Abstract
Prior work proposed a shortened version of the Social Responsiveness Scale (SRS), a commonly used quantitative measure of social communication traits. We used data from 3,031 participants (including 190 ASD cases) from the Environmental Influences on Child Health Outcomes (ECHO) Program to compare distributional properties and criterion validity of 16-item “short” to 65-item “full” SRS scores. Results demonstrated highly overlapping distributions of short and full scores. Both scores separated case from non-case individuals by approximately two standard deviations. ASD prediction was nearly identical for short and full scores (area under the curve values of 0.87, 0.86 respectively). Findings support comparability of shortened and full scores, suggesting opportunities to increase efficiency. Future work should confirm additional psychometric properties of short scores.
Deficits in social communication, and the presence of restricted, repetitive behaviors or interests, are the hallmarks of an autism spectrum disorder (ASD) diagnosis. Although ASD is a categorical diagnosis, the continuum of the ASD-related phenotype extends into the general population (Constantino & Todd, 2005; Robinson, St Pourcain, & Anttila, 2016). The study of ASD-related traits as a continuous distribution in population samples therefore holds promise for more fully gauging the population impact of impairment in social communication. Moreover, trait-based research is more consistent with the Research Domain Criteria conceptualization of neuropsychopathology, hypothesized to relate more closely to the underlying biology of these conditions (Cuthbert & Insel, 2013).
The Social Responsiveness Scale (SRS) is one of the most widely-used quantitative trait measures of the ASD-related phenotype, both for ASD screening and phenotype assessment purposes as well as to capture broader variation in social communication across unaffected samples (Constantino & Gruber, 2012). It is frequently used in both epidemiological and clinical settings, and has been translated into over 10 languages. This 65-item informant-report measure has favorable psychometric properties and has been previously validated against ASD diagnosis (Bolte, Westerwald, Holtmann, Freitag, & Poustka, 2011; Constantino et al., 2003). In addition, research has demonstrated that the SRS is able to capture variation in ASD-related traits and social communication across general population samples (Constantino & Todd, 2003; Lyall et al., 2014). Evidence for intergenerational transmission of traits as measured by the SRS (Constantino & Todd, 2005; Lyall et al., 2014; Page et al., 2016), as well as heritability estimates comparable to those for ASD diagnosis (Frazier et al., 2014), and relationships with genetic and neurologic features of ASD (Blanken et al., 2015; Duvall et al., 2007; Lowe, Werling, Constantino, Cantor, & Geschwind, 2015), provide further support for construct validity and utility of the SRS as a quantitative trait measure in etiologic studies.
Despite strong support for the validity of the SRS, some concerns have been raised regarding the specificity of the measure to ASD, given suggestions that other neurodevelopmental conditions and demographic factors influence scores (Havdahl, Hus, Huerta et al, 2016; Hus, Bishop, Gotham, Huerta, & Lord, 2013). Specifically, several studies have reported SRS scores that approach or overlap those seen for ASD populations in conditions commonly co-occurring with ASD and/or according to features not central to ASD diagnosis, leading to some debate as to whether this represents bias in capturing etiologically distinct features or rather expected overlap due to potential shared etiology (Constantino & Frazier, 2013; Hus, Bishop, Gotham, Huerta, & Lord, 2013). Recently, a “short form” of the SRS, including 16 of the published SRS’s 65 items, was developed to increase efficiency and psychometric performance of the SRS for use as a quantitative measure of social communication based on items with potentially reduced bias due to non-core ASD symptoms (Sturm, Kuhfeld, Kasari, & McCracken, 2017). While several other studies have utilized subsets of SRS items, these shortened versions were created primarily for screening purposes or to focus on ASD severity, and were not developed considering the SRS’s utility as a quantitative trait measure (Duku et al., 2013; Moul, Cauchi, Hawes, Brennan, & Dadds, 2015; Reiersen, Constantino, Volk, & Todd, 2007). Sturm and colleagues’ short score was developed using methods to optimize performance of items for social communication assessment in the largest sample to date. However, these scores have seen little application (Kaat & Farmer, 2017) (Nguyen et al., 2019), were derived from data over-representing ASD-affected individuals and families, and have not been validated in large general population samples. These latter points, as noted elsewhere (Kaat & Farmer, 2017), represent a key consideration for use as a continuous measure of quantitative ASD-related traits. Thus, in order to address the use of the SRS both as a way to capture social communication deficits spanning the population (including unaffected individuals) and as a potential screener for ASD phenotype, comparison to full SRS score distributions and examination of predictive ability in large, heterogeneous samples that include individuals and families without ASD diagnoses are needed. We had the opportunity to address these goals by pooling existing data from cohorts participating in the Environmental Influences on Child Health Outcomes (ECHO) program.
Methods
Study population
ECHO is a large collaborative project that seeks to examine environmental factors that impact child health. Detailed methods have been previously published (Gillman & Blaisdell, 2018). Briefly, ECHO is comprised of individual study cohorts across the US who have already enrolled participants. These participants are then followed in a common protocol assessing various aspects of child development. Here, we used data previously collected by individual cohorts. Of 82 ECHO cohorts that participated in the first phase of the program, 11 had collected at least one SRS on the child and had individual item scores available at the time of our analyses. Individual participants were excluded if they did not have item-level scores from the SRS (n=1,725 from 1 cohort), were missing child sex (n=42), had > 10% of SRS items missing (n=559), or were outside the SRS age range for inclusion (<2.5 or > 18 years old, n=37). Thus, 3,031 participants were included in the analytic dataset (Online Resource Figure 1). For ROC analyses, participants with no information on ASD diagnosis were also excluded (n=608). All participants provided informed consent, and local IRB approvals were obtained at all study sites.
Participants were also grouped according to source population to allow for additional comparisons by background risk groups in secondary analyses. Groups included: “enriched familial risk,” participants from studies following younger siblings of a child with ASD (owing to presumed increased genetic risk (Messinger et al., 2015; Ozonoff, Young, et al., 2011)); “non-familial enriched risk,” participants from a preterm birth cohort (owing to the increased risk of ASD and ASD-related traits with lower gestational age (Korzeniewski et al., 2017; Leavey, Zwaigenbaum, Heavner, & Burstyn, 2013)); and participants drawn from groups without increased baseline risk. Further details on ECHO cohorts included, and these groupings, are provided in Online Resource Table 1.
