Abstract
Uterine cervical adenosquamous carcinoma in situ was originally defined as having either a uniform population of cells with features intermediate in appearance between glandular and squamous cells, or a mixture of distinct glandular and squamous components within a single lesion. The former type would likely be reclassified today as stratified mucin-producing intraepithelial lesion (SMILE), while the latter type is vanishingly rare. Here, we report a novel case of bona fide adenosquamous carcinoma in situ, which exhibits two morphologically and immunophenotypically distinct components: 1) an inner glandular component composed of a single layer of p40-negative, ciliated, mucin-producing dysplastic columnar cells and 2) an outer p40-positive, stratified dysplastic squamous component otherwise identical to CIN-3. Both components show block-positive staining for p16 and are positive for high-risk HPV RNA by in situ hybridization (ISH). Our finding expands the histological spectrum of HPV-associated pre-invasive cervical lesions while also providing further evidence that HPV-driven processes can exhibit ciliated morphology.
Keywords: Ciliated, HPV, Adenosquamous, Cervix, Carcinoma, In Situ
Introduction
The squamocolumnar junction of the uterine cervix is well known for being uniquely susceptible to human papillomavirus (HPV) infection1 and gives rise to HPV-associated pre-invasive lesions with a range of phenotypes, including those with glandular differentiation, squamous differentiation and indeterminate differentiation2. Adenosquamous carcinoma in situ is a rare preinvasive lesion classically defined as having either a uniform population of cells with features intermediate in appearance between glandular and squamous cells, or a mixture of distinct glandular and squamous components3. Today, the former type would be undoubtedly reclassified as “stratified mucin-producing intraepithelial lesion” (SMILE)2. True examples of the latter type of adenosquamous carcinoma in situ are likely extremely rare. Here we report a bona fide example of adenosquamous carcinoma in situ of the latter type with some unusual features, including conspicuous cilia and goblet cell-like mucin in the glandular component. This unusual adenosquamous carcinoma in situ was accompanied by both conventional adenocarcinoma in situ as well as conventional high-grade squamous intraepithelial lesion/cervical intraepithelial neoplasia-3 (HSIL/CIN-3). Diagnostic implications as well as the similarity of this variant in situ carcinoma to the recently-described invasive ciliated HPV-related carcinoma of the head and neck4,5 are discussed.
Case Report
The patient, a 29-year-old previously healthy woman, had a Pap test showing HSIL. A subsequent cervical biopsy confirmed the presence of HSIL/CIN-3. However, an excisional procedure was not performed at that time as the patient was lost to follow-up for 14 months, after which time a repeat Pap test showed persistent HSIL. Thirty-four months after her initial diagnosis of HSIL, the patient underwent a loop electrocautery excision (LEEP) procedure and endocervical curettage.
