Abstract
Context
There are sparse patient-level data available for children with novel coronavirus disease (COVID-19). Therefore, there is an urgent need for an updated systematic literature review that analyzes individual children rather than aggregated data in broad age groups.
Methods
Six databases (MEDLINE, Scopus, Web of Science, CINAHL, Google Scholar, medRxiv) were searched for studies indexed from January 1 to May 15, 2020 with MeSH terms: children, pediatrics, COVID-19, SARS-CoV-2. 1241 records were identified, of which only unique papers in English with individual patient information and documented COVID-19 testing were included. This review of 22 eligible studies followed Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data guidelines.
Results
123 patients from five countries were identified. 46% were females. Median age was five years (IQR=8). At presentation, 62% had fever, 32% had cough, 58% had a single symptom and 21% were asymptomatic. Abnormal chest imaging was seen in 62% (65/105) of imaged and 76.9% (20/26) of asymptomatic children. A minority of children had elevated platelets, CRP, lactate dehydrogenase, and d-dimer.
Conclusion
Data from this independent participant data systematic review revealed that the majority of children with COVID-19 presented with either no symptoms or a single, non-respiratory symptom.
Introduction
SARS-CoV-2 is a novel coronavirus first identified in Wuhan, China in 2019. This virus causes the clinical syndrome Coronavirus disease-2019 (COVID-19) and is an ongoing global pandemic. The Chinese Center for Disease Control reported in February 2020 that of the first 72,000 cases, only 2% were children (1).
Early studies identified a range of symptoms in children including acute upper respiratory tract infection, pneumonia, and gastrointestinal symptoms (2). Subsequent systematic reviews compiled key findings from aggregated cohort studies of children and adolescents with COVID-19 and confirmed that disease tended to be milder than has been reported in adult patients (3–6). However, the description of multi-system inflammatory syndrome in children (MIS-C), a systemic inflammatory syndrome with overlapping features of Kawasaki disease, has highlighted the need for more robust analysis of the presenting features of COVID-19 in children (7–9). Here, we offer a detailed description of the clinical presentation and outcomes of pediatric patients using an individual patient data approach to visualize trends by age (10).
Methods
Protocol is publicly available in the Open Science Framework database.
Search Strategy
A systematic search of MEDLINE (via PubMed), Web of Science, CINAHL, Google Scholar, Scopus, and medRxiv was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) individual participant data guidelines (11). Three authors (B.C., B.G.M., and R.O.) independently identified studies by searching the databases using the following exploded MeSH terms: (“pediatric” OR “child”) AND (“COVID-19” OR “SARS-CoV-2” OR “coronavirus”) to identify records indexed from January 1, 2020 to May 15, 2020. Identified references were exported to Sciwheel (formerly F1000Workspace, London, UK).
Inclusion and Exclusion Criteria
Articles were included if they fulfilled the following criteria: included patients older than 1 month and younger than 19 years with RT-PCR-confirmed SARS-CoV-2 or positive serology and a clinical syndrome compatible with COVID-19; were originally published in or translated into English; described clinical characteristics and laboratory tests relating to SARS-CoV-2 infection; and were indexed from January 1 to May 15, 2020. Studies that recruited based on consecutively identified children or convenience sampling were included. Exclusion criteria were lack of primary individual patient data; individual case reports, meta-analyses, or guidelines; fewer than three patient data points reported; and lack of individual patient ages. Single case reports were excluded because of the wide variability of data provided. Three authors (B.C., B.G.M., and R.O.) independently screened all identified articles to remove duplicates and those that did not meet inclusion criteria based on the title or abstract. Full-text articles were then read by two authors and eligibility of each paper was assessed independently; consensus was reached on all included articles. No articles from medRxiv fulfilled the inclusion criteria of this review.
Data extraction and Quality Assessment
Data from each article was extracted using a standardized form as follows: (1) study details including population, location, and quality of analysis; and (2) individual patient information including demographic data, presenting clinical symptoms, laboratory results, radiological findings, clinical course and outcome data. No issues were identified in extracting IPD. The methodological quality of studies evaluated was based on a modified form of the Oxford Centre for Evidence-Based Medicine schema (12). Five articles were initially evaluated and discussed in detail by the authors to standardize the approach to scoring. Remaining articles were evaluated independently by two authors. The authors confirmed that no patients were included in more than one study by comparing location, age and description of illness.