Social Responsiveness Scale (SRS)
The SRS is a 65-item questionnaire assessing social communication and restricted repetitive behaviors, developed for use in both ASD-affected and unaffected individuals. Item scores (ranging from 0 to 3) are summed to yield a total score ranging from 0 to 195. Higher values indicate greater expression of the ASD-related phenotype. Subscale scores assessing specific components of ASD-related behavior can also be calculated based on item subsets. Raw scores can be converted to age and sex-normed T-scores to facilitate clinical utility. The SRS has well-established psychometric properties in both the general population (Constantino & Todd, 2003) and in ASD families (Constantino et al., 2006), with high internal validity, reliability, reproducibility, and score stability (Constantino & Gruber, 2012; Constantino et al., 2003). It has also been validated against a ‘gold standard’ for diagnosis, the Autism Diagnostic Interview-Revised (ADI-R), with strong results (r=0.7 for SRS scores and ADI-R algorithm scores for DSM-IV criteria) (Constantino et al., 2003). Particularly in general population samples, SRS scores are unimodal, continuously distributed, and not related to intelligence quotient (IQ) or age (within form) (Constantino & Gruber, 2012). However, relationships between scores and nonverbal IQ have been reported in affected samples (Hus et al., 2013; Sturm et al., 2017). Established SRS thresholds reliably distinguish ASD children from both non-affected children and those with other conditions such as intellectual disability (Constantino et al., 2003), though the influence of other neuropsychiatric conditions on scores has been questioned (Aldridge, Gibbs, Schmidhofer, & Williams, 2012; Hus et al., 2013).
The 16-item short SRS score was developed by Sturm and colleagues (Sturm et al., 2017) based on item response theory (IRT) analyses. The goal of their analyses was to increase score efficiency (i.e., near-equivalent score precision in fewer items) and reduce potential biases related to age, sex, and expressive language. Analyses were conducted based on extant 65-item SRS data from autism registries (Simons Simplex, Interactive Autism Network, the National Database for Autism Research, and Autism Genetic Resource Exchange). From these analyses, 16 items were selected (Table 1) based on high factor loadings and low evidence for differential functioning, as well as expert consideration of content validity.
Table 1.
SRS items included in short scores (as identified in Sturm et al., 2017 “Short Form”)
| SRS item number | Subscale and broad content assessed1 |
|---|---|
| 4 | Autistic mannerisms; rigid behavior |
| 7 | Social awareness; perception of others’ feelings2 |
| 8 | Autistic mannerisms; odd behavior |
| 13 | Social communication; awkward peer interactions3 |
| 16 | Social communication; eye contact avoidance3 |
| 18 | Social communication; difficulty initiating friendships3 |
| 22 | Social communication; appropriate peer play 2 |
| 23 | Social motivation; lack of group activities |
| 29 | Autistic mannerisms; regarded as odd3 |
| 30 | Social cognition; overstimulation |
| 33 | Social communication; socially awkward |
| 37 | Social communication; difficulty relating to peers3 |
| 38 | Social communication; response to others’ emotions2 |
| 39 | Autistic mannerisms; narrow interests3 |
| 42 | Social cognition; sensory sensitivity |
| 54 | Social awareness; aloof interpersonal interactions |
The SRS is copyrighted and published by Western Psychological Services (WPS). Reproduction of specific items is not permitted; we therefore provide a summary of item content, which is not intended to be used for administrations.
Reverse-coded items.
Item also included in the Generation R Study’s 18-item SRS.
Full and short SRS scores in ECHO
Participating cohorts in ECHO used the SRS-1 (24%) (Constantino & Gruber, 2005), a prepublication preschool version (4.1%), or the SRS-2 (71.4%)(Constantino & Gruber, 2012). Differences between versions are limited to relatively minor wording changes. Basic quality control checks were conducted to ensure accuracy of obtained SRS data. Missing SRS items were imputed with median item values according to publisher recommendations (missing <18% for SRS-1 and <10% of the 65 items for SRS-2, n=157 participants). 65-item SRS scores, referred to here as “full” SRS scores, were calculated according to publisher guidelines. The 16-item score, hereafter referred to as the “short score”, was calculated by summing the individual items identified in the Sturm et al. analysis.
We used raw scores in all analyses due to lack of population-based T-score norms for short scores and minor norming differences across SRS forms and versions. To enable direct comparison of short and full SRS scores, we re-scaled scores to range from 0 to 100. Original raw scores were divided by their potential total (i.e., 195 and 48 for the full and short, respectively) and multiplied by 100. This scaling method holds the advantage of reflecting group differences proportional to the original scale (Cohen, Cohen, Aiken, & West, 2010).
Diagnostic information
Information on child diagnoses was obtained from each of the participating cohorts (see Online Resource Table 1). In 38% of all study participants, ASD diagnoses and typical development were confirmed according to clinical evaluations, with ASD assessed using gold-standard research measures and assessing consistency with DSM-IV and/or DSM-5 criteria. Diagnosis was reported according to medical record and/or parental report (30% had both sources) in the remaining cohorts. Children were categorized as non-ASD if typical development was confirmed according to evaluation or no diagnosis was reported. Information on other diagnoses, including learning disability, anxiety, depression, language delays, intellectual disability, attention deficit hyperactivity disorder, was also requested from cohorts for use in secondary analyses, according to caregiver report, medical records, or clinical confirmation. These diagnoses were grouped together as non-ASD psychiatric and neurodevelopmental conditions given low degree of reporting.
Statistical analyses
Basic descriptive statistics were calculated for characteristics of the study population and their raw and scaled (as described above) SRS scores. Due to non-normality of scores, Spearman correlation coefficients were calculated between raw full and short scores. The distributions of full and short SRS scores were examined using density plots. These plots and descriptive statistics for SRS scores were also examined by source population (i.e., general population, ASD familial- or non-familial enriched risk), SRS form (preschool or school-age), sex (male vs female child), and ASD status. To compare criterion validity of full and short SRS scores with ASD diagnosis, we used Receiver Operating Characteristic (ROC) curves and calculated area under the curve (AUC) for each score. We also conducted several sensitivity analyses of these ROC comparisons. We conducted 50 permutations of scores of 16 randomly-selected items from the SRS, and compared the range of AUCs of these scores to those of the published full SRS and Sturm et al. short scores. Further, to assess whether the short SRS score is less-influenced by other (non-ASD) disorders than the full score, we compared the AUC for the short and full score for available non-ASD conditions (listed above under diagnostic information).
Analyses were conducted using SAS Studio version 3.71 (SAS Institute, Inc., Cary, North Carolina). Density plots and ROC curves were plotted using R Studio version 1.1.463.
Results
The majority of participants were from studies drawn from the general population (66.2%; Table 2). In addition, most participant mothers were non-Hispanic white and had a college degree or higher. Owing to inclusion of several ASD enriched-risk cohorts, as well as a case-control study, 6% of our sample had an ASD diagnosis, and 91% of these were confirmed according to clinical assessment. Overall, 57% of participants had completed the school-aged SRS form (average age= 7.4; range= 2.5–18; 8 cohorts used the preschool form, 9 the school-aged, and 6 used both depending on participant age), though this differed by study population (92% of participants from enriched familial risk cohorts used preschool forms).
