Abstract
Objective:
Constipation is highly prevalent in advanced chronic kidney disease (CKD), due in part to dietary (e.g., fiber) restrictions, and is often managed by laxatives; however, the effect of laxative use on kidney function in advanced CKD remains unclear. We aimed to examine the association of laxative use with longitudinal change in estimated glomerular filtration rate (eGFR) in patients with advanced CKD.
Design and Methods:
In a retrospective cohort of 43,622 US veterans transitioning to end-stage renal disease (ESRD) from 2007–2015, we estimated changes in eGFR (slope) by linear mixed-effects models using ≥2 available outpatient eGFR measurements during the 2-year period before transition to ESRD. The association of laxative use with change in eGFR was examined by testing the interaction of time-varying laxative use with time for eGFR slope in the mixed-effects models with adjustment for fixed and time-varying confounders.
Results:
Laxatives were prescribed in 49.8% of patients during the last 2-year pre-ESRD period. In the crude model, time-varying laxative use was modestly associated with more progressive eGFR decline compared with non-use of laxatives (median [IQI], −7.1 [−11.9, −4.3] vs. −6.8 [−11.6, −4.0] mL/min/1.73 m2/year, P<0.001). After multivariable adjustment, a faster eGFR decline associated with laxative use (vs. non-use of laxatives) remained statistically significant, although the between-group difference in eGFR slope was minimal (median [IQI], −8.8 [−12.9, −5.9] vs. −8.6 [−12.6, −5.6] mL/min/1.73 m2/year, P<0.001). The significant association was no longer evident across different types of laxatives (i.e., stool softeners, stimulants, or hyperosmotics).
Conclusion:
There was a clinically negligible association of laxative use with change in eGFR during the last 2-year pre-ESRD period, suggesting the renal safety profile of laxatives in advanced CKD patients.
Keywords: chronic kidney disease, constipation, diet, estimated glomerular filtration rate, gut microbiota, laxative
INTRODUCTION
Constipation is highly prevalent in patients with chronic kidney disease (CKD), especially in its advanced stages, due in part to their dietary restrictions (e.g., limited fiber and/or fluid intake), high prevalence of comorbidities (e.g., diabetes mellitus), concomitant medication use (e.g., phosphate binders and diuretics), and altered gut microbiota.1–6 Although constipation is usually perceived as a benign, self-limited condition, its chronic symptoms negatively affect patients’ quality of life and impose a considerable social and economic burden.7,8 Furthermore, recent epidemiological studies have revealed that constipation is independently associated with adverse renal outcomes, such as CKD progression and end-stage renal disease (ESRD), potentially through processes mediated by altered gut microbiota and/or increased production of fecal metabolites.5,9
Nonpharmacological treatments such as increased dietary fiber intake and physical activity are traditionally considered the first step of comprehensive constipation management.10 However, due to the complexity of predisposing factors in patients with CKD (e.g., high prevalence of hyperkalemia and cardiovascular diseases), these nonpharmacological approaches may not always be practical and effective, and hence pharmacological interventions are often required for constipation management in this particular population.11 Currently, a wide range of pharmacological agents are available, including commonly used laxative compounds (e.g., stool softeners, hyperosmotics, stimulants, bulk-formers, and lubricants) and relatively new laxatives with more physiological mechanisms of action (e.g., chloride channel activators, guanylate cyclase C receptor agonists, selective serotonin 5-HT4 receptor agonists, and ileal bile acid transporter inhibitors).12,13 While these pharmacological agents have substantially contributed to the management of constipation in patients with CKD, the relatively easy access to these agents can facilitate their long term-use, inappropriate dosing, and even abuse, which in turn could lead to serious health consequences.14–16 In particular, among patients with advanced stages of CKD who are highly vulnerable to physiological stress and adverse drug reactions,17 electrolyte and fluid disturbances and/or potential drug toxicity associated with laxative use could induce kidney injury, potentially contributing to progressive loss of kidney function. Meanwhile, given the greater risk of adverse kidney events associated with constipation and the fact that the gut plays increasing roles in acid-base and mineral homeostasis and disposal of nitrogenous waste products with declining kidney function,18 the use of laxatives may enhance these ancillary gastrointestinal roles and thereby exert potential renoprotective effects in patients with advanced CKD.
Despite these plausible mechanisms, to the best of our knowledge no previous studies have investigated the effect of laxative use on longitudinal change in kidney function among patients with advance stages of CKD. Our aim was to examine the association of laxative use with change in estimated glomerular filtration rate (eGFR) during the last 2-years before transition to ESRD using a large nationally representative cohort of US veterans with advanced non-dialysis dependent CKD (NDD-CKD) transitioning to dialysis.
METHODS
Study Population
We analyzed longitudinal data from a nationally representative retrospective cohort study of US veterans transitioning to ESRD.19–21 In the present study, a total of 102,477 US veterans who transitioned to ESRD from October 1, 2007 through March 31, 2015 were identified from the USRDS as a source population. Among these, 60,187 patients with at least one outpatient eGFR measurement recorded at any VA facility prior to dialysis initiation were identified. After excluding patients without ≥2 outpatient eGFR measurements during the last 2-year pre-ESRD period (i.e., baseline period) (n = 15,384), those who did not have any prescription information during the same 2-year baseline period (n = 414), and those with missing baseline covariates (n = 767), 43,622 patients were included in the final analytical cohort (Figure 1).
