.6).
The results for the objective image quality assessment by use of ROI measurements are given in Table 2; those for subjective image quality are given in Table 3.
Table 2:
Results of ROI measurements
| Group | 80 kV | 100 kV | 120 kV | Study Group Complete | Control Group (120 kV) | |
|---|---|---|---|---|---|---|
| n | 18 | 24 | 10 | 53 | 45 | |
| ROI carotid | Mean | 488.4 ± 93.3a | 340.9 ± 73.8a | 243 ± 83.2a | 372.5 ± 122.1a | 293.9 ± 58 |
| SD | 12.7 ± 2.8a | 12.1 ± 2.7a | 7.9 ± 3.1 | 11.5 ± 3.3a | 8.8 ± 1.8 | |
| SNR | 40.1 ± 12.3 | 29.9 ± 9.4 | 33.7 ± 13 | 34.1 ± 11.8 | 35.2 ± 11.7 | |
| CNR | 34.9 ± 11.5a | 24.4 ± 8.9 | 25 ± 12.7 | 28.1 ± 11.5 | 28.3 ± 10.9 | |
| ROI muscle | Mean | 61.4 ± 7.8 | 62.2 ± 11.2 | 61.4 ± 9.8 | 61.8 ± 9.7 | 58.9 ± 6.3 |
| SD | 9.7 ± 2.7a | 9.6 ± 2.8a | 6.5 ± 2.4 | 9.1 ± 2.9a | 6.7 ± 1.4 | |
| ROI air | Mean | −1000.2 ± 1.8a | −999.1 ± 2.2 | −999.2 ± 1.3 | −999.5 ± 2 | −998.7 ± 2.2 |
| SD | 6.2 ± 1.9a | 6.5 ± 2.2a | 4.1 ± 1.3 | 5.9 ± 2.1 | 4.6 ± 1 |
Note:—Values correspond to Hounsfield units.
P < .05.
Table 3:
Results of the subjective image quality assessment
| Group | 80 kV | 100 kV | 120 kV | Study Group | Control Group (120 kV) |
|---|---|---|---|---|---|
| n | 18 | 25 | 10 | 53 | 45 |
| Grade 3, excellent | 14/15 | 17/15 | 10/9 | 41/39 | 37/38 |
| Grade 2, good | 4/3 | 8/10 | 0/1 | 12/14 | 8/7 |
| Grade 1, diagnostic | 0 | 0 | 0 | 0 | 0 |
| Grade 0, not diagnostic | 0 | 0 | 0 | 0 | 0 |
| Average | 2.8 | 2.6a | 2.9 | 2.8 | 2.8 |
Note:—Counts are given for both observers (observer 1/observer 2).
P < .05.
In the study group, diagnostic findings included 24 with >70% stenosis or occlusion (22.6%; 13 right, 11 left) of the internal carotid arteries; 16 (15.1%; 9 right, 7 left) with stenosis between 50–70%; and in 66 (62.3%; 31 right, 35 left), stenosis <50% or no stenosis. Severe calcified plaques of the carotid bifurcation occurred in 52.8%.
In the control group, diagnostic findings included 37 with >70% stenosis or occlusion (41.1%; 19 right, 18 left) of the internal carotid arteries; 10 (11.1%; 3 right, 7 left) with stenosis between 50–70%; and in 43 cases (47.8%; 23 right, 20 left), <50% stenosis or no stenosis. Severe calcified plaques of the carotid bifurcation occurred in 53.3%.
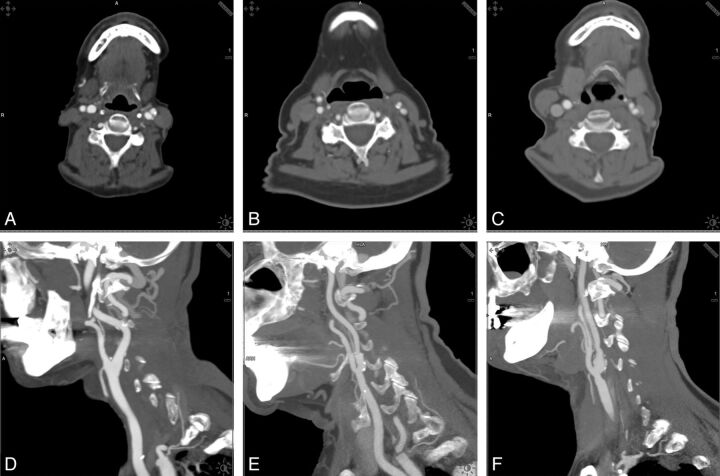
Examples of the different kilovolt groups, comparing examinations by use of 80, 100, and 120 kV in the axial plane and sagittal maximum intension projections are shown in Fig 1.