Table 2:
Basic characteristics of the study population (N= 3,031)
| N (%) or Mean (SD) | |
|---|---|
| Cohort Source Population | |
| ASD familial enriched risk | 257 (8.5%) |
| ASD non-familial enriched risk | 677 (22.3%) |
| General population | 2,097 (69.2%) |
| Maternal race/ethnicity | |
| Hispanic | 256 (8.5%) |
| Non-Hispanic White | 2,212 (73.0%) |
| Non-Hispanic Black | 280 (9.2%) |
| Non-Hispanic Other | 180 (6.3%) |
| Missing | 103 (3.4%) |
| Maternal education | |
| ≤ High school degree | 520 (17.2%) |
| Some college | 585 (19.3%) |
| ≥ Bachelor’s degree | 1,856 (61.2%) |
| Missing | 70 (2.3%) |
| Parity | |
| No prior child | 1,324 (43.7%) |
| ≥ 1 prior child | 1,580 (52.1%) |
| Missing | 127 (4.2%) |
| Neurodevelopmental Outcomes | |
| ASD diagnosis | 190 (6.3%) |
| No ASD | 2,233 (73.7%) |
| Missing ASD diagnostic information2 | 608 (20.1%) |
| Other non-ASD neurodevelopmental or psychiatric condition1 | 621 (27.8%) |
| Maternal age3 | 31.3 (5.8) |
| Paternal age3 | 33.6 (6.1) |
| Child age at SRS in years | 7.4 (4.9) |
| Gestational age3 | 35.8 (5.8) |
Abbreviations: ASD, Autism Spectrum Disorder; SRS, Social Responsiveness Scale
Other condition including: Intellectual disability, non-verbal disability, ADD or ADHD, learning disability, anxiety, depression, language disability. Includes 94 individuals with ASD diagnosis. Percent totals do not sum to 100 due to overlap between groups.
These individuals were not included in ROC analyses.
Number of individuals with missing values: maternal age (n=31); paternal age (n=909); gestational age (n=46)
Full and short score descriptive statistics are presented in Table 3. Full raw scores in our total sample were similar to those seen in general population samples, with a slightly higher mean (32.5) likely due to the higher proportion of ASD cases than in the general population. Scaled full score means were higher across all comparisons than short score means, except for ASD cases. Both short and full scores were, on average, higher in enriched risk cohorts than in those drawn from the general population. Scores in males were on average approximately 4 scaled points higher than in females for both full and short scores. Scores derived from preschool forms were on average lower by a few scaled points for both full and short scores. Both the full and short scores yielded approximately a 2 standard deviation difference in mean score for those with vs without an ASD diagnosis, consistent with prior work for full scores (Constantino & Gruber, 2012). Short and full SRS scores were higher in those with Hispanic ethnicity, Black race, and with lower education. The correlation between short and full scores in the total study population was high (Spearman correlation coefficient=0.90); this did not differ by study type. Subscale score correlation ranged from 0.60 (for social cognition) to 0.86 (for autistic mannerisms and social communication).
Table 3.
Descriptive statistics of SRS full and short scores
| N1 | SRS full scores – Raw score | SRS full scores –Scaled score2 | SRS short score –Scaled score2 | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (1st, 3rd Quartile) | Mean (SD) | Median (1st, 3rd Quartile) | Mean (SD) | Median (1st, 3rd Quartile) | ||
| Total analytic sample | 3,031 | 32.5 (24.4) | 26 (16, 40.5) | 16.7 (12.5) | 13 (8,21) | 14.2 (14.8) | 10 (4, 19) |
| Cohort source population | |||||||
| General | 2,097 | 29.5 (21.3) | 25 (16, 36) | 15.1 (10.9) | 13 (8,18) | 12.6 (13.1) | 8 (4, 17) |
| ASD Familial Enriched Risk | 257 | 37.3 (26.7) | 32 (18, 46) | 19.1 (13.7) | 16 (9, 24) | 16.4 (16.1) | 10 (6, 19) |
| ASD Non-Familial Enriched Risk | 677 | 40.1 (29.9) | 30 (16, 58) | 20.5 (15.3) | 15 (8, 30) | 18.6 (17.8) | 13 (4, 27) |
| ASD diagnosis3 | |||||||
| Diagnosed with ASD | 190 | 74.7 (34.6) | 78 (44.2, 97.8) | 38.3 (17.8) | 40 (23, 50) | 41.2 (21.8) | 42 (23, 56) |
| Not diagnosed with ASD | 2,233 | 30.4 (22.2) | 25.5 (18.0, 34.2) | 15.6 (11.3) | 13 (8, 20) | 13.0 (13.0) | 8 (4, 17) |
| Child sex | |||||||
| Boys | 1,563 | 35.4 (26.0) | 28 (18, 45) | 19 (14.3) | 15 (9, 25) | 17.1 (17.0) | 13 (6, 23) |
| Girls | 1,468 | 29.4 (22.1) | 24 (14, 37) | 15.7 (12.2) | 12 (7, 20) | 13.1 (14.2) | 8 (4, 17) |
| SRS form type | |||||||
| Preschool | 1,302 | 29.4 (18.2) | 26 (17, 36) | 15.1 (9.3) | 13 (9, 18) | 11.9 (11.0) | 8 (4, 15) |
| School-Age | 1,729 | 34.8 (27.9) | 26 (14, 46) | 17.9 (14.3) | 13 (7, 24) | 16 (16.8) | 10 (4, 23) |
| Among general population | |||||||
| Preschool | 980 | 27.4 (15.1) | 25 (17, 34) | 14.1 (7.7) | 13 (9, 17) | 10.9 (9.3) | 8 (4, 15) |
| School-age | 1,025 | 31.6 (26.1) | 24 (14, 41) | 16.3 (13.4) | 12 (7, 21) | 14.4 (16.0) | 8 (4, 19) |
| Maternal race/ethnicity4 | |||||||
| Hispanic | 256 | 41.9 (28.0) | 35 (22, 52.2) | 21.5 (14.3) | 18 (11, 27) | 19 (16.5) | 15 (8, 25) |
| Non-Hispanic White | 2,212 | 29.9 (22.8) | 24 (15, 37) | 15.4 (11.7) | 12 (8, 19) | 13 (14.1) | 8 (4, 17) |
| Non-Hispanic Black | 280 | 44.2 (28.1) | 37 (23, 61) | 22.6 (14.4) | 19 (12, 31) | 19.7 (16.7) | 15 (6, 27) |
| Non-Hispanic Other | 180 | 34.9 (25.7) | 28 (18, 43) | 17.9 (13.1) | 14 (9, 22) | 15.7 (15.9) | 10 (5.5, 21) |
| Maternal education4 | |||||||
| ≤ High school graduate | 520 | 44.5 (28.1) | 37 (23, 61) | 22.8 (14.4) | 19 (12, 31) | 20 (17) | 15 (7.5, 27) |
| Some college | 585 | 38.9 (29.8) | 30 (18, 49) | 19.9 (15.3) | 15 (9, 25) | 17.7 (18.2) | 13 (6, 23) |
| ≥ Bachelor’s degree | 1,856 | 27 (19.3) | 23 (14, 34) | 13.9 (9.9) | 12 (7, 17) | 11.5 (12) | 8 (4, 15) |
| Other non-ASD neurodevelopmental or psychiatric conditions5 | |||||||
| Reported | 527 | 44.2 (28.3) | 37 (23, 60.5) | 22.6 (14.5) | 19 (12, 31) | 20.4 (16.9) | 15 (8, 29) |
| Not Reported | 2314 | 26.4 (16.8) | 23 (15, 34) | 13.6 (8.6) | 12 (8, 17) | 10.6 (10) | 8 (4, 15) |
N remains same across columns.