Figure 1.
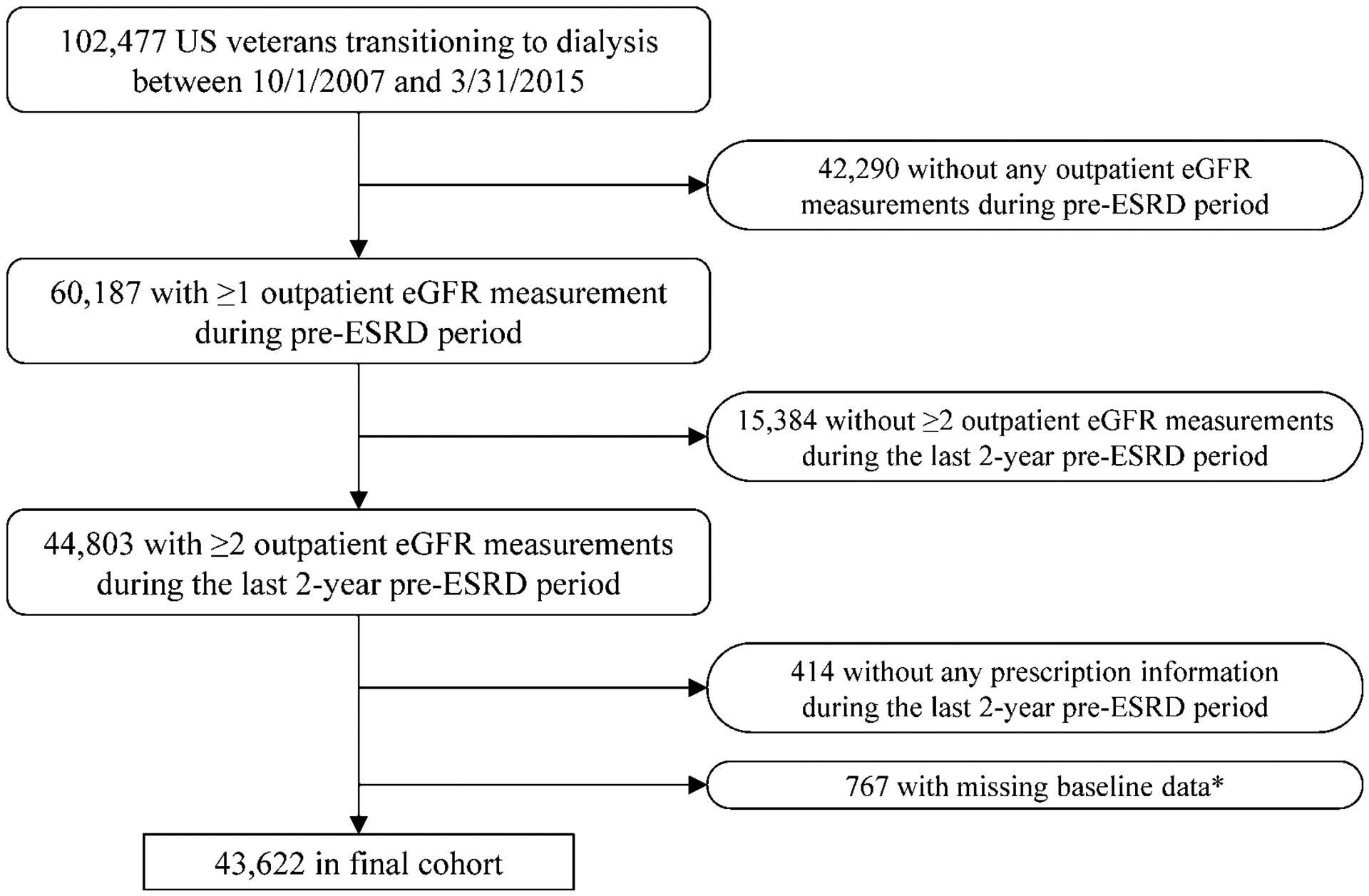
Algorithm used to define the analytical cohort
*Except for laboratory data (i.e., hemoglobin, serum albumin, serum phosphorus, serum bicarbonate, and urine albumin-creatinine ratio).
Abbreviations: eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease
Exposure variable
The primary exposure of interest was laxative use during the last 2-year pre-ESRD period. Given the time-varying nature of laxative use (due in part to symptom improvement and/or adverse events such as diarrhea) and its potential influence on the change in eGFR over time, laxative use was treated as a time-varying exposure in this study. Time-varying laxative use was defined based on the prescription status at the time of each outpatient eGFR measurement during the last 2-year pre-ESRD period (i.e., whether or not the day of eGFR measurement was covered by any prescribed laxative). Laxative agents were ascertained according to prescription information for the following six types of laxatives: stool softeners, hyperosmotics, stimulants, bulk formers, chloride channel activator, and lubricants (Supplementary Table 1).