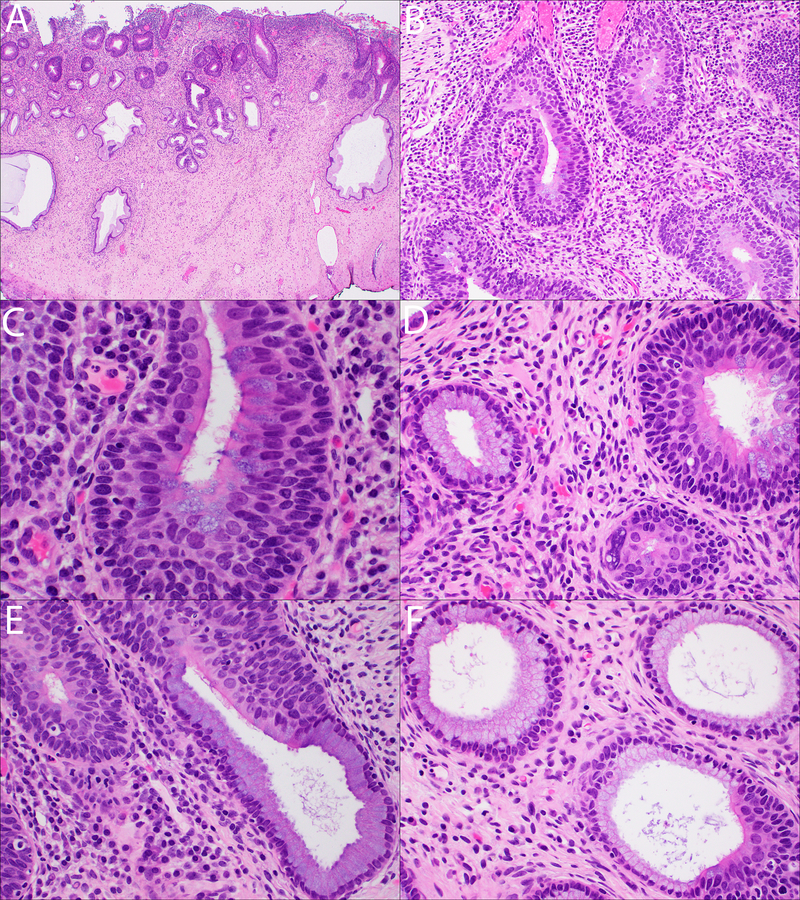
Sections from the LEEP specimen revealed several foci of adenosquamous carcinoma in situ, which involved both the overlying surface epithelium as well as the underlying endocervical glands (Figures 1A–1B). The adenosquamous carcinoma in situ was consistently composed of two very orderly components: 1) an inner glandular component composed of a single layer of atypical columnar cells with an extensively ciliated surface and scattered goblet cell-like mucin-filled vacuoles and 2) an immediately subjacent outer dysplastic stratified squamous component without any mucin vacuoles (Figures 1C–1D). The degree of cytologic atypia in the inner glandular component was moderate at best, with slightly enlarged, rounded nuclei with occasional nucleoli and smooth chromatin. While occasional luminally located mitotic figures and focal apoptotic bodies were seen, they were not as conspicuous those of conventional adenocarcinoma in situ (AIS). The nuclei were notably larger than those of the benign adjacent endocervical glandular cells; some of these adjacent benign endocervical glandular cells also showed focal cilia, implying the presence of focal tubal metaplasia (Figures 1D–1F). The cytological features of the subjacent, outer squamous component were identical to those of conventional CIN-3 (Figures 1C–1F). Where the adenosquamous carcinoma in situ partially involved the endocervical glands, its dual differentiation became most evident, as both the glandular and squamous components “traveled together” right up until the point where the lesional epithelium met the uninvolved glandular epithelium (Figures 1E–1F).
Figures 1.
A–B: Low magnification views of ciliated mucin-producing adenosquamous carcinoma in situ (A: H&E, 20x; B: H&E, 200x).
C: Ciliated mucin-producing adenosquamous carcinoma in situ, characterized by two components: 1) an inner single layer of ciliated, mucin-producing dysplastic glandular cells and 2) a subjacent stratified dysplastic squamous component (H&E, 600x).
D: Histological features of mucin-producing adenosquamous carcinoma in situ (right) and focal ciliated epithelium in the adjacent normal endocervical glands (left) (H&E, 400x).
E-F: Ciliated mucin-producing adenosquamous carcinoma in situ is seen partially involving endocervical glands (both images: H&E, 400x).