Scaled scores according to percent of maximum possible, as described in text.
Among participants with information available on ASD diagnosis.
Among participants with non-missing maternal race/ethnicity or maternal education.
Excluding participants with an ASD diagnosis (n=190).
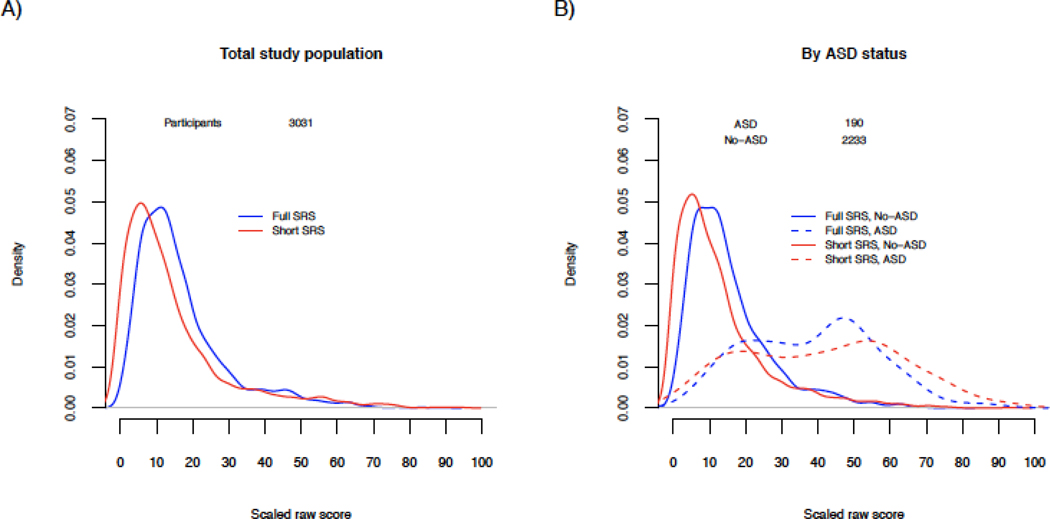
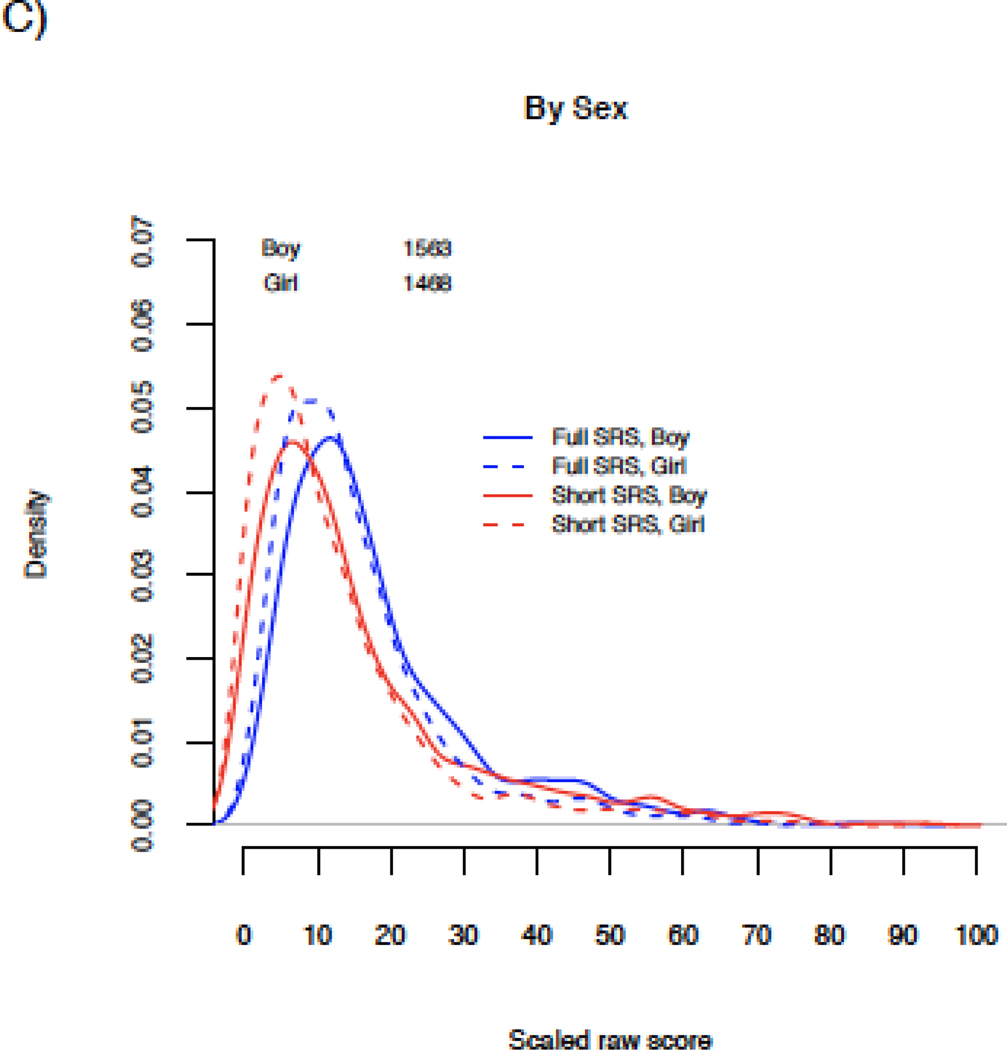
Raw short and full scores were unimodal and right skewed (Online Resource Figure 2). Comparing scores on the same scale, distributions were highly overlapping overall as well as by ASD status and sex (Figure 1). The mode was shifted slightly to the left for short scores in the total population (Figure 1A). ASD case distributions were more bimodal, and there was somewhat greater separation between the ASD and non-ASD modes based on short scores as compared to full scores (corresponding to approximately 5-scaled-point greater separation; Figure 1B). Distributions were similar by sex (Figure 1C), though male scores were shifted slightly higher than female scores for both short and full scores. Full and short score comparisons were similar when examined by cohort type (Online Resource Figure 3). Enriched risk populations had distributions shifted slightly higher relative to the general population for both the full and short scores. Full and short scores from familial enriched risk cases were highly overlapping and lower than for cases from other study types, with a distribution shifted to the left. Examining by form (Online Resource Figure 4; Table 3), preschool scores had narrower distributions than scores derived from school-aged forms for both short and full scores.
Figure 1. Distributions of full and short SRS scores in the study population.