Covariates
Patient demographic characteristics, including age, sex, and self-identified race, were ascertained from the following three national databases: the USRDS, Veterans Affairs (VA), and Centers for Medicare and Medicaid Services (CMS). Data on marital and smoking status were obtained from VA records only.22,23 Pre-existing comorbidities were identified from the VA Inpatient and Outpatient Medical SAS Datasets, using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic and procedure codes and Current Procedural Terminology codes, as well as from VA/CMS data.24 The Charlson Comorbidity Index (CCI) score was calculated using the Deyo modification for administrative datasets, without including kidney disease.25 Cardiovascular disease was defined as the presence of diagnostic codes for coronary artery disease, angina, myocardial infarction, or cerebrovascular disease.26 Prescribed medications were ascertained using both inpatient and outpatient prescriptions sourced from CMS Medicare Part D and VA pharmacy dispensation records,27 and patients with at least one prescription over the 2-year pre-ESRD baseline period were recorded as treated with the medication. Select medications were also treated as time-varying covariates similar to the use of laxatives (as described above). Data on body mass index (BMI), blood pressure, and laboratory tests were obtained from VA research databases as previously described,28,29 and their time-averaged values (defined as the average of each covariate during the 2-year pre-ESRD period) were used to characterize patients and to model fixed exposure. Time-varying systolic blood pressure (SBP) was defined using SBP values on the same day as outpatient eGFR measurements. Time-varying laboratory variables (i.e., hemoglobin, serum albumin, serum phosphorus, serum bicarbonate, and urine albumin-creatinine ratio) were defined using respective laboratory values measured within +/−7 days of the eGFR measurements. The eGFR was calculated with the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation using outpatient serum creatinine and demographic data.30
Outcome Assessment
The primary outcome of interest was the yearly rate of change in eGFR (slope) over the last 2-year pre-ESRD period. The eGFR slope was assessed by linear mixed-effects models using all available outpatient eGFR measurements during the 2-year pre-ESRD period.
Statistical Analysis
Baseline patient characteristics were summarized for the entire analytical population (n = 43,622) and presented as number (percentages) for categorical variables and mean (standard deviation [SD]) for continuous variables with a normal distribution or median (interquartile interval [IQI]) for those with a skewed distribution. In order to examine the association of time-varying laxative use with change in eGFR, we accounted for time-varying laxative use in the adjusted linear mixed-effects models for eGFR slope estimates (i.e., crude model). Changes in eGFR were calculated separately by laxative use status (i.e., with or without laxative use), and the difference in eGFR slope by laxative use status was evaluated by testing the interaction of time-varying laxative use with time for change in eGFR in the linear mixed-effects models. Subsequently, to assess the impact of potential confounders, we additionally performed multivariable-adjusted mixed-effects models by further accounting for fixed and time-varying characteristics along with their interactions with time based on theoretical considerations and their availability in this study. The fixed (time-independent) confounders included age, sex, race, smoking status, BMI averaged over the 2-year pre-ESRD period, comorbidities (diabetes, congestive heart failure, cardiovascular disease, cerebrovascular disease, mild liver disease, moderate/severe liver disease, and constipation), and cumulative length of hospital stay during the 2-year pre-ESRD period; while, the time-varying confounders included the use of renin-angiotensin system inhibitor (RASi), sodium polystyrene sulfonate, bicarbonate, loop diuretics, thiazide diuretics, potassium-sparing diuretics, calcium channel blockers, phosphate binders, non-opioid analgesics, and opioid analgesics. Given the possibility that the effects of diuretics on eGFR slope may vary depending on the other diuretic agents they are being combined with, the potential interaction between loop, thiazide, and potassium-sparing diuretics were also accounted for in the multivariable-adjusted model. Each covariate included in the multivariable adjustment was grand-mean centered as appropriate.
We performed several sensitivity analyses to evaluate the robustness of our main findings. To assess the effect of laxative use on eGFR slopes across different types of laxatives, we identified three major types of laxatives (i.e., stool softeners, hyperosmotics, and stimulants, irrespective of concomitant use of other laxatives) and categorized the status of time-varying laxative use into three groups as the use of laxative of interest, other laxative use, and non-use of laxatives. The association of each laxative use status with change in eGFR was then examined in the crude and multivariable-adjusted mixed-effects models. Due to the relatively high proportion of missing information for time-varying SBP and laboratory variables (i.e., hemoglobin, serum albumin, serum phosphorus, serum bicarbonate, and urine albumin-creatinine ratio) during the 2-year pre-ESRD period, these variables were additionally included in the main model as a sensitivity analysis. Given the fact that sodium biphosphate and magnesium-containing laxatives should generally be avoided in advanced CKD due to their potential adverse effects, we repeated our main analyses after excluding 3,133 patients who had had at least one prescription of such laxatives (i.e., sodium biphosphate, magnesium citrate, or magnesium sulphate) from the final analytical cohort. Since the use of some types of laxative formulations (e.g., oral liquid and liquid enema) may not be captured at the time of eGFR measurement due to the nature of their prescriptions and may thus be underestimated, our main analysis was repeated by treating laxative use as a fixed exposure. Fixed laxative use was defined as having at least two prescriptions of any types of laxatives that were >30 days apart during the 2-year pre-ESRD period. Similarly, three major types of laxatives (i.e., stool softeners, hyperosmotics, and stimulants) were treated as fixed exposures and their association with eGFR slopes were evaluated.
A two-sided P value of <0.05 was used as a threshold of statistical significance for all analyses. All analyses were conducted in SAS Enterprise guide v7.1 (SAS Institute; Cary, NC) and STATA/MP Version 15 (STATA Corporation, College Station, TX). The study was approved by the Institutional Review Boards of the VA Medical centers, with exemption from informed consent.