Fig 1.
Examples of the different kilovolt groups. Comparison of examinations by use of 80, 100, and 120 kV in the axial plane and sagittal maximum intension projections.
Discussion
CTA is frequently used in the evaluation of carotid artery stenosis29 and is meant to be the reference standard.30 Anzidei et al31 reported that CTA was the most accurate technique for evaluating carotid stenosis in a large study group (n = 170 patients), with a slightly better performance than MRA (97% versus 95% for steady-state MRA and 92% for first-pass MRA) and a greater accuracy than color Doppler ultrasonography (97% versus 76%). Although patients scheduled for carotid artery imaging are predominantly in the higher age group and radiation exposure is thought to be less critical, the ALARA (as low as reasonably achievable) concept should still be followed, and a variety of dose-reduction techniques in CT imaging have become available in recent years.32–35 There are abundant data that the low-kilovolt technique can significantly reduce radiation exposure and maintain image quality; however, until now, it is still not often used in clinical routine.
The main reason that most CT examinations including CTA are still performed with the 120-kV setting is that many users are not familiar with the required tube current adaption when scanning at low kilovolts. This adaption is important to keep the image noise level within an appropriate range. Elevating the tube current, on the other hand, limits or reverses the dose-reduction potential; therefore, scanning at 80 or 100 kV in every patient is not reasonable.
The software under evaluation is fully integrated in the scan protocols, tailoring the kilovolt and milliampere settings to the respective examination situation and individual patient anatomy. No further user interaction is required. In most (81.2%), adaptions allowed overall dose reduction. The predefined image quality level (120 kV, 140 mA) was kept constant throughout the study and the control group. Objective image quality was comparably high, with no significant differences between the study group and the control group, though a significant reduction in dose (overall CTDIvol reduction in the study group, 33.7%) was achieved. CTDIvol reduction was 55.8% in the examinations at 80 kV and 31.6% at 100 kV.
The noise measurements revealed significantly increased image noise levels in the 80-kV and 100-kV examinations but also significantly higher iodine contrast. The CNR in the study group and the control group was comparable; in the 80-kV group, CNR increased. To compensate for the different image contrasts, the window-level setting also must be adapted. This is of special importance to differentiate calcified plaque from luminal contrast and to restore the familiar image aspect in low-kilovolt images, but this step can also be performed automatically by the software.
Subjective image quality was high in all CTA examinations, without any equivocal or nondiagnostic results. Image noise in the 100-kV group was similar to that at 80 kV on average, resulting in higher SNR and CNR and higher subjective scores for 80-kV examinations.
The fact that the HU values in the carotid arteries were significantly lower in the study group scanned at 120 kV as compared with the control group, in which all scans were performed at 120 kV, may be attributed to the small sample size (n = 10) and patient-specific factors.36
The algorithm is designed to provide optimal parameter adjustment by the attenuation profile of the scout scan; thus, patient size is supposed to be one of the main influencing factors. In imaging the trunk, the body mass index (BMI) influences the kilovolt setting.20 BMI does not play that role in carotid CTA or CT of the neck, because a short neck or elevated, muscular shoulders influence attenuation more profoundly and do not correlate well with the BMI. In our study cohort, patients up to a BMI of 42 (height, 185 cm; weight, 145 kg) were scanned successfully at 100 kV.
Low-kilovolt scanning is limited by tube output. If higher pitch values are applied, the high attenuation at the level of the shoulders in larger persons may preclude the application of 80 kV. Iterative reconstruction has been demonstrated to compensate image noise in low-kilovolt scanning, maintaining high image quality.24,37 Adding iterative reconstruction techniques to 100-kV scanning may further improve dose reduction by compensation of the higher image noise. We did not investigate this option because we wanted to exclude all confounding effects from the different techniques. Another limitation may be that we used an aggressive setting of the algorithm, which balances image noise and vascular enhancement but leads to decreased CNR and SNR of tissue with less or no contrast material uptake. This makes the success of the approach also dependent on optimal contrast timing, which can be achieved with the use of either a test bolus or a bolus-tracking technique.
Conclusions
We demonstrated that an attenuation-based kilovolt selection with automated milliampere adaption in CTA of the carotid arteries results in a significant dose reduction while preserving image quality. The significantly increased noise level of the images is compensated by increased iodine contrast, resulting in a comparably high or improved CNR. Further dose reduction might be achieved by combining the algorithm with other dose-saving techniques such as iterative reconstruction algorithms.