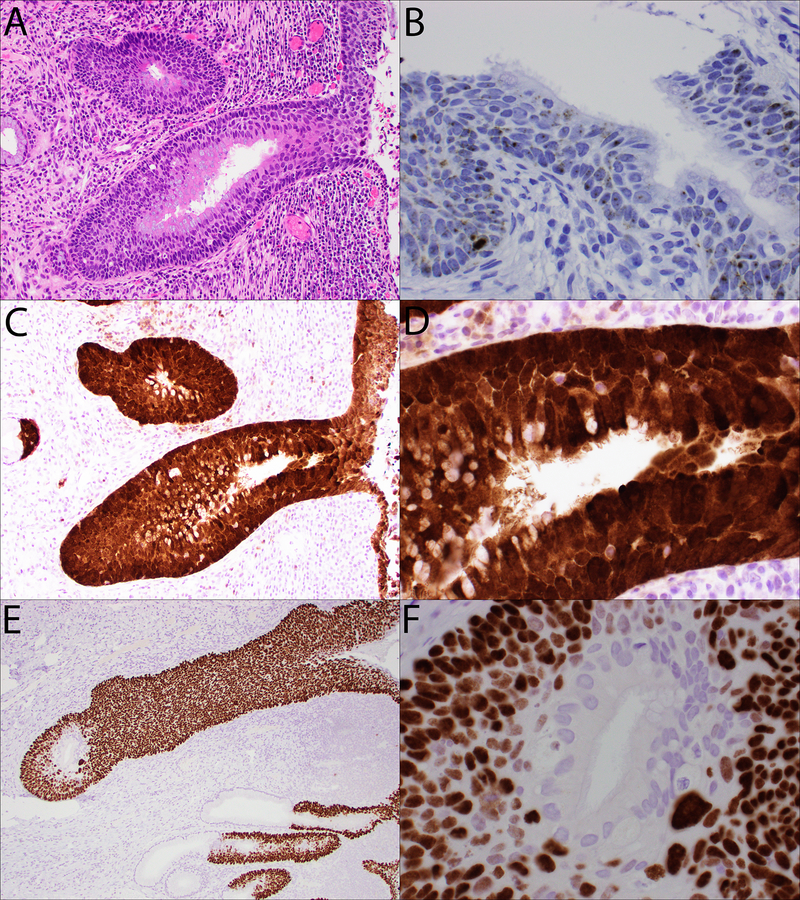
By immunohistochemistry, the inner glandular component was completely negative for p40, while the subjacent outer squamous component was diffusely and strongly positive for p40 (Figures 2E–2F). Both components showed unequivocal block-positive staining pattern for p16 (Figures 2C–2D). RNA in situ hybridization (ISH) testing for high-risk HPV subtypes was positive in both the glandular and squamous components (Figure 2B).
Figure 2.
A:H&E-stained section of ciliated mucin-producing adenosquamous carcinoma in situ for reference (200x).
B: High-risk HPV RNA ISH analysis shows positive staining with punctate cytoplasmic and nuclear signals in both components of the ciliated mucin-producing adenosquamous carcinoma in situ (400x).
C-D: An immunohistochemical stain for p16 shows block-positive immunoreactivity in both the ciliated, mucin-producing glandular component and the subjacent squamous component (C: 200x; D: 600x).
E-F: An immunohistochemical stain for p40 is negative in the ciliated, mucin-producing glandular component, but is strongly and diffusely positive in the subjacent squamous component (E: 200x; F: 600x).
Minor components of conventional AIS and HSIL were present in the LEEP specimen.
HSIL extended to the endocervical and deep margins, but the margins were negative for adenosquamous carcinoma in situ and conventional AIS. No dysplasia was seen in endocervical curettage. A cold knife conization procedure was scheduled but was delayed due to the COVID 19 pandemic.
Materials and Methods
Formalin-fixed paraffin-embedded (FFPE) tissue sectioned at 5 μm increments was stained with hematoxylin and eosin (H&E) and immunohistochemical stains. Immunohistochemistry was performed on a Benchmark Ultra autostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA), using commercially available antibodies for p16 (E6H4, predilute, Ventana, AZ, USA) and p40 (BC28(m), predilute, BioCare Medical, CA, USA). Unstained slides (FFPE, 5 μm sections) were sent to Mayo Clinic Laboratories for high risk HPV E6/E7 RNA in situ hybridization testing, performed according to previously published guidelines6.