RESULTS
Patient characteristics
Baseline patient characteristics in the entire analytical cohort are summarized in Table 1. Among 43,622 patients, the mean (SD) age was 69.5 (11.1) years; 98.0% of patients were male; 28.6% were African American; and 76.1% were diabetic. Loop diuretics (76.4%), calcium channel blockers (74.6%), RASi (69.4%), and opioid analgesics (56.8%) were the most commonly prescribed medications. There were a median (IQI) of 8 (4, 15) outpatient eGFR measurements per patient over the 2-year pre-ESRD baseline period, and the mean time-averaged outpatient eGFR was 26.1 mL/min/1.73m2. Laxatives were prescribed in 49.8% of patients, while, 22.5% had a diagnosis of constipation.
Table 1.
Baseline patient characteristics
| Characteristic | N = 43,622 |
|---|---|
| Age, mean (SD), year | 69.5 (11.1) |
| Male sex, n (%) | 42,760 (98.0) |
| Race, n (%) | |
| White | 29,840 (68.4) |
| African American | 12,491 (28.6) |
| Others | 1,291 (3.0) |
| Smoking status, n (%) | |
| Current | 15,656 (35.9) |
| Past | 14,540 (33.3) |
| Never | 13,426 (30.8) |
| Body mass index*, mean (SD), kg/m2 | 29.3 (6.3) |
| Systolic blood pressure*, mean (SD), mmHg | 142.9 (15.5) |
| Comorbidities, n (%) | |
| Diabetes | 33,173 (76.1) |
| Cardiovascular disease | 32,954 (75.5) |
| Congestive heart failure | 27,331 (62.6) |
| Cerebrovascular disease | 18,777 (43.0) |
| Chronic pulmonary disease | 23,771 (54.5) |
| Connective tissue disease | 3,029 (6.9) |
| Mild liver disease | 6,894 (15.8) |
| Moderate/severe liver disease | 1,612 (3.7) |
| Malignancies | 13,354 (30.6) |
| Depression | 15,382 (35.3) |
| Constipation | 9,800 (22.5) |
| Charlson comorbidity index, median (IQI) | 5 (3, 7) |
| Cumulative length of hospitalization, median (IQI), days | 6 (0, 19) |
| Medications, n (%) | |
| RASi | 30,279 (69.4) |
| Calcium channel blockers | 32,545 (74.6) |
| Loop diuretics | 33,308 (76.4) |
| Thiazide diuretics | 15,245 (34.9) |
| Potassium-sparing diuretics | 5,806 (13.3) |
| Phosphate binders | 15,042 (34.5) |
| Sodium polystyrene sulphonate | 9,786 (22.4) |
| Non-opioid analgesics | 14,557 (33.4) |
| Opioid analgesics | 24,793 (56.8) |
| Laxatives | 21,739 (49.8) |
| Time-averaged eGFR*, mean (SD), mL/min/1.73m2 | 26.1 (17.3) |
| First eGFR†, mean (SD), mL/min/1.73m2 | 32.8 (20.1) |
| Number of eGFR measurements, median (IQI) | 8 (4, 15) |
| Hemoglobin*, mean (SD), g/dL | 11.2 (1.6) |
| Serum albumin*, mean (SD), g/dL | 3.5 (0.5) |
| Serum phosphorus*, mean (SD), mg/dL | 4.7 (1.0) |
| Serum bicarbonate*, mean (SD), mEq/L | 23.9 (3.5) |
| Urine albumin-creatinine ratio*, median (IQI), mg/g | 346.5 (27.5, 1,655.8) |
Note: Baseline was defined based on the last 2-year pre-ESRD period.
Values are time-averaged over the 2-year pre-ESRD period.
The first eGFR measured during the 2-year pre-ESRD period.
Abbreviations: eGFR = estimated glomerular filtration rate; IQI = interquartile interval; RASi = renin-angiotensin system inhibitor; SD = standard deviation
Laxative use and eGFR slope
Table 2 shows changes in eGFR associated with time-varying laxative use over the last 2-year pre-ESRD period. In the crude model, the use of laxatives was modestly but significantly associated with more progressive eGFR decline compared with non-use of laxatives (median [IQI], −7.1 [−11.9, −4.3] vs. −6.8 [−11.6, −4.0] mL/min/1.73 m2/year for laxative use and non-use, respectively, P<0.001). After adjustment for various potential confounders, a faster eGFR decline associated with laxative use (vs. non-use of laxatives) remained statistically significant, although the difference in eGFR slope between the two groups was remarkably small (median [IQI], −8.8 [−12.9, −5.9] vs. −8.6 [−12.6, −5.6] mL/min/1.73 m2/year for laxative use and non-use, respectively, P<0.001).
Table 2.
Changes in eGFR associated with time-varying laxative use status during the last 2-year pre-ESRD period (n = 43,622)
| Changes in eGFR (median [IQI], mL/min/1.73 m2 per year) | P value * | ||
|---|---|---|---|
| Laxative use | Non-use of laxatives | ||
| Crude model | −7.1 (−11.9, −4.3) | −6.8 (−11.6, −4.0) | <0.001 |
| Multivariable-adjusted model | −8.8 (−12.9, −5.9) | −8.6 (−12.6, −5.6) | <0.001 |
Note: eGFR slopes were estimated using crude and multivariable-adjusted mixed-effects models. Crude model accounted only for time-varying laxative use, and multivariable-adjusted model was additionally adjusted for fixed (age, sex, race, smoking status, BMI averaged over the 2-year pre-ESRD period, comorbidities [diabetes, CHF, CVD, cerebrovascular disease, mild liver disease, moderate/severe liver disease, and constipation], and cumulative length of hospital stay during the 2-year pre-ESRD period) and time-varying confounders (RASi, sodium polystyrene sulfonate, bicarbonate, loop diuretics†, thiazide diuretics†, potassium-sparing diuretics†, calcium channel blockers, phosphate binders, non-opioid analgesics, and opioid analgesics use).