Distributional plots of full 65-item (blue lines) and short 16-item (red lines) SRS scores in the total study population (A), by ASD status (B), by child’s sex (C) and by ASD status and child’s sex (D). Scores were scaled from 0–100 to allow for comparison on the same scale.
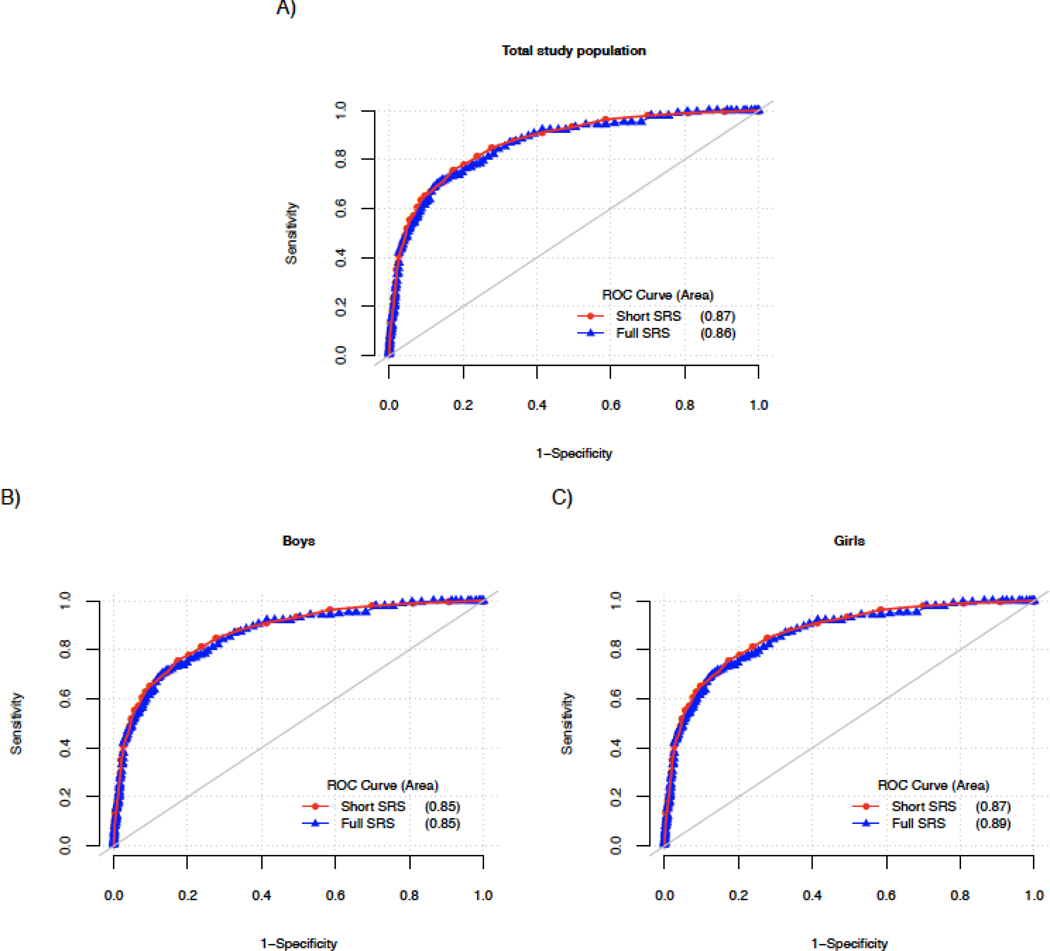
ROC analyses suggested nearly identical performance of full and short SRS scores in predicting ASD diagnosis, including by sex (Figure 2; AUC values of 0.86 and 0.87 for full and short scores, respectively). AUC values were similar, though slightly lower, for scores of randomly selected 16-item subsets of the SRS (range 0.81–0.86 for 50 iterations; Online Resource Figure 5A). ASD prediction for full and short SRS scores was also similar by cohort type and form (Online Resource Figure 5B-F). Higher AUC values were seen in general population and non-familial enriched risk cohorts (0.92–0.93) than in enriched familial risk cohorts. In addition, higher AUC was noted with school-aged forms than for preschool forms (which comprise the vast majority of enriched familial risk scores). AUC values were comparable in studies with clinical confirmation of diagnostic status (0.84 for full and 0.85 for short scores).
Figure 2. ROC Curves of full and short SRS scores in the study population.
ROC curve predicting ASD status in our study population for full 65-item (blue line) and short 16-item (red line) SRS scores. AUC values shown in parenthesis.
Overall, score cut-offs identified here are similar to published standards (e.g., broadly consistent with a T-score ~60), yielded strong sensitivity and specificity, and were remarkably similar across short and full scores (Table 4; Online Resource Table 2 shows AUC results further stratified by sex and form). Exceptions were cut-offs approximately 1–1.5 standard deviations lower than published norms (suggesting T-score cut-offs ~45–50) in enriched familial risk cohorts and according to preschool forms. Among pre-school children in the general population, sensitivity and especially specificity were markedly lower (0.75 and 0.64 respectively), compared with performance among school aged children (0.85 and 0.90 respectively), though performance was similar by short and full scores.
Table 4.
Area under the curve (AUC) values and cutoff points for SRS full and short score ROC analyses in association with ASD diagnosis
| Full SRS | Short SRS | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | AUC | Raw score cutoff | T-score2 (boy, girl) | Scaled3 score cutoff | AUC | Raw score cutoff | Scaled3 score cutoff | Sensitivity | Specificity | |
| Total ROC analytic sample | 2,423 | 0.8623 | 52.00 | 57,60 | 26.67 | 0.8708 | 12.6 | 26.26 | 0.72 | 0.86 |
| Cohort source population | ||||||||||
| General | 1,495 | 0.9118 | 54.00 | 58,61 | 27.69 | 0.9246 | 13.53 | 28.18 | 0.79 | 0.91 |
| ASD Familial Enriched Risk | 251 | 0.7858 | 42.00 | 50 | 21.54 | 0.8119 | 9.09 | 18.94 | 0.63 | 0.82 |
| ASD Non-Familial Enriched Risk | 677 | 0.9158 | 63.00 | 62,64 | 32.31 | 0.9228 | 15.91 | 33.14 | 0.90 | 0.83 |
| Child sex | ||||||||||
| Boys | 1,251 | 0.8500 | 52.00 | 57 | 26.67 | 0.8542 | 12.64 | 26.34 | 0.73 | 0.83 |
| Girls | 1,172 | 0.8720 | 45.00 | 57 | 23.08 | 0.8902 | 10.47 | 21.80 | 0.76 | 0.83 |
| SRS form type | ||||||||||
| Preschool | 765 | 0.7916 | 34.00 | 47 | 17.44 | 0.8063 | 6.94 | 14.45 | 0.77 | 0.70 |
| School-Age | 1,658 | 0.9168 | 54.00 | 58,61 | 27.69 | 0.9282 | 13.45 | 28.03 | 0.87 | 0.85 |
| Among general population | ||||||||||
| Preschool | 524 | 0.6959 | 29.00 | 45 | 14.87 | 0.7237 | 5.57 | 11.61 | 0.86 | 0.61 |
| School-age | 971 | 0.9316 | 54.00 | 58,61 | 27.69 | 0.9432 | 13.61 | 28.36 | 0.86 | 0.90 |
N indicates the number of individuals with information on ASD diagnosis.