Discussion
Based on classical descriptions of adenosquamous carcinoma in situ3, it is likely that the vast majority of these lesions would be reclassified today as stratified mucin-producing intraepithelial lesion (SMILE)2. However, SMILE is debatably not at all an adenosquamous carcinoma in situ in the purest sense, as it shows very limited expression of squamous markers such as p40 and p63 (typically restricted to the basal aspect) and is also negative for markers of adenocarcinoma in situ, such as IMP37. Here, we report a bona fide, biphasic example of adenosquamous carcinoma in situ with distinct squamous and glandular elements, with an unusual ciliated and goblet cell morphology in the glandular component. The presence of cilia raises the obvious objection that this appearance is simply due to endocervical glandular involvement by CIN-3, with tuboendometrioid metaplasia of the overlying glandular epithelium. However, several lines of evidence militate against this alternative hypothesis. First, the glandular cells in this lesion are both atypical (albeit only moderately so) and mitotically active. Second, the glandular component is diffusely and strongly positive for p16 with a block-positive pattern; a valid objection would be that tuboendometrioid metaplasia may also show considerable staining for p168,9, though we would argue that the level of staining seen in the current lesion exceeds even that expected for tuboendometrioid metaplasia8,9. Third, the presence of intestinal-type goblet cells in HPV-associated neoplasia is well established10 and typically not seen in benign endocervical glands. We attempted to perform additional immunohistochemistry for estrogen and progesterone receptors since they are typically positive in normal endocervical glands and tuboendometrioid metaplasia, while negative in HPV associated glandular neoplasia. Unfortunately, the lesion was no longer present on subsequent sections. Perhaps the most compelling argument in this case is the transcriptional activity of high-risk HPV identified in the glandular component by in situ hybridization (ISH) testing.
While the ISH results in this case establish the dysplastic nature of both components of this lesion, they do not entirely rule out the possibility that the its appearance could be the result of HSIL undermining pre-existing ciliated, mucin-producing AIS. While we cannot entirely exclude this possibility, a number of findings in this case militate against this hypothesis. First of all, while separate small foci of AIS were present in this case without an associated dysplastic squamous component, these were exclusively of the conventional, usual type and lacked cilia or goblet cells. By contrast, the ciliated, mucin-producing glandular component of this biphasic lesion was never seen by itself, unaccompanied by the dysplastic squamous component; rather, the two components were inextricably paired together. Nowhere was this more dramatically apparent than when the lesion partially involved endocervical glands (Figures 1E–1F). In these foci, the transition between benign endocervical glandular epithelium to dysplastic epithelium was marked by the immediate acquisition of a biphasic appearance, even when the involvement was extremely focal (in particular, Figure 1F).
In an attempt to identify additional cases with this morphology, two of the authors (J.X. and P.W.) screened slides from an additional 121 archived cases of cervical excisional specimens with a diagnosis of HSIL or AIS diagnosed at our institution between 2012–2017. However, we could not identify a second case with this variant morphology.
Compared to previously published reports, this lesion does show considerable similarity to “endocervical adenocarcinoma in situ of tubal type” described by Schlesinger and Silverberg 11. However, in their description and the photographs shown in their publication, an orderly, biphasic glandular and squamous arrangement was not evident. Furthermore, abundant mucin vacuoles (seen in the inner glandular component of our case) were not a feature described by these authors. In their discussion, however, Schlesinger and Silverberg postulated that differentiation along endometrial lines could explain the cilia seen in their cases11. Indeed, since endometrioid tumors and even non-neoplastic endometrial glands can show both ciliated and mucinous metaplasia12, it is possible that the glandular component of this adenosquamous carcinoma in situ is simply showing divergent endometrial-type differentiation.