Ethics statement
The Weill Cornell Medicine Institutional Review Board classified this study as exempt because it collected and synthesized non-identifiable, publicly accessible data from previously published studies.
Results
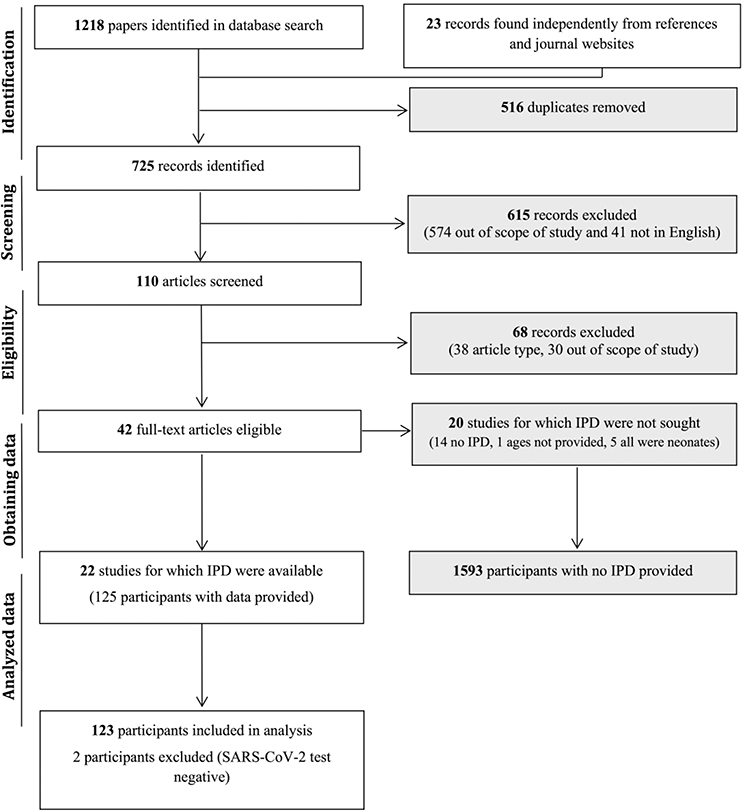
Our search yielded 1,218 records from six databases with an additional 23 records found independently by looking through references of other included papers and review of journal websites’ articles in press (Figure 1). A total of 1,131 records were excluded based on their titles and abstracts alone because they were duplicates, topics were outside the scope of the present study, patient ages did not meet criteria, or they were not in English. Full-text review led to the exclusion of an additional 88 papers. For final analysis, 22 studies were included in this systematic review (Table 1).
Figure 1.
Flowchart of study selection process for individual patient data extraction. Study selection was done in accordance with the PRISMA IPD guidelines.
Table 1.
Studies included for systematic analysis
| Study | n | Cities | Country | Study period | Publication |
|---|---|---|---|---|---|
| Ji et al. (20) | 2 | Beijing | China | prior to Jan 25 | Mar 16 |
| Li et al.(17) | 5 | Zhuhai City | China | Jan 28 - Feb 8 | Mar 11 |
| Liu H et al.(23) | 4 | Hubei Province | China | Jan 27 - Feb 14 | Mar 21 |
| Liu M et al.(25) | 5 | Chongqing | China | Mar 25 | |
| Liu W et al. (29) | 6 | Wuhan, Huangshi | China | Jan 7 - Jan 15 | Apr 2 |
| Lou et al. (24) | 3 | Zhengzhou | China | Mar 22 | |
| Paret et al. (35) | 2 | New York City, NY | United States | late March | Apr 17 |
| Parri et al. (37) | 9 | Florence, Rome, Milan, Brescia, Genova, Padua, Bergamo, Turin, Lodi, Udine, Verona, Lavagna, Pistoia, Varese, Bologna, Pordenone, Catanzaro, Cosenza | Italy | Mar 3 - Mar 27 | May 4 |
| Rahimzadeh et al. (36) | 9 | Chalus city, Sari city, Gonabad city, Tehran city | Iran | April | |
| See et al. (34) | 4 | Sungai Buloh, Langkawi, Seremban | Malaysia | prior to Feb 29 | Apr 15 |
| Shen et al. (31) | 9 | Changsha | China | Jan 8 - Feb 19 | Apr 7 |
| Su et al. (26) | 9 | Jinan, Shandong | China | Jan 24 - Feb 24 | Mar 25 |
| Sun et al. (22) | 8 | Wuhan | China | Jan 24 - Feb 24 | Mar 19 |
| Tan et al. (33) | 10 | Hunan | China | Jan 27 - Mar 10 | Apr 10 |
| Verdoni et al. (38)a | 8 | Bergamo | Italy | March-April | May 13 |
| Wei et al. (15) | 9 | Beijing, Hainan, Guangdong, Anhui, Shanghai, Zhejiang, Guizhou | China | Dec 8 - Feb 6 | Feb 14 |
| Xing et al. (18) | 3 | Qingdao | China | Jan 17 - Feb 23 | Mar 13 |
| Xu R et al. (14) | 2 | Guizhou province | China | 27-Jan | Feb 29 |
| Xu Y et al. (19) | 10 | Guangzhou | China | Jan 22 - Feb 20 | Mar 13 |
| Zhang T et al. (28) | 3 | Tianjin | China | Feb 3 - Feb 17 | Mar 29 |
| Zheng et al. (39) | 2 | Hubei | China | Feb 1 - Feb 10 | Mar 24 |
n, patients studied