T-score corresponding to raw score cutoff, using preschool form for ASD familial enriched risk and school-age form (boys, girls) for total analytic sample, general population, ASD Non-Familial Enriched Risk.
Scaled scores as described in text.
When exploring score prediction of available non-ASD diagnoses (representing 28% of the study population) in secondary analyses, performance was nearly identical between the full and short SRS scores (AUC 0.70 and 0.68 respectively; Online Resource Figure 6); notably, these AUC values were lower than those for ASD diagnosis (Figure 2).
Discussion
Using data from the large ECHO Program, we evaluated the performance of newly proposed 16-item short SRS scores relative to full (65-item) SRS scores, and considered score properties across different types of study populations. We found a high degree of similarity between these SRS scores in our sample. Short scores maintained similar distributional properties and strong prediction of ASD diagnosis, as well as modest established sex differences characteristic of social communication traits. However, we note some caution in concluding independently-administered short and long measures are equivalent for assessment of quantitative social communication traits, given short scores considered here were drawn from 65-item administrations, and due to the need to address performance according to other properties. These and additional points are further discussed below.
Prior work has not examined comparability of distributions of these short SRS scores (or, to our knowledge, other abbreviated SRS versions) and full 65-item SRS scores in order to address their use as a quantitative trait measure capturing variation across the population. In our analyses, overall, distributions of full and short SRS scores were overlapping, though there was some evidence for floor effects with the shortened SRS score as overall distributions were shifted slightly to the lower end. Both scores had an approximately two standard deviation difference in mean scores between cases and controls, demonstrating ability to separate these groups. There was somewhat greater separation of the distributions between individuals with and without ASD diagnoses for short scores, although the difference did not translate into markedly improved ASD prediction in our ROC analyses. Additional studies may consider the impact of any modest distributional differences on etiologic research.
We observed slight differences in the distribution of scores across types of study populations, though these largely did not differ by full or short scores. Populations with higher risk for ASD had distributions shifted toward greater ASD-related traits (higher SRS scores), consistent with observations in prior work (Virkud, Todd, Abbacchi, Zhang, & Constantino, 2009; Page et al., 2016). However, agreement with ASD diagnosis according to either score was poorer in familial enriched risk studies. This may have been due to differences in scores according to preschool forms (which also had reduced AUC values) and their higher use in these cohorts, and/or well-described challenges in determining ASD-related behaviors in younger children (Ozonoff et al., 2011), or potential differences in reporting of ASD-related traits in the younger siblings of ASD cases. Prior work has noted on average lower scores according to the preschool form (Constantino & Gruber, 2012) as well as reduced reliability of diagnoses in children age 3 or younger (Martinez-Pedraza Fde & Carter, 2009). Results from previous enriched familial risk studies have also suggested the potential for phenotypic heterogeneity in quantitative traits across siblings (Girault et al., 2020).
Our findings suggest nearly identical criterion validity of short and full SRS scores. This implies improved efficiency and valid prediction of ASD diagnosis from short scores. Results of a recent Vietnamese validation study (which derived short scores from full school-aged SRS administrations) were also supportive of the short scores’ performance (AUC 0.98, sensitivity 0.9, specificity 0.98) and agreement with full scores (Nguyen et al., 2019). However, we cannot rule out potential over-estimation in results regarding the similarity between 16- and 65-item scores, due to having drawn 16 item scores from 65-item administrations and potential influences therein from correlated response patterns. We also examined predictive ability of a series of randomly-selected 16 item scores, for comparison to performance of the specific 16 items selected based on IRT analyses in Sturm et al. Results of these analyses suggested similarity between scores of the randomly-selected subsets and the full and short scores, though ranges for AUC values were lower than for the Sturm et al. short score in most comparisons. In addition, across these randomly selected subsets, we did not observe items that were uniformly selected across all scores with the highest AUC values. While these findings could indicate any of a minimal subset of items within the SRS may be sufficient to broadly predict ASD, prior work (and consideration of clinical content) would suggest that certain items do hold greater importance for the measurement of ASD phenotype (Duku et al., 2013; Reiersen et al., 2007; Sturm et al., 2017).
Development of the Sturm et al. 16-item-SRS scores stemmed from concerns regarding the potential influence of non-core ASD symptoms on SRS scores. We found modest gains in ASD prediction and specificity according to scores based on their item selection. Secondary analyses predicting other, non-ASD psychiatric and neurodevelopmental conditions here suggested markedly lower prediction than for ASD. These analyses support specificity of SRS scores to ASD above other conditions, with similarity for short and full scores. While prior work has demonstrated higher SRS scores among individuals with other developmental and behavioral conditions, on average, scores of these groups tend to be lower than those with ASD diagnoses (Constantino & Gruber, 2012; Griffiths, Farrell, Waters, & White, 2017; Reiersen et al., 2007). There is a high prevalence of co-occurring conditions in ASD-affected individuals (Hossain et al., 2020). Evidence of the interactive relationship between social communication and other behavioral factors in the developmental progression of ASD traits (Hawks, Marrus, Glowinski, & Constantino, 2019; Liew, Thevaraja, Hong, & Magiati, 2015) and shared genetic liability across disorders (Pohl et al., 2019) suggests challenges in attempts to separate these factors.
While our study has several strengths, including the ability to examine scores across different types of study populations drawn from both the general population and high-risk cohorts, several limitations should be considered. As noted, we based score comparisons on 65-item administrations and it is not known how this may influence findings. Prior studies using other subsets of SRS items have reported similarly high correlation between shortened and full scores, though have also drawn shortened scores from 65-item administrations (Blanken et al., 2015; Duku et al., 2013). Notably, the Generation R cohort in the Netherlands has administered an 18-item version of the SRS to its nearly 10,000 participants (Roman et al., 2013). While validation studies have not specifically compared scores from these 18-item administrations to those from the full SRS in the same participants, these scores’ separation of case and non-case distributions is similar to that of 65-item scores (T. White, personal communication), suggesting at least some broad support for similarity of short SRS scores administered independently. Initial development of the SRS, as well as attempts to abbreviate the Autism Screening Questionnaire (Berument, Rutter, Lord, Pickles, & Bailey, 1999), suggested reduced ability to separate from other psychiatric disorders when attempting to reduce the number of items, while examination of shortened versions of other psychometric scales has provided evidence supporting performance and comparability for some measures (Roberti, Harrington, & Storch, 2011) and sample-dependent performance for others (Marvin, Marvin, Lipkin, & Law, 2017). This evidence suggests the need to clarify the performance of short form independent administration in future work. While we examined several key psychometric properties of 16-item-SRS scores here, we were not able to examine test-re-test reliability, and future work should assess whether reliability is affected when removing redundancy of certain items built into the published SRS. ASD diagnoses were not uniformly defined across studies included in our analyses. However, AUC values here are similar to those in other studies with clinical confirmation for all participants (Constantino & Gruber, 2012). Information on other disorders was limited and likely underreported. We did not have sufficient numbers to examine properties according to ADHD (or other individual conditions) specifically, as may be useful to address in future work. We did not have the ability to examine the role of behavioral and clinical features in short vs full score comparisons in ASD cases due to limited information. Finally, our cohorts with research participants drawn from the general population are not representative of the US general population; while score differences by race and ethnicity were not found in SRS standardization samples (Constantino & Gruber, 2012), work has suggested differences by maternal education and family income (Moody et al., 2017). Thus, future work is needed to consider differential item functioning by demographic characteristics and potential impact on short versus full SRS scores.