P values were for the difference in eGFR slope by time-varying laxative use status.
Potential interactions between these diuretics were accounted for.
Abbreviations: BMI = body mass index; CHF = congestive heart failure; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease; IQI = interquartile interval; RASi = renin-angiotensin system inhibitor
When the effect of laxative use on eGFR slope was assessed separately for three specific types of laxatives (i.e., stool softeners, stimulants, or hyperosmotics), a significant difference in eGFR slope was observed only for stool softeners in the crude model, with the decline in eGFR for stool softener use being slightly faster than that for non-use of laxatives (median [IQI], −7.0 [−11.8, −4.2] vs. −6.9 [−11.6, −4.0] mL/min/1.73 m2/year for stool softener use and non-use, respectively; Table 3). After multivariable adjustment, no significant between-group differences (laxative of interest vs. non-use of laxatives) were observed in eGFR slopes across different types of laxatives (Table 3).
Table 3.
Changes in eGFR associated with time-varying (A) stool softeners, (B) stimulants, and (C) hyperosmotics use status during the last 2-year pre-ESRD period (n = 43,622)
| (A) Stool softeners | |||
|---|---|---|---|
| Changes in eGFR (median [IQI], mL/min/1.73 m2 per year) | |||
| Stool softener use | Other laxative use | Non-use of laxatives | |
| Crude model | −7.0 (−11.8, −4.2) | −7.2 (−12.0, −4.4)* | −6.9 (−11.6, −4.0)* |
| (B) Stimulants | |||
| Changes in eGFR (median [IQI], mL/min/1.73 m2 per year) | |||
| Stimulant use | Other laxative use | Non-use of laxatives | |
| Crude model | −7.0 (−11.8, −4.2) | −7.1 (−11.9, −4.3) | −6.8 (−11.6, −4.0) |
| (C) Hyperosmotics | |||
| Changes in eGFR (median [IQI], mL/min/1.73 m2 per year) | |||
| Hyperosmotic use | Other laxative use | Non-use of laxatives | |
| Crude model | −6.9 (−11.7, −4.1) | −7.2 (−11.9, −4.3) | −6.8 (−11.6, −4.0) |
Note: eGFR slopes were estimated using crude and multivariable-adjusted mixed-effects models. Crude model accounted only for time-varying laxative use, and multivariable-adjusted model was additionally adjusted for fixed (age, sex, race, smoking status, BMI averaged over the 2-year pre-ESRD period, comorbidities [diabetes, CHF, CVD, cerebrovascular disease, mild liver disease, moderate/severe liver disease, and constipation], and cumulative length of hospital stay during the 2-year pre-ESRD period) and time-varying confounders (RASi, sodium polystyrene sulfonate, bicarbonate, loop diuretics†, thiazide diuretics†, potassium-sparing diuretics†, calcium channel blockers, phosphate binders, non-opioid analgesics, and opioid analgesics use).
P values <0.05 versus eGFR slope associated with the use of laxative of interest. Unless otherwise specified, no significant difference was observed for eGFR slopes between laxative of interest and other laxative or non-use of laxatives.
Potential interactions between these diuretics were accounted for.
Abbreviations: BMI = body mass index; CHF = congestive heart failure; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease; IQI = interquartile interval; RASi = renin-angiotensin system inhibitor
Changes in eGFR associated with laxative use status were essentially similar even after further accounting for time-varying SBP and laboratory variables in the multivariable-adjusted model and after excluding those with at least one prescription of sodium biphosphate, magnesium citrate, or magnesium sulphate, showing no statistical significance in most (Supplementary Tables 2 and 3). With a few notable exceptions, when laxative use was treated as a fixed exposure, the faster decline in eGFR associated with laxative use (vs. non-use) was more evident, with hyperosmotics use (vs. other laxative use) being significantly associated with faster decline in eGFR; while stool softeners use (vs. other laxative use) being significantly associated with slower eGFR decline, even after multivariable adjustment (Supplementary Tables 4 and 5).
DISCUSSION
In this large national cohort of US veterans transitioning to dialysis, we examined the association of laxative use with change in eGFR during the last 2-year pre-ESRD period, and found that laxative use was only modestly associated with faster eGFR decline compared with non-use of laxatives, after accounting for various potential confounders. While some of the associations between laxative use and eGFR slope described in our study were statistically significant, their magnitude was negligibly small and hence the biological significance of these findings is questionable.