ABBREVIATIONS:
- CTDIvol
CT dose index volume
- DLP
dose-length product
- HU
Hounsfield unit
- CNR
contrast-to-noise ratio
- BMI
body mass index
Footnotes
Disclosures: Michael Uder—UNRELATED: Payment for Lectures (including service on speakers bureaus): Bracco, Siemens, Bayer-Schering, Medtronic; Patents (planned, pending or issued): Siemens. Michael Lell—RELATED: Grant: German government, BMBF*; UNRELATED: Siemens, Bracco, Bayer-Schering.
This paper was supported by the German Government, Bundesministerium für Bildung und Forschung, BMBF (01EX1012B, “Spitzencluster Medical Valley”).
References
- 1. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the management of patients with extracranial carotid and vertebral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery, developed in collaboration with the American Academy of Neurology and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2011;57:e16–94 [DOI] [PubMed] [Google Scholar]
- 2. Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 2009;169:2071–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuefner MA, Grudzenski S, Schwab SA, et al. DNA double-strand breaks and their repair in blood lymphocytes of patients undergoing angiographic procedures. Invest Radiol 2009;44:440–46 [DOI] [PubMed] [Google Scholar]
- 4. Brenner DJ, Hall EJ. Computed tomography: an increasing source of radiation exposure. N Engl J Med 2007;357:2277–84 [DOI] [PubMed] [Google Scholar]
- 5. Narvid J, Do HM, Blevins NH, et al. CT angiography as a screening tool for dural arteriovenous fistula in patients with pulsatile tinnitus: feasibility and test characteristics. AJNR Am J Neuroradiol 2011;32:446–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Gils MJ, Vukadinovic D, van Dijk AC, et al. Carotid atherosclerotic plaque progression and change in plaque composition over time: a 5-year follow-up study using serial CT angiography. AJNR Am J Neuroradiol 2012;33:1267–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lobrich M, Rief N, Kuhne M, et al. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci U S A 2005;102:8984–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coakley FV, Gould R, Yeh BM, et al. CT radiation dose: what can you do right now in your practice? AJR Am J Roentgenol 2011;196:619–25 [DOI] [PubMed] [Google Scholar]
- 9. Kalender WA, Buchenau S, Deak P, et al. Technical approaches to the optimisation of CT. Phys Med 2008;24:71–79 [DOI] [PubMed] [Google Scholar]
- 10. Kalra MK, Maher MM, Toth TL, et al. Strategies for CT radiation dose optimization. Radiology 2004;230:619–28 [DOI] [PubMed] [Google Scholar]
- 11. Manninen AL, Isokangas JM, Karttunen A, et al. A comparison of radiation exposure between diagnostic CTA and DSA examinations of cerebral and cervicocerebral vessels. AJNR Am J Neuroradiol 2012;33:2038–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soderberg M, Gunnarsson M. Automatic exposure control in computed tomography: an evaluation of systems from different manufacturers. Acta Radiol 2010;51:625–34 [DOI] [PubMed] [Google Scholar]
- 13. van Straten M, Deak P, Shrimpton PC, et al. The effect of angular and longitudinal tube current modulations on the estimation of organ and effective doses in x-ray computed tomography. Med Phys 2009;36:4881–89 [DOI] [PubMed] [Google Scholar]
- 14. Bittencourt MS, Schmidt B, Seltmann M, et al. Iterative Reconstruction in Image Space (IRIS) in cardiac computed tomography: initial experience. Int J Cardiovasc Imaging 2011;27:1081–87 [DOI] [PubMed] [Google Scholar]
- 15. Leipsic J, Heilbron BG, Hague C. Iterative reconstruction for coronary CT angiography: finding its way. Int J Cardiovasc Imaging 2012;28:613–20 [DOI] [PubMed] [Google Scholar]
- 16. Haberland U, Klotz E, Abolmaali N. Performance assessment of dynamic spiral scan modes with variable pitch for quantitative perfusion computed tomography. Invest Radiol 2010;45:378–86 [DOI] [PubMed] [Google Scholar]
- 17. Singh S, Kalra MK, Moore MA, et al. Dose reduction and compliance with pediatric CT protocols adapted to patient size, clinical indication, and number of prior studies. Radiology 2009;252:200–08 [DOI] [PubMed] [Google Scholar]
- 18. Kalender WA, Deak P, Kellermeier M, et al. Application- and patient size-dependent optimization of x-ray spectra for CT. Med Phys 2009;36:993–1007 [DOI] [PubMed] [Google Scholar]