Conclusions
Quantitative measures like the SRS allow for consideration of traits across the population, including the ability to capture both subclinical deficits and ranges in clinical severity. Furthermore, the SRS allows for determination of ASD-related traits in studies where clinical assessment is either not possible or not feasible due to large sample sizes and limited resources. Our findings leveraging existing data from ECHO cohorts demonstrate a high degree of similarity between shortened 16-item and full 65-item SRS scores and suggest promise for use of this abbreviated measure. However, validation studies are needed to further confirm psychometric properties of stand-alone administrations of shortened scores, examine their heritability, and consider their use as a quantitative trait measure in etiologic work.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under Award Number U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), as well as 1U2COD023375-02 (Lyall), UH3OD023342 (Newschaffer), UH3OD023275 (Karagas), UG3/UH3OD023365 (Hertz-Picciotto), 5UH3OD023348-04 (O’Shea), 1U24OD023319-01 (Gershon & Cella), 1UG3OD023271-01 and 4UH3OD023271-03 (Karr), 1UG3OD023305-01 and 4UH3OD023305-03 (Trasande), UG3/UH3OD023328 (Duarte); UG3/UH3OD023286 (Oken). Funding support for original data collections was also received from the NIH under R01MH068398 (Ozonoff), R01HD055741 (Piven); R01ES016863 (Swan), R01ES25169 (Swan); from the National Institute of Environmental Health Sciences under P01ES022832 and US EPA US EPA: RD83544201 (Karagas), and from the National Institute of Child Health and Human Development under R01HD057284 (Messinger and Stone). The authors wish to thank our ECHO colleagues, the medical, nursing and program staff, as well as the children and families participating in the ECHO cohorts. We would also like to recognize the contributions of our collaborators, including SH Swan, ES Barrett, RHN Nguyen (TIDES study PIs). Dr Constantino reported having received royalties from Western Psychological Services from the distribution of the SRS. No other disclosures were reported. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix
ECHO Collaborators
The authors wish to thank our ECHO colleagues, the medical, nursing and program staff, as well as the children and families participating in the ECHO cohorts.
ECHO Components- Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Benjamin DK, Smith PB, Newby KL; Data Analysis Center: Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland: Jacobson LP; Research Triangle Institute, Durham, North Carolina: Parker CB; Person-reported Outcomes-Core: Northwestern University, Evanston, Illinois: Gershon R, Cella D; Children’s Health and Exposure Analysis Resource: Icahn School of Medicine at Mount Sinai, New York City, New York: Teitelbaum S; Wright RO; Wadsworth Center, Albany, New York: Aldous, KM, RTI International, Research Triangle Park, North Carolina: Fennell T; University of Minnesota, Minneapolis, Minnesota: Hecht SS, Peterson L; Westat, Inc., Rockville, Maryland: O’Brien B; Idea States Network: University of Arkansas for Medical Sciences, Little Rock: Lee JY, Snowden J.
ECHO Awardees and Cohorts (see also Online Resource Table 1):
The following awards contributed data to this manuscript: Columbia University, New York, NY: Duarte C; University of California, Davis, CA: Hertz-Picciotto I; Dartmouth College, Hanover, NH: Karagas M; University of Washington, Seattle, WA: Karr K and Trasande L; Drexel University, Philadelphia, PA : Newschaffer C; Harvard Pilgrim Healthcare, Boston MA: Oken E; and the University of Chapel Hill, North Carolina: O’Shea M
References
- Aldridge FJ, Gibbs VM, Schmidhofer K, & Williams M. (2012). Investigating the clinical usefulness of the Social Responsiveness Scale (SRS) in a tertiary level, autism spectrum disorder specific assessment clinic. J Autism Dev Disord, 42(2), 294–300. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, & Bailey A. (1999). Autism screening questionnaire: diagnostic validity. Br J Psychiatry, 175, 444–451. [DOI] [PubMed] [Google Scholar]
- Blanken LM, Mous SE, Ghassabian A, Muetzel RL, Schoemaker NK, El Marroun H, . . . White T. (2015). Cortical morphology in 6- to 10-year old children with autistic traits: a population-based neuroimaging study. Am J Psychiatry, 172(5), 479–486. [DOI] [PubMed] [Google Scholar]
- Bolte S, Westerwald E, Holtmann M, Freitag C, & Poustka F. (2011). Autistic traits and autism spectrum disorders: the clinical validity of two measures presuming a continuum of social communication skills. J Autism Dev Disord, 41(1), 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Aiken LS, & West SG (2010). The Problem of Units and the Circumstance for POMP. Multivariate Behavioral Research, 34(3), 315–346. [Google Scholar]
- Constantino JN, Gruber C. (2005). The Social Responsiveness Scale (SRS). Los Angeles, CA: Western Pyschological Services. [Google Scholar]
- Constantino JN, Gruber C. (2012). Social Responsiveness Scale, Second Edition. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Constantino JN , & Gruber C. (2012). Social Responsiveness Scale, Second Edition (SRS-2) [Manual]. Torrance, CA: Western Psychological Services. [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, . . . Reich W. (2003). Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord, 33(4), 427–433. [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Frazier TW (2013). Commentary: The observed association between autistic severity measured by the social responsiveness scale (SRS) and general psychopathology--a response to Hus et al.(2013). J Child Psychol Psychiatry, 54(6), 695–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Todd RD (2003). Autistic traits in the general population: a twin study. Arch Gen Psychiatry, 60(5), 524–530. [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Todd RD (2005). Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry, 57(6), 655–660. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, & Insel TR (2013). Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med, 11, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duku E, Vaillancourt T, Szatmari P, Georgiades S, Zwaigenbaum L, Smith IM, . . . Bennett T. (2013). Investigating the measurement properties of the social responsiveness scale in preschool children with autism spectrum disorders. J Autism Dev Disord, 43(4), 860–868. [DOI] [PubMed] [Google Scholar]