Two patients were excluded from Verdoni et al. because all three samples tested from different sites were negative for the virus
Study characteristics and demographics
The 22 included studies were published in peer-reviewed journals over the span of three and a half months in 2020—three in February (13–16), 11 in March (17–28), six in April (29–36), and two in May (37,38). The 123 children identified were cared for between December 8, 2019 and April 19, 2020. These studies enrolled children from 48 cities and provinces in China, Italy, United States, Iran, and Malaysia (Table 2).
Table 2.
Patient Demographics
| Total, n | 123 |
| Age, years, median (IQR) | 5 (8) |
| <1 | 27 (22.0%) |
| 1–4 | 29 (23.6%) |
| 5–9 | 46 (37.4%) |
| 10–14 | 16 (13.0%) |
| 15–19 | 5 (4.1%) |
| Sex, n (%) | |
| Female | 56 (45.5%) |
| Male | 67 (54.5%) |
| Underlying Conditions,a n (%) | 3 (2.4%) |
| Country, n (%) | |
| China | 100 (81.3%) |
| Italy | 14 (11.4%) |
| United States | 2 (1.6%) |
| Malaysia | 4 (3.3%) |
| Iran | 3 (2.4%) |
Underlying conditions included asthma (no. 106), autism (no. 112), and epileptic encephalopathy with tracheostomy dependence (no. 118).
The median age was 5 years (interquartile range=8), and infants accounted for 21.6% of patients (n=27; Table 2). Of the children with recorded sex, 56/123 (45.5%) were females. Only three (2.4%) patients had comorbidities, including asthma (no. 106), autism (no. 112), and epileptic encephalopathy with tracheostomy dependence (no. 118) (Supplemental Table).
SARS-CoV-2 Virus Exposure and Testing
A majority of patients (n=104, 83.2%) had known exposure to the SARS-CoV-2 virus: a family member or close contact (n=83, 67.5%), or from travel to or residence in Wuhan, China. Twenty-one patients (16.8%) had unknown or unavailable exposure history. Of these patients, 19 presented with at least one symptom. The other two other patients with no known exposure were asymptomatic, and one had abnormal lung findings on CT.
All patients in our analysis tested positive for SARS-CoV-2 by reverse transcriptase polymerase chain reaction (RT-PCR) for SARS-CoV-2. The samples—from multiple anatomic sites in some children—were collected from the nasopharynx (n=56; (3,14,15,20,22,26,35,37), nose (n=20; (33,34,38), oropharynx (n=4; (20), throat (n=60; (18,21,23–25,28–30,32,34,39), pharynx (n=77; (33,36,40), respiratory tract (n=10; (13), sputum (n=9; (26), or feces (n=3; (28) Two children tested negative on presentation but were positive on subsequent testing (Supplemental Table, no. 15, 113; (25). Serology testing for SARS-CoV-2 IgG and IgM was performed on five (4%) patients; IgG+IgM− was detected in three patients, IgG+IgM+ in two patients (38). Rectal or stool RT-PCR for SARS-CoV-2 was performed in 21 patients, was positive in 15 patients (71.4%) and remained positive one month after negative respiratory PCR for some.