In general, the majority of drugs used for constipation treatment are considered safe when used in the absence of contraindications, without abuse, and under medical supervision in cases where prolonged use is required.31 According to the results from ten clinical trials that evaluated the safety profiles of commonly prescribed laxatives including stool softeners, stimulants, and hyperosmotics,32–41 the overall incidence of drug-related adverse events was highest (up to 72%) for bisacodyl (one of the stimulant laxatives),35 and the most frequently reported adverse events were mild gastrointestinal-related symptoms, such as diarrhea, abdominal pain, flatulence, and nausea. Of note, except for one trial that reported an increase in serum creatinine in 3 out of 49 (6.1%) patients who were treated with lactulose (one of the hyperosmotic laxatives),36 no adverse kidney events were reported in any of these trials, suggesting the renal safety of commonly used laxatives. These trials, however, have investigated the safety of laxatives with relatively short follow-up periods (e.g., 4 weeks) in relatively small cohorts, and hence the long-term safety of these agents in the general population remains unknown. More importantly, most of the previous trials have traditionally excluded patients with abnormal kidney function, as well as those with dehydration, abnormal electrolytes, and secondary constipation caused by underlying diseases and medications,33–35 all of which are typical features of patients with advanced CKD.
Outside the settings of clinical trials, adverse kidney events related to the use of laxatives have been reported mainly in case reports and series, most of which are introducing cases with acute phosphate nephropathy associated with the use of sodium phosphate-containing laxatives42,43 and those with hypokalemic nephropathy associated with laxative abuse.44–46 In addition to these relatively rare adverse kidney events, it is intuitively plausible that laxatives can cause ischemic kidney injury due to diarrhea and resultant dehydration, potentially contributing to progressive loss of kidney function, particularly in patients with advanced CKD who are vulnerable to fluid imbalance. Nevertheless, the effect of laxatives on longitudinal changes in kidney function has been scarcely documented. In the present study, we therefore estimated eGFR slopes using a sophisticated modeling technique in a large and unique cohort of patients with advanced NDD-CKD transitioning to dialysis, and for the first time demonstrated the association of laxative use with change in eGFR during the 2-year period before transitioning to ESRD.
On the whole, our results showing clinically negligible differences (irrespective of statistical significance) in eGFR slope by laxative use status imply that the use of laxatives did not have any influence on kidney function in this group of patients with advanced CKD, which is reassuring given the above mentioned theoretical concerns about the safety of these agents. On the other hand, given the recent evidence on the risk of progressive eGFR decline associated with constipation,5 it is possible that the lack of difference in eGFR slopes between laxative users and non-users was a reflection of a favorable treatment effect of laxatives on kidney function among laxative users through the improvement of underlying constipation. Additionally, albeit still speculative, the potential renoprotective benefit associated with laxative use might be partly through the improvement of altered gut microbiota related to constipation and/or the enhancement of the roles of the gut in disposing toxic fecal metabolites and maintaining mineral homeostasis, which in turn could be a novel pharmacological property of laxatives relevant to patients with advanced CKD. In this context, our findings from different types of laxatives may be of particular value, with a few clinical and research implications. When laxative use was treated as a fixed exposure, we observed that the use of hyperosmotics and stool softeners (vs. other laxative use) was significantly associated with faster and slower eGFR declines, respectively, even after multivariable adjustment. Although we cannot eliminate the possibility of residual and unmeasured confounders (e.g., diet and lifestyle) that might have affected these associations, the finding may suggest the need for careful monitoring of kidney function when treating constipation with hyperosmotics in advanced CKD patients, and also suggests a potentially less nephrotoxic effect of stool softeners, which act by enhancing interaction of water and lipids with stool and appear to have less adverse effects.47 Since a few types of laxatives, such as a chloride channel activator (lubiprostone) and a guanylate cyclase C receptor agonist (linaclotide), have been shown to have unique renoprotective properties in animal studies,48,49 the effect of these relatively new agents on kidney function in advanced CKD may deserve future investigation.
Despite the advantages of this study including its large sample size of patients with advanced CKD, our results must be interpreted in light of some limitations. Our patients were US veterans who were mostly male, and hence the results may not apply to women or non-US veterans. All patients in this cohort survived to the point of initiating dialysis therapy and thus were conditioned on CKD progression. Results may therefore not apply to patients with CKD who do not have progressive loss of kidney function. Due to the relatively high proportion of missing information, time-varying SBP and laboratory variables were not fully accounted for in the main analytical cohort. Information about laxatives prescribed by non-VA/CMS providers and/or obtained over-the-counter was not available; therefore, it is possible that patients using laxatives only from these non-VA/CMS sources were misclassified as non-use of laxatives. In addition, although laxatives were treated as a time-varying exposure, the continuity of laxative exposure during the 2-year pre-ESRD period could not be accounted for. It may also be important to note that the use of laxatives did not necessarily reflect constipation status, especially given the lack of information about subjective symptoms of constipation and the fact that only a minority of patients with constipation seeks medical care.50 In fact, the prevalence of laxative use was substantially higher in our cohort than the prevalence of constipation diagnosis, suggesting that the sensitivity of the ICD code-based constipation diagnosis is limited.
In conclusions, in this large nationwide cohort of 43,622 patients who transitioned to dialysis, we found a clinically negligible association of time-varying laxative use with change in eGFR during the last 2-year pre-ESRD period. Further studies are warranted to clarify whether active interventions with laxatives can provide any therapeutic benefits beyond their conventional indication in patients with advanced CKD.
PRACTICAL APPLICATION
The results of the present study suggest the renal safety profile of laxatives in patients with advanced CKD, whose constipation management relying largely on pharmacological interventions due to their severe dietary restrictions. Furthermore, given the recent evidence on the greater risk of adverse kidney outcomes associated with constipation, our results may also support active interventions with laxatives to treat constipation in advanced CKD patients.