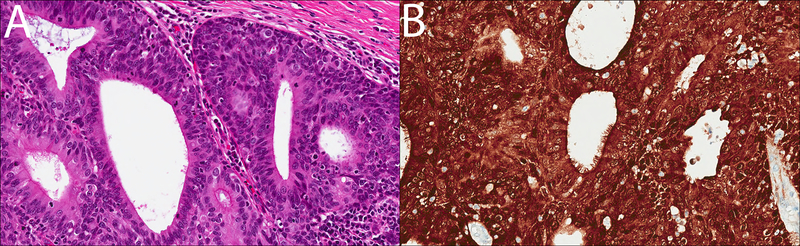
The greatest similarity to this case can actually be seen best in the Head and Neck literature. The shared morphology between our HPV-related ciliated adenosquamous carcinoma in situ, the invasive HPV-related ciliated carcinoma described by Bishop and Westra4 and the ciliated adenosquamous carcinoma described by Radkay-Gonzalez et al5 is hard to overlook. As in our case, these lesions show a biphasic, malignant HPV-driven adenosquamous proliferation with an outer squamous component and an inner ciliated, glandular component (Figures 3A–B). Of course, an obvious caveat is that our patient’s lesion is an in situ carcinoma and the lesions described by these authors are invasive carcinomas.
Figure 3.
A–B: A case of ciliated HPV-associated carcinoma of the head and neck, for comparison. Histologically, ciliated HPV-associated carcinoma of the head and neck also shows a biphasic appearance, with a focally ciliated inner glandular component and a subjacent stratified squamous component (A; H&E, 400x), both of which show block-positive immunoreactivity for p16 (B; 400x). Images courtesy of Dr. Bin Xu.
Radkay-Gonzalez et al point out the similarity of their adenosquamous carcinomas to mucoepidermoid carcinoma of the salivary gland type5. This point is relevant to gynecologic pathologists, since mucoepidermoid carcinoma of the cervix has also been described2. However, all of the lesions in the series from Radkay-Gonzalez et al were negative for MAML2 gene rearrangements, the defining molecular genetic marker for mucoepidermoid carcinomas. Furthermore, as in our case, the lesions in both head and neck series were diffusely positive for p16 by immunohistochemistry and showed positive signals in both the squamous and glandular components by high-risk HPV in situ hybridization4,5, confirming their HPV association, while simultaneously militating against a fusion-driven salivary gland type tumor.
The shared common ground in our case, the cervical lesions described by Schlesinger and Silverberg11 and the invasive head and neck carcinomas described by Bishop and Westra4 and Radkay-Gonzalez et al5 is the presence of cilia in HPV-associated neoplasia. This feature underscores the morphological plasticity of HPV-associated neoplasia at various anatomic sites, which includes carcinomas resembling adenoid cystic carcinoma or other salivary gland neoplasms13, adenoid basal carcinomas2, adenosquamous carcinomas5,14, glassy cell carcinomas2, both in situ and invasive stratified mucin-producing carcinomas7 intestinal-type mucinous adenocarcinoma10, lymphoepithelioma-like carcinomas2,14 and high-grade neuroendocrine carcinomas2,15. It is likely that the presence of cilia in HPV-associated carcinomas simply represents another manifestation of this divergent differentiation.
Importantly, the presence of cilia in the head and neck and in the gynecologic tract has traditionally been ascribed to benign processes4,5,11. While the presence of cilia in glandular lesions in the head and neck has long been associated with branchial cleft cysts4,5 and, in the cervix, with benign tuboendometrioid metaplasia11, HPV-associated malignant and premalignant lesions with glandular differentiation at both locations can indeed produce cilia, albeit rarely. It is important to recognize that one should not discount a lesion as benign solely based on the presence of cilia.
In summary, we report a rare, novel form of HPV-associated cervical adenosquamous carcinoma in situ with abundant cilia and goblet cells that joins the ranks of ciliated malignancies associated with HPV infection4,5,11.