Co-infection was rarely found (n=7, 1.8%). Pathogens detected in the respiratory tract were Mycoplasma pneumoniae (n=3; no. 39, 88, 102), influenza A (n=2; no. 55, 86), respiratory syncytial virus (n=1; no. 3), and Enterobacter aerogenes (n=1; no. 30). No children had bacteremia.
Clinical findings at presentation
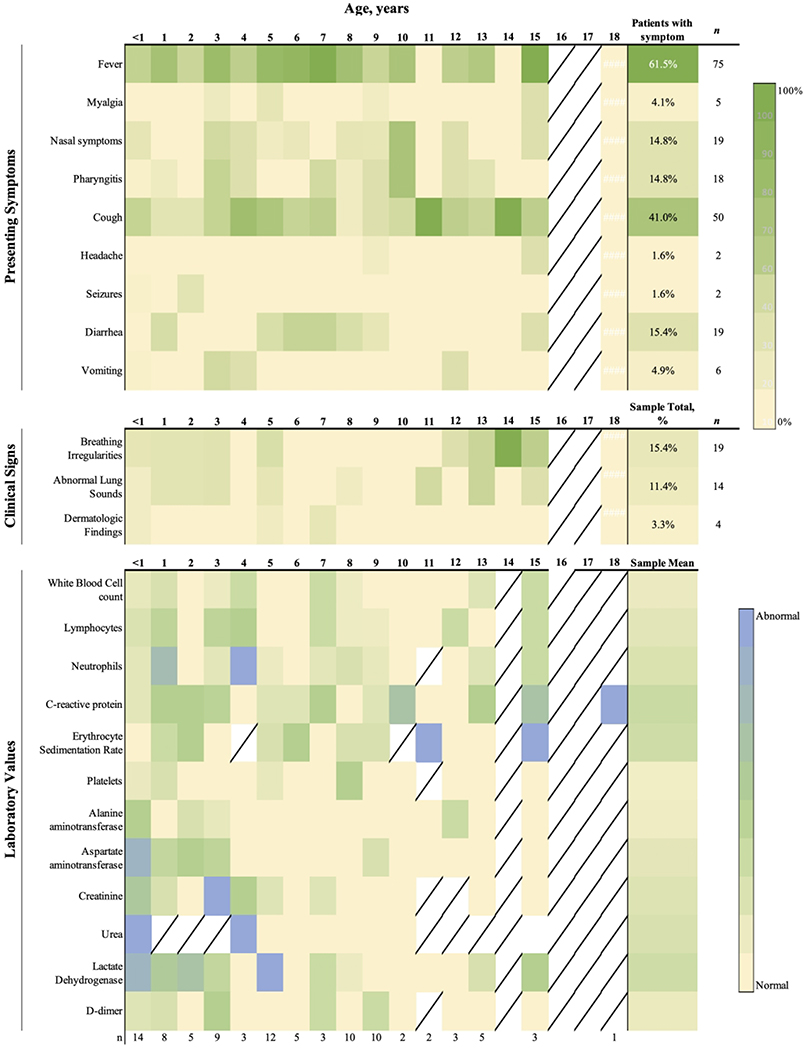
All patients presented to the emergency department and were then hospitalized. The symptomatic period before clinical encounter ranged from 0 to 17 days (no. 90). Upon initial testing, 14.6% patients were asymptomatic (n=18). Similar proportions of asymptomatic patients were found across age groups: 11% infants (n=3), 21% between 1–4 years (n=6), 13% between 5–9 (n=6), 13% between 10–14 (n=2), 20% between 15–18 (n=2). 41.6% of patients had more than one symptom. Fever was the most common symptom (n=78, 62.4%), and 29 (23.2%) experienced fever as the only symptom (Figure 2A). The proportion of children with fever varied by age: 48.2% of infants (n=14), 62% of those 1–4 years (n=18), 78.5% of those 5–9 years (n=33), 43.2% of those 10–14 (n=7) and 80% of adolescents older than 15 years (n=4). The second most common symptom across ages was cough (n=40, 32.0%), seven (5.6%) of whom presented with cough as their only symptom. Diarrhea occurred in 13 patients (10.4%), two (1.6%) of whom presented with diarrhea as their only symptom. Vomiting was only described for six patients (4.8%). Children with vomiting or diarrhea were mostly under nine years old (84%).