Supplementary Material
Acknowledgements:
Drs. Kovesdy, Kalantar-Zadeh, Streja and Molnar are employees of the US Department of Veterans affairs. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of Veterans Affairs or the US government. The results of this paper have not been published previously in whole or part.
Support:
This study is supported by grant 5U01DK102163 from the National Institute of Health (NIH) to KKZ and CPK, and by resources from the US Department of Veterans Affairs. The data reported here have been supplied in part by the United States Renal Data System (USRDS). Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure:
None of the authors have relevant conflicts of interest.
REFERENCES
- 1.Shirazian S, Radhakrishnan J. Gastrointestinal disorders and renal failure: exploring the connection. Nat Rev Nephrol. 2010;6(8):480–492. [DOI] [PubMed] [Google Scholar]
- 2.Wu MJ, Chang CS, Cheng CH, et al. Colonic transit time in long-term dialysis patients. Am J Kidney Dis. 2004;44(2):322–327. [DOI] [PubMed] [Google Scholar]
- 3.Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am J Kidney Dis. 2016;67(3):483–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quigley EM. The enteric microbiota in the pathogenesis and management of constipation. Best Pract Res Clin Gastroenterol. 2011;25(1):119–126. [DOI] [PubMed] [Google Scholar]
- 5.Sumida K, Molnar MZ, Potukuchi PK, et al. Constipation and Incident CKD. J Am Soc Nephrol. 2017;28(4):1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumida K, Kovesdy CP. The gut-kidney-heart axis in chronic kidney disease. Physiol Int. 2019;106(3):195–206. [DOI] [PubMed] [Google Scholar]
- 7.Sun SX, Dibonaventura M, Purayidathil FW, Wagner JS, Dabbous O, Mody R. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sci. 2011;56(9):2688–2695. [DOI] [PubMed] [Google Scholar]
- 8.Guerin A, Carson RT, Lewis B, Yin D, Kaminsky M, Wu E. The economic burden of treatment failure amongst patients with irritable bowel syndrome with constipation or chronic constipation: a retrospective analysis of a Medicaid population. J Med Econ. 2014;17(8):577–586. [DOI] [PubMed] [Google Scholar]
- 9.Lu CY, Chen YC, Lu YW, Muo CH, Chang RE. Association of Constipation with risk of end-stage renal disease in patients with chronic kidney disease. BMC Nephrol. 2019;20(1):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tack J, Muller-Lissner S, Stanghellini V, et al. Diagnosis and treatment of chronic constipation--a European perspective. Neurogastroenterol Motil. 2011;23(8):697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumida K, Yamagata K, Kovesdy CP. Constipation in CKD. Kidney Int Rep. 2020;5(2):121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andresen V, Layer P. Medical Therapy of Constipation: Current Standards and Beyond. Visc Med 2018;34(2):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109 Suppl 1:S2–26; quiz S27. [DOI] [PubMed] [Google Scholar]
- 14.Roerig JL, Steffen KJ, Mitchell JE, Zunker C. Laxative abuse: epidemiology, diagnosis and management. Drugs. 2010;70(12):1487–1503. [DOI] [PubMed] [Google Scholar]
- 15.Moriarty KJ, Silk DB. Laxative abuse. Dig Dis. 1988;6(1):15–29. [DOI] [PubMed] [Google Scholar]
- 16.Baker EH, Sandle GI. Complications of laxative abuse. Annu Rev Med. 1996;47:127–134. [DOI] [PubMed] [Google Scholar]
- 17.Sumida K, Kovesdy CP. Disease Trajectories Before ESRD: Implications for Clinical Management. Semin Nephrol. 2017;37(2):132–143. [DOI] [PubMed] [Google Scholar]
- 18.Poesen R, Meijers B, Evenepoel P. The colon: an overlooked site for therapeutics in dialysis patients. Semin Dial. 2013;26(3):323–332. [DOI] [PubMed] [Google Scholar]
- 19.Sumida K, Molnar MZ, Potukuchi PK, et al. Blood Pressure Before Initiation of Maintenance Dialysis and Subsequent Mortality. Am J Kidney Dis. 2017;70(2):207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumida K, Molnar MZ, Potukuchi PK, et al. Association between vascular access creation and deceleration of estimated glomerular filtration rate decline in late-stage chronic kidney disease patients transitioning to end-stage renal disease. Nephrol Dial Transplant. 2017;32(8):1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumida K, Molnar MZ, Potukuchi PK, et al. Association of Slopes of Estimated Glomerular Filtration Rate With Post-End-Stage Renal Disease Mortality in Patients With Advanced Chronic Kidney Disease Transitioning to Dialysis. Mayo Clin Proc. 2016;91(2):196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Streja E, Gosmanova EO, Molnar MZ, et al. Association of Continuation of Statin Therapy Initiated Before Transition to Chronic Dialysis Therapy With Mortality After Dialysis Initiation. JAMA Netw Open. 2018;1(6):e182311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13(12):1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumida K, Molnar MZ, Potukuchi PK, et al. Prognostic significance of pre-end-stage renal disease serum alkaline phosphatase for post-end-stage renal disease mortality in late-stage chronic kidney disease patients transitioning to dialysis. Nephrol Dial Transplant. 2018;33(2):264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 26.Sumida K, Molnar MZ, Potukuchi PK, et al. Pre-end-stage renal disease visit-to-visit systolic blood pressure variability and post-end-stage renal disease mortality in incident dialysis patients. J Hypertens. 2017;35(9):1816–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Department of Veterans Affairs, VA Information Resource Center (VIReC),. VIReC Research User Guide: VHA Pharmacy Prescription Data. 2 ed: Hines, IL: VIReC; 2008. [Google Scholar]