- Duvall JA, Lu A, Cantor RM, Todd RD, Constantino JN, & Geschwind DH (2007). A quantitative trait locus analysis of social responsiveness in multiplex autism families. Am J Psychiatry, 164(4), 656–662. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Thompson L, Youngstrom EA, Law P, Hardan AY, Eng C, & Morris N. (2014). A twin study of heritable and shared environmental contributions to autism. J Autism Dev Disord, 44(8), 2013–2025. doi: 10.1007/s10803-014-2081-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW, & Blaisdell CJ (2018). Environmental influences on Child Health Outcomes, a Research Program of the National Institutes of Health. Curr Opin Pediatr, 30(2), 260–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JB, Swanson MR, Meera SS, Grzadzinski RL, Shen MD, Burrows CA, . . . Piven J. (2020). Quantitative trait variation in ASD probands and toddler sibling outcomes at 24 months. J Neurodev Disord, 12(1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths DL, Farrell LJ, Waters AM, & White SW (2017). ASD Traits Among Youth with Obsessive-Compulsive Disorder. Child Psychiatry Hum Dev, 48(6), 911–921. [DOI] [PubMed] [Google Scholar]
- Havdahl KA, Hus Bal V, Huerta M, et al. Multidimensional Influences on Autism Symptom Measures: Implications for Use in Etiological Research. Journal of the American Academy of Child and Adolescent Psychiatry. 2016;55(12):1054–1063.e1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawks ZW, Marrus N, Glowinski AL, & Constantino JN (2019). Early Origins of Autism Comorbidity: Neuropsychiatric Traits Correlated in Childhood Are Independent in Infancy. J Abnorm Child Psychol, 47(2), 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, Khan N, Sultana A, Ma P, McKyer ELJ, Ahmed HU, & Purohit N. (2020). Prevalence of comorbid psychiatric disorders among people with autism spectrum disorder: An umbrella review of systematic reviews and meta-analyses. Psychiatry Res, 287, 112922. [DOI] [PubMed] [Google Scholar]
- Hus V, Bishop S, Gotham K, Huerta M, & Lord C. (2013). Factors influencing scores on the social responsiveness scale. J Child Psychol Psychiatry, 54(2), 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaat AJ, & Farmer C. (2017). Commentary: Lingering questions about the Social Responsiveness Scale short form. A commentary on Sturm et al. (2017). J Child Psychol Psychiatry, 58(9), 1062–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewski SJ, Joseph RM, Kim SH, Allred EN, O’Shea TM, Leviton A, & Kuban KCK (2017). Social Responsiveness Scale Assessment of the Preterm Behavioral Phenotype in 10-Year-Olds Born Extremely Preterm. J Dev Behav Pediatr, 38(9), 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavey A, Zwaigenbaum L, Heavner K, & Burstyn I. (2013). Gestational age at birth and risk of autism spectrum disorders in Alberta, Canada. J Pediatr, 162(2), 361–368. [DOI] [PubMed] [Google Scholar]
- Liew SM, Thevaraja N, Hong RY, & Magiati I. (2015). The relationship between autistic traits and social anxiety, worry, obsessive-compulsive, and depressive symptoms: specific and non-specific mediators in a student sample. J Autism Dev Disord, 45(3), 858–872. [DOI] [PubMed] [Google Scholar]
- Lowe JK, Werling DM, Constantino JN, Cantor RM, & Geschwind DH (2015). Social responsiveness, an autism endophenotype: genomewide significant linkage to two regions on chromosome 8. Am J Psychiatry, 172(3), 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Constantino JN, Weisskopf MG, Roberts AL, Ascherio A, & Santangelo SL (2014). Parental social responsiveness and risk of autism spectrum disorder in offspring. JAMA Psychiatry, 71(8), 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pedraza Fde L, & Carter AS (2009). Autism spectrum disorders in young children. Child Adolesc Psychiatr Clin N Am, 18(3), 645–663. doi: 10.1016/j.chc.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin AR, Marvin DJ, Lipkin PH, & Law JK (2017). Analysis of Social Communication Questionnaire (SCQ) Screening for Children Less Than Age 4. Curr Dev Disord Rep, 4(4), 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger DS, Young GS, Webb SJ, Ozonoff S, Bryson SE, Carter A, . . . Zwaigenbaum L. (2015). Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Mol Autism, 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody EJ, Reyes N, Ledbetter C, Wiggins L, DiGuiseppi C, Alexander A, . . . Rosenberg SA (2017). Screening for Autism with the SRS and SCQ: Variations across Demographic, Developmental and Behavioral Factors in Preschool Children. J Autism Dev Disord, 47(11), 3550–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moul C, Cauchi A, Hawes DJ, Brennan J, & Dadds MR (2015). Differentiating autism spectrum disorder and overlapping psychopathology with a brief version of the social responsiveness scale. Child Psychiatry Hum Dev, 46(1), 108–117. doi: 10.1007/s10578-014-0456-4 [DOI] [PubMed] [Google Scholar]
- Nguyen PH, Ocansey ME, Miller M, Le DTK, Schmidt RJ, & Prado EL (2019). The reliability and validity of the social responsiveness scale to measure autism symptomology in Vietnamese children. Autism Res, 12(11), 1706–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, . . . Stone WL (2011). Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics, 128(3), e488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Young GS, Hepburn S, Thompson M, Colombi C, . . . Rogers SJ (2011). Onset patterns in autism: correspondence between home video and parent report. J Am Acad Child Adolesc Psychiatry, 50(8), 796–806 e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page J, Constantino JN, Zambrana K, Martin E, Tunc I, Zhang Y, . . . Messinger D. (2016). Quantitative autistic trait measurements index background genetic risk for ASD in Hispanic families. Mol Autism, 7(1), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl A, Jones WR, Marrus N, Zhang Y, Klin A, & Constantino JN (2019). Behavioral predictors of autism recurrence are genetically independent and influence social reciprocity: evidence that polygenic ASD risk is mediated by separable elements of developmental liability. Transl Psychiatry, 9(1), 202. doi: 10.1038/s41398-019-0545-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, & Todd RD (2007). Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry, 48(5), 464–472. [DOI] [PubMed] [Google Scholar]
- Roberti JW, Harrington LN, & Storch EA (2011). Further psychometric support for the 10-item version of the perceived stress scale. Journal of College Counseling, 9, 35–47. [Google Scholar]
- Robinson EB, St Pourcain B, & Anttila V. (2016). Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VW, Hofman A, de Rijke YB, . . . Tiemeier H. (2013). Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol, 74(5), 733–742. [DOI] [PubMed] [Google Scholar]
- Sturm A, Kuhfeld M, Kasari C, & McCracken JT (2017). Development and validation of an item response theory-based Social Responsiveness Scale short form. J Child Psychol Psychiatry, 58(9), 1053–1061. [DOI] [PubMed] [Google Scholar]
- Virkud YV, Todd RD, Abbacchi AM, Zhang Y, & Constantino JN (2009). Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am J Med Genet B Neuropsychiatr Genet, 150B(3), 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.