Figure 2.
Trends for pediatric COVID-19 patients by age. Heat map representing the proportion of patients with each presenting symptom (A), clinical sign (B) or abnormal lab values (C) compared to all patients of that age who had information available. Diagonal line indicates that no patients of that age had information available.
The most common concurrent symptoms at presentation were fever and cough (n=10, 8.0%); fever and diarrhea (n=8, 6.4%); fever, cough, and vomiting (n=3, 2.4%); fever and nasal symptoms (n=3, 2.4%); fever, nasal symptoms, and cough (n=3, 2.4%); fever, pharyngitis, and cough (n=2, 1.6%); fever, pharyngitis, and diarrhea (n=2, 1.6%).
Other reported symptoms were headache (n=2; no. 94, 120), seizure despite not having an underlying seizure disorder (n=2; no. 21 and 39), and dermatologic findings such as papular rash or skin changes around the oral cavity (n=4; no. 1, 7, 61, 78; Figure 2A). Seven children had a presentation similar to classic (n=3, no. 61, 64, 65) or incomplete Kawasaki Disease (n=4, no. 76, 78, 79, 100) (38). No differences in presenting symptoms were noted when comparing patients from different countries.
Radiographic findings
Chest imaging was obtained in 105 of the 123 patients (84%). A chest radiograph was acquired for 57 (46.3%) patients, CT scan for 72 (58.5%) patients, and both for 25 patients (23.8%). An abnormal chest radiograph or CT scan was described for 62% (n=65) of imaged patients of whom only 19 presented with a cough; two in respiratory distress (no. 27, 112); and seven with abnormal findings on lung auscultation. Two cases had normal chest radiographs but abnormal CT scans (no. 5, 122). Of the 26 asymptomatic patients, 20 (76.9%) had abnormal imaging findings.
Ground glass opacities (GGO) were noted in 62.5% patients with CT scans across all age groups (n=45/72). Bilateral lung abnormalities affected 25% of patients with CT scans (n=18/72). Other imaging findings included consolidation (n=14, 11.2%), pneumonia (n=13, 10.4%), patchy shadows (n=13, 10.4%), mottling (n=10, 8%), bronchitis (n=2, 1.6%), and perihilar opacities (n=2, 1.6%). Lung ultrasound was performed in two patients who required respiratory support and revealed interstitial syndrome and multiple B-lines (37).
Laboratory Values
The most common laboratory values reported included white blood cell count, platelet count, erythrocyte sedimentation rate, C-reactive protein, urea, creatinine, alanine aminotransferase, and aspartate aminotransferase (Figure 2C). Elevated white blood cell count was seen in 10.4% (n=13), elevated ESR in 7.2% (n=13), and elevated CRP in 24.8% (n=31). White blood cell count was elevated in 11.1% of infants (n=3), in 13.8% 1–4 year olds (n=4), 6.5% 5–9 year olds (n=3), 12.5% 10–14 year olds (n=2), and 20% of 15–19 year olds (n=1). Thrombocytosis was seen in 3.3% of patients (n=4) while 1.6% (n=2) patients were thrombocytopenic. Elevated lactate dehydrogenase was reported for 12.2% of (n=15) patients and elevated d-dimer in 6.5% (n=8) patients.
Treatment
No consistent treatment strategy was reported. Antiviral treatment was given to 23.6% (n=29) patients with lopinavir/ritonavir (n=11), oseltamivir (n=11), or ribavirin (n=7). Twenty-four patients, all in China, received inhaled alpha-interferon while in the hospital. Antibiotics were used as the sole treatment in 17 patients who were treated solely with antibiotics (Supplemental Table). Intravenous immunoglobulin was administered in 17 patients. A total of 30 (24%) children received oxygen therapy. Twenty-five patients were additionally given traditional Chinese medicine.