- 19. Bjorkdahl P, Nyman U. Using 100- instead of 120-kVp computed tomography to diagnose pulmonary embolism almost halves the radiation dose with preserved diagnostic quality. Acta Radiol 2010;51:260–70 [DOI] [PubMed] [Google Scholar]
- 20. Eller A, May MS, Scharf M, et al. Attenuation-based automatic kilovolt selection in abdominal computed tomography: effects on radiation exposure and image quality. Invest Radiol 2012;47:559–65 [DOI] [PubMed] [Google Scholar]
- 21. Matsuoka S, Hunsaker AR, Gill RR, et al. Vascular enhancement and image quality of MDCT pulmonary angiography in 400 cases: comparison of standard and low kilovoltage settings. AJR Am J Roentgenol 2009;192:1651–56 [DOI] [PubMed] [Google Scholar]
- 22. Schindera ST, Graca P, Patak MA, et al. Thoracoabdominal-aortoiliac multidetector-row CT angiography at 80 and 100 kVp: assessment of image quality and radiation dose. Invest Radiol 2009;44:650–55 [DOI] [PubMed] [Google Scholar]
- 23. Wildberger JE, Mahnken AH, Schmitz-Rode T, et al. Individually adapted examination protocols for reduction of radiation exposure in chest CT. Invest Radiol 2001;36:604–11 [DOI] [PubMed] [Google Scholar]
- 24. Winklehner A, Goetti R, Baumueller S, et al. Automated attenuation-based tube potential selection for thoracoabdominal computed tomography angiography: improved dose effectiveness. Invest Radiol 2011;46:767–73 [DOI] [PubMed] [Google Scholar]
- 25. Ramgren B, Bjorkman-Burtscher IM, Holtas S, et al. CT angiography of intracranial arterial vessels: impact of tube voltage and contrast media concentration on image quality. Acta Radiol 2012;53:929–34 [DOI] [PubMed] [Google Scholar]
- 26. Marin D, Nelson RC, Samei E, et al. Hypervascular liver tumors: low tube voltage, high tube current multidetector CT during late hepatic arterial phase for detection: initial clinical experience. Radiology 2009;251:771–79 [DOI] [PubMed] [Google Scholar]
- 27. Ramgren B, Bjorkman-Burtscher IM, Holtas S, et al. CT angiography of intracranial arterial vessels: impact of tube voltage and contrast media concentration on image quality. Acta Radiol 2012;53:929–34 [DOI] [PubMed] [Google Scholar]
- 28. Beitzke D, Wolf F, Edelhauser G, et al. Computed tomography angiography of the carotid arteries at low kV settings: a prospective randomised trial assessing radiation dose and diagnostic confidence. Eur Radiol 2011;21:2434–44 [DOI] [PubMed] [Google Scholar]
- 29. Byrnes KR, Ross CB. The current role of carotid duplex ultrasonography in the management of carotid atherosclerosis: foundations and advances. Int J Vasc Med 2012. March 7 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Babiarz LS, Romero JM, Murphy EK, et al. Contrast-enhanced MR angiography is not more accurate than unenhanced 2D time-of-flight MR angiography for determining ≥70% internal carotid artery stenosis. AJNR Am J Neuroradiol 2009;30:761–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anzidei M, Napoli A, Zaccagna F, et al. Diagnostic accuracy of colour Doppler ultrasonography, CT angiography and blood-pool-enhanced MR angiography in assessing carotid stenosis: a comparative study with DSA in 170 patients. Radiol Med 2012;117:54–71 [DOI] [PubMed] [Google Scholar]
- 32. Alkadhi H, Leschka S. Radiation dose of cardiac computed tomography: what has been achieved and what needs to be done. Eur Radiol 2011;21:505–09 [DOI] [PubMed] [Google Scholar]
- 33. Kim JE, Newman B. Evaluation of a radiation dose reduction strategy for pediatric chest CT. AJR Am J Roentgenol 2010;194:1188–93 [DOI] [PubMed] [Google Scholar]
- 34. McCollough CH, Bruesewitz MR, Kofler JM, Jr. CT dose reduction and dose management tools: overview of available options. Radiographics 2006;26:503–12 [DOI] [PubMed] [Google Scholar]
- 35. Silva AC, Lawder HJ, Hara A, et al. Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. AJR Am J Roentgenol 2010;194:191–99 [DOI] [PubMed] [Google Scholar]
- 36. Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010;256:32–61 [DOI] [PubMed] [Google Scholar]
- 37. Hou Y, Xu S, Guo W, et al. The optimal dose reduction level using iterative reconstruction with prospective ECG-triggered coronary CTA using 256-slice MDCT. Eur J Radiol 2012;81:3905–11 [DOI] [PubMed] [Google Scholar]