Acknowledgments
Sources of Support: Department of Pathology and Laboratory Medicine, University of Wisconsin Hospital and Clinics, Madison, WI
Footnotes
Financial disclosure: All authors declare no conflict of interest
References
- 1.Herfs M, Yamamoto Y, Laury A, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012;109(26):10516–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurman RJ, International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: International Agency for Research on Cancer; 2014. [Google Scholar]
- 3.Zaino RJ. Symposium part I: adenocarcinoma in situ, glandular dysplasia, and early invasive adenocarcinoma of the uterine cervix. Int J Gynecol Pathol. 2002;21(4):314–326. [DOI] [PubMed] [Google Scholar]
- 4.Bishop JA, Westra WH. Ciliated HPV-related Carcinoma: A Well-differentiated Form of Head and Neck Carcinoma That Can Be Mistaken for a Benign Cyst. Am J Surg Pathol. 2015;39(11):1591–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radkay-Gonzalez L, Faquin W, McHugh JB, Lewis JS Jr., Tuluc M, Seethala RR. Ciliated Adenosquamous Carcinoma: Expanding the Phenotypic Diversity of Human Papillomavirus-Associated Tumors. Head Neck Pathol. 2016;10(2):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol 2012;36(12):1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lastra RR, Park KJ, Schoolmeester JK. Invasive Stratified Mucin-producing Carcinoma and Stratified Mucin-producing Intraepithelial Lesion (SMILE): 15 Cases Presenting a Spectrum of Cervical Neoplasia With Description of a Distinctive Variant of Invasive Adenocarcinoma. Am J Surg Pathol. 2016;40(2):262–269. [DOI] [PubMed] [Google Scholar]
- 8.Cameron RI, Maxwell P, Jenkins D, McCluggage WG. Immunohistochemical staining with MIB1, bcl2 and p16 assists in the distinction of cervical glandular intraepithelial neoplasia from tubo-endometrial metaplasia, endometriosis and microglandular hyperplasia. Histopathology. 2002;41(4):313–321. [DOI] [PubMed] [Google Scholar]
- 9.O’Neill CJ, McCluggage WG. p16 expression in the female genital tract and its value in diagnosis. Adv Anat Pathol. 2006;13(1):8–15. [DOI] [PubMed] [Google Scholar]
- 10.McCluggage WG, Shah R, Connolly LE, McBride HA. Intestinal-type cervical adenocarcinoma in situ and adenocarcinoma exhibit a partial enteric immunophenotype with consistent expression of CDX2. Int J Gynecol Pathol. 2008;27(1):92–100. [DOI] [PubMed] [Google Scholar]
- 11.Schlesinger C, Silverberg SG. Endocervical adenocarcinoma in situ of tubal type and its relation to atypical tubal metaplasia. Int J Gynecol Pathol. 1999;18(1):1–4. [DOI] [PubMed] [Google Scholar]
- 12.Kurman RJ. Blaustein’s pathology of the female genital tract. New York, NY: Springer Berlin Heidelberg; 2019. [Google Scholar]
- 13.Bishop JA, Andreasen S, Hang JF, et al. HPV-related Multiphenotypic Sinonasal Carcinoma: An Expanded Series of 49 Cases of the Tumor Formerly Known as HPV-related Carcinoma With Adenoid Cystic Carcinoma-like Features. Am J Surg Pathol. 2017;41(12):1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens TM, Bishop JA. HPV-related carcinomas of the head and neck: morphologic features, variants, and practical considerations for the surgical pathologist. Virchows Arch. 2017;471(2):295–307. [DOI] [PubMed] [Google Scholar]
- 15.Thompson ED, Stelow EB, Mills SE, Westra WH, Bishop JA. Large Cell Neuroendocrine Carcinoma of the Head and Neck: A Clinicopathologic Series of 10 Cases With an Emphasis on HPV Status. Am J Surg Pathol. 2016;40(4):471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]