Course and outcome
Of the 123 children in this review, 29 (23.6%) patients remained asymptomatic, 17 (13.8%) were diagnosed with pneumonia, and 66 (52.8%) reported upper respiratory infection symptoms. We found no differences in these presentations across age groups. Length of stay ranged from 2 (no. 96, 119) to 25 days (no. 102), but 12 patients were still in the hospital for greater than 24 days at the time of publication for some studies. The median time to discharge was 12 days (IQR=7). Four patients were treated in the intensive care unit; one was discharged and three were still being cared for at the time of publication after 20 days in the ICU (no. 21 86, 114). There were no reported deaths among this group of 123 patients.
Discussion
Our study sought to identify characteristic clinical and laboratory features of SARS-CoV-2 infection by age using patient-level pooled data from pediatric studies. We found that children under 7 years tended to present with fever, diarrhea, and vomiting more than older children who experienced more cough, although the proportion of asymptomatic patients was similar for all ages (11–21%).
In April the United States Center for Disease Control and Prevention (CDC) reported that children under age one and older than ten years comprised the highest percentage of COVID-19associated hospitalizations in the pediatric population. Case and hospitalization rates in children older than 10 years old were also higher than the mean for all pediatric patients, suggesting that COVID-19 disease severity follows a bimodal age distribution among pediatric patients (41). Data from Washington, D.C. and Korea identified a similar bimodal distribution (42,43). In the pooled sample examined here, the largest proportion of patients were between 5–9 years old whereas only 4% were adolescents 15–19 years. The fact that many of the studies contributed cases from a single or a small number of institutions could have contributed to a skewed patient age distribution.
We found that 20.8% of patients in this sample were asymptomatic, mostly identified by contact tracing, household exposure to the virus, or targeted screening efforts. This highlights that efforts to increase testing in children are important to identify these cases in order to limit transmission in the community, although the role of asymptomatic transmission by children remains a topic of investigation (41,42).
Our review showed that 41.6% of cases presented with only one non-specific symptom. Therefore, symptom-based screening is neither sensitive nor specific as a tool to detect SARS-CoV-2 infection in children; for example, only 62.4% of patients presented with fever. Furthermore, children presented with a variety of other non-specific symptoms referable to the upper respiratory tract (fever, cough, nasal congestion), gastrointestinal tract (vomiting, diarrhea), or neurologic system (headache, seizure). A recent meta-analysis also described fever and cough as the most common presenting symptoms in children with diarrhea occurring in a minority of patients (43). While anosmia and ageusia have been described as early symptoms of COVID-19 in adults, we did not identify a single case in our study that reported these symptoms in children. However, this may be due to a reporting bias as young children may have difficulty articulating these symptoms. As several cases had prolonged shedding of viral material in fecal samples detected by PCR assays, the role of fecal-oral transmission also needs further investigation.
The time between symptom onset to presentation to the hospital ranged from 0–17 days, although this may be due to late identification early in the outbreak before widespread testing became available or due to quarantine delaying presentation to a medical facility. In the United States, up to 23% of childhood cases have been reported to the CDC as having an underlying condition, however only three of the patients analyzed in this review had comorbid conditions (41). Our results are consistent with other systematic reviews examining patients primarily from China and other countries where few patients were reported with comorbid conditions (44). Only four patients required transfer to the intensive care unit, and the mean time to discharge for hospitalized patients was 12 days (IQR=7). These differences may be due to variations in virulence of circulating viral strains, population structure or host characteristics such as nutritional status.
The radiological findings frequently did not match the patient’s clinical presentation, as 76.9% of asymptomatic patients had abnormal lung images and almost one-third of children with chest imaging done had no abnormal radiographic findings. Similar to findings in adults, ground-glass opacities were the most frequent abnormality identified on CT, but in contrast to adults, children were not identified as having interlobular septal thickening. Cough as a presenting symptom did not appear to correlate with abnormal lung findings more so than any other presenting symptom. Due to the variability of imaging findings, some have called into question the use of CT in the workup of children with suspected COVID-19.