- 28.Kovesdy CP, Norris KC, Boulware LE, et al. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132(16):1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovesdy CP, Alrifai A, Gosmanova EO, et al. Age and Outcomes Associated with BP in Patients with Incident CKD. Clin J Am Soc Nephrol. 2016;11(5):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serrano-Falcon B, Rey E. The safety of available treatments for chronic constipation. Expert Opin Drug Saf. 2017;16(11):1243–1253. [DOI] [PubMed] [Google Scholar]
- 32.Rider JA. Treatment of acute and chronic constipation with bisoxatin acetate and bisacodyl. Double-blind crossover study. Curr Ther Res Clin Exp. 1971;13(6):386–392. [PubMed] [Google Scholar]
- 33.Mueller-Lissner S, Kamm MA, Wald A, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. Am J Gastroenterol. 2010;105(4):897–903. [DOI] [PubMed] [Google Scholar]
- 34.Kienzle-Horn S, Vix JM, Schuijt C, Peil H, Jordan CC, Kamm MA. Comparison of bisacodyl and sodium picosulphate in the treatment of chronic constipation. Curr Med Res Opin. 2007;23(4):691–699. [DOI] [PubMed] [Google Scholar]
- 35.Kamm MA, Mueller-Lissner S, Wald A, Richter E, Swallow R, Gessner U. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol. 2011;9(7):577–583. [DOI] [PubMed] [Google Scholar]
- 36.Huang CH, Lin JS, Li TC, et al. Comparison of a Chinese Herbal Medicine (CCH1) and Lactulose as First-Line Treatment of Constipation in Long-Term Care: A Randomized, Double-Blind, Double-Dummy, and Placebo-Controlled Trial. Evid Based Complement Alternat Med. 2012;2012:923190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seinela L, Sairanen U, Laine T, Kurl S, Pettersson T, Happonen P. Comparison of polyethylene glycol with and without electrolytes in the treatment of constipation in elderly institutionalized patients: a randomized, double-blind, parallel-group study. Drugs Aging. 2009;26(8):703–713. [DOI] [PubMed] [Google Scholar]
- 38.Kinnunen O, Winblad I, Koistinen P, Salokannel J. Safety and efficacy of a bulk laxative containing senna versus lactulose in the treatment of chronic constipation in geriatric patients. Pharmacology. 1993;47 Suppl 1:253–255. [DOI] [PubMed] [Google Scholar]
- 39.Passmore AP, Wilson-Davies K, Stoker C, Scott ME. Chronic constipation in long stay elderly patients: a comparison of lactulose and a senna-fibre combination. BMJ. 1993;307(6907):769–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677–680. [DOI] [PubMed] [Google Scholar]
- 41.MacLennan WJ, Pooler A. A comparison of sodium picosulphate (“Laxoberal”) with standardised senna (“Senokot”) in geriatric patients. Curr Med Res Opin. 1974;2(10):641–647. [DOI] [PubMed] [Google Scholar]
- 42.Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16(11):3389–3396. [DOI] [PubMed] [Google Scholar]
- 43.Desmeules S, Bergeron MJ, Isenring P. Acute phosphate nephropathy and renal failure. N Engl J Med. 2003;349(10):1006–1007. [DOI] [PubMed] [Google Scholar]
- 44.Lee EY, Yoon H, Yi JH, Jung WY, Han SW, Kim HJ. Does hypokalemia contribute to acute kidney injury in chronic laxative abuse? Kidney Res Clin Pract. 2015;34(2):109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menahem SA, Perry GJ, Dowling J, Thomson NM. Hypokalaemia-induced acute renal failure. Nephrol Dial Transplant. 1999;14(9):2216–2218. [DOI] [PubMed] [Google Scholar]
- 46.Copeland PM. Renal failure associated with laxative abuse. Psychother Psychosom. 1994;62(3–4):200–202. [DOI] [PubMed] [Google Scholar]
- 47.Wong MYW, Hebbard G, Gibson PR, Burgell RE. Chronic constipation and abdominal pain: Independent or closely interrelated symptoms? J Gastroenterol Hepatol. 2020. [DOI] [PubMed] [Google Scholar]
- 48.Mishima E, Fukuda S, Shima H, et al. Alteration of the Intestinal Environment by Lubiprostone Is Associated with Amelioration of Adenine-Induced CKD. J Am Soc Nephrol. 2015;26(8):1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nanto-Hara F, Kanemitsu Y, Fukuda S, et al. The guanylate cyclase C agonist linaclotide ameliorates the gut-cardio-renal axis in an adenine-induced mouse model of chronic kidney disease. Nephrol Dial Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 50.Galvez C, Garrigues V, Ortiz V, Ponce M, Nos P, Ponce J. Healthcare seeking for constipation: a population-based survey in the Mediterranean area of Spain. Aliment Pharmacol Ther. 2006;24(2):421–428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