Post-infection sequelae
Unique to SARS-CoV-2, reports have highlighted the risk for severe post-infectious sequelae (45–47). Verdoni et al. reported eight patients in Italy with Kawasaki-like disease (38). More recent reports from the United States, United Kingdom and Europe characterizing the now recognized multi-system inflammatory syndrome (MIS-C) in children were not included in this search because criteria focused on primary infection with SARS-CoV-2 (48–51). Because it appears that MIS-C may present weeks after initial SARS-CoV-2 infection, it is likely that the patients included in this study were not followed for a time course that was ample enough to detect these symptoms and relate them to COVID-19 (52–53). Future investigations on MIS-C should aim to correlate primary COVID-19 signs and symptoms with MIS-C incidence and presentation to identify and validate useful predictive factors for developing this potentially life threatening condition. We found that younger children tended to have greater elevations in WBC, CRP, ESR, AST, LDH, D-dimer than older children. While thrombocytopenia and lymphopenia has been correlated with disease severity in adults, although these findings were infrequently reported in children. More research is needed into the long-term consequences of SARS-CoV-2 infection during childhood.
Limitations
Our study has several limitations. Our search strategy across six databases may have excluded some publications published prior to May 15 that were not indexed in databases at the time of the literature review. Few studies included individual patient details or clinical follow-up; thus, our findings may not reflect the true spectrum of disease or post-infection inflammatory sequelae. The small number of reported adolescents for this review limits our ability to describe the full spectrum of clinical features in that age group. Data was not collected prospectively in most studies, so recruitment bias likely exists. Furthermore, a majority of the patients were from China during the early phase of the pandemic, so we are unable to analyze differences in presentation by country. The time span covered by these papers coincides with large outbreaks in a handful of countries prior to April 19, 2020. However, since then more studies have provided data from other countries, so our limited sample may introduce bias to our findings.
Conclusion
Unlike other systematic reviews of children that summarized aggregated data, this review compiled individual participant data for 125 children with documented SARS-CoV-2 infection. The information was derived from 22 publications from January 1 to May 15, 2020 according to standardized PRISMA guidelines. Children from China, Italy, Malaysia, Iran, and the United States were included in the study. Our findings indicated that no individual presenting symptom, sign, laboratory test or radiological finding could reliably predict a diagnosis of COVID-19. At the time of testing, one-fifth of children had asymptomatic infections, under two-thirds had only one symptom. Notably, chest radiographs or CT scans were abnormal in three-quarters of asymptomatic children. Therefore, widespread community-based testing will be needed to diagnose, isolate and possibly treat infected children to effectively combat the COVID-19 pandemic while awaiting a highly effective vaccine. The findings highlight the challenges of diagnosing COVID-19 in pediatric patients due to the wide variety of symptoms and the seemingly poor correlation of imaging findings with symptomatic disease. These challenges emphasize the importance of having a low threshold for testing pediatric patients for SARS-CoV-2.
Supplementary Material
Impact.
This systematic review revealed that the majority of children with COVID-19 presented with either no symptoms or a single, non-respiratory symptom.
By using an independent participant data approach, this analysis underscores the challenge of diagnosing COVID-19 in pediatric patients due to the wide variety of symptoms and seemingly poor correlation of imaging findings with symptomatic disease.
The data presented from individual patients from case series or cohort studies adds more granularity to the current description of pediatric COVID-19.
Acknowledgments
Financial Support: BC was supported by a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the National Institutes of Health under award number T32GM007739 to the Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD–PhD Program. BGM and ICM were supported by the National Institute of Allergies and Infectious Diseases of the National Institutes of Health award number R25AI140490.
Footnotes
Disclosure Statement: No financial relationships or conflicts of interest to disclose.
Category of Study: Systematic Review
Consent: Patient consent was not required for this study as it analyzed anonymized, publicly-available data.
References
- 1.Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. (2020). [DOI] [PubMed] [Google Scholar]
- 2.Dong Y et al. Epidemiology of COVID-19 Among Children in China. Pediatrics. 145, (2020). [DOI] [PubMed] [Google Scholar]
- 3.Chang T-H, Wu J-L, Chang L-Y. Clinical characteristics and diagnostic challenges of pediatric COVID-19: A systematic review and meta-analysis. J Formos Med Assoc. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castagnoli R et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr. (2020). [DOI] [PubMed] [Google Scholar]
- 5.Mustafa NM, Selim LA. Characterisation of COVID-19 Pandemic in Paediatric Age Group: A Systematic Review and Meta-Analysis. J Clin Virol. 128, 104395 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minotti C, Tirelli F, Barbieri E, Giaquinto C, Donà D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones VG et al. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hospital Peds. (2020). [DOI] [PubMed] [Google Scholar]
- 9.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 395, 1607–8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. (Wiley; 2008). [Google Scholar]
- 11.Stewart LA et al. PRISMA-IPD Development Group. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 313:1657–65 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Durieux N, Pasluea F, Howick J. OCEBM Levels of Evidence-CEBM.
- 13.Cai J et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu R et al. CT imaging of one extended family cluster of corona virus disease 2019 (COVID-19) including adolescent patients and “silent infection.” Quant Imaging Med Surg. 10, 800–4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei M et al. Novel coronavirus infection in hospitalized infants under 1 year of age in china. JAMA. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu H et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Ped. 9, 51–60 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol. 50, 796–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xing et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 26, 502–5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji L-N et al. Clinical features of pediatric patients with COVID-19: a report of two family cluster cases. World J Pediatr. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y et al. Infants Born to Mothers With a New Coronavirus (COVID-19). Frontiers in pediatrics. 8, 104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun D et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center observational study. World J Pediatr 1–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H et al. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J Infect. 80, e7–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou XX, Shi CX, Zhou CC, Tian YS. Three children who recovered from novel coronavirus 2019.pneumonia. J Paediatr Child Health. 56, 650–1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M, Song Z, Xiao K. High-Resolution Computed Tomography Manifestations of 5 Pediatric Patients With 2019 Novel Coronavirus. J of Comp Assist Tomo. 44, 311–3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su L et al. The different clinical characteristics of corona virus disease cases between children and their families in China - the character of children with COVID-19. Emerg Microbes Infect. 9, 707–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng L et al. Neonatal Early-Onset Infection With SARS-CoV-2 in 33 Neonates Born to Mothers With COVID-19 in Wuhan, China. JAMA Pediatr. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang T et al. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu W et al. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 382, 1370–1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen Q, Guo W, Guo T, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 55, 1424–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang P et al. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. 127, 104356 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan Y-P et al. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. 127, 104353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.See KC et al. COVID-19: Four Paediatric Cases in Malaysia. Int J Infect Dis. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paret M et al. SARS-CoV-2 infection (COVID-19) in febrile infants without respiratory distress. Clin Infect Dis. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahimzadeh G et al. COVID-19 Infection in Iranian Children: A Case Series of 9 Patients. J Pediatr Rev. 139–44 (2020). [Google Scholar]
- 37.Parri N, Lenge M, Buonsenso D, and Pediatric Emergency Departments (CONFIDENCE) Research Group CI. Children with Covid-19 in Pediatric Emergency Departments in Italy. N Engl J Med. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verdoni L et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng F et al. Clinical Characteristics of Children with Coronavirus Disease 2019 in Hubei, China. Current Medical Science. 40, 275–80 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia W et al. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 55, 1169–74 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludvigsson JF. Children are unlikely to be the main drivers of the COVID-19 pandemic - A systematic review. Acta Paediatr. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies NG et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. (2020). [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A et al. Coronavirus disease 2019 (COVID-19) in children and/or adolescents: a meta-analysis. Pediatr Res. (2020). [DOI] [PubMed] [Google Scholar]
- 44.Mehta NS et al. SARS-CoV-2 (COVID-19): What do we know about children? A systematic review. Clin Infect Dis. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirzaee SMM, Gonçalves FG, Mohammadifard M, Tavakoli SM, Vossough A. Focal Cerebral Arteriopathy in a COVID-19 Pediatric Patient. Radiology. 202197, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dallan C et al. Septic shock presentation in adolescents with COVID-19. Lancet Child Adolesc Health. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toubiana J et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 369, m2094 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiotos K et al. Multisystem Inflammatory Syndrome in Children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis Soc (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19: scientific brief, 15 May 2020. World Health Organization; 2020. [Google Scholar]
- 50.Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capone CA et al. Characteristics, Cardiac involvement, and Outcomes of Multisystem Inflammatory Disease of Childhood (MIS-C) Associated with SARS-CoV-2 Infection. J Pediatr. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dufort E et al. Multisystem Inflammatory Syndrome in Children in New York State. New Eng J Med. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldstein LR et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. New Eng J Med. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.