Abstract
BACKGROUND AND PURPOSE:
Previous studies demonstrated that carotid plaques analyzed by CTA can show contrast plaque enhancement. The purpose of this preliminary work was to evaluate the possible association between the fissured fibrous cap and contrast plaque enhancement.
MATERIALS AND METHODS:
Forty-seven consecutive (men = 25; average age = 66.8 ± 9 years) symptomatic patients studied by use of a multidetector row CT scanner were prospectively analyzed. CTA was performed before and after contrast and radiation doses were recorded; analysis of contrast plaque enhancement was performed. Patients underwent carotid endarterectomy en bloc; histologic sections were prepared and evaluated for fissured fibrous cap and microvessel attenuation. The Mann-Whitney test was performed to evaluate the differences between the 2 groups. A multiple logistic regression analysis was performed to assess the effect of fissured fibrous cap and microvessel attenuation on contrast plaque enhancement. Receiver operating characteristic curve and area under the curve were also calculated.
RESULTS:
Twelve patients had fissured fibrous cap. In 92% (11/12) of fissured fibrous cap–positive plaques, we found contrast plaque enhancement, whereas in 69% (24/35) of the plaques without fissured fibrous cap contrast plaque enhancement was found. The Mann-Whitney test showed a statistically significant difference between the contrast enhancement in plaques with fissured fibrous cap (Hounsfield units = 22.6) and without fissured fibrous cap (Hounsfield units = 12.9) (P = .011). On the regression analysis, both fissured fibrous cap and neovascularization were associated with contrast plaque enhancement (P = .0366 and P = .0001). The receiver operating characteristic curve confirmed an association between fissured fibrous cap and contrast plaque enhancement with an area under the curve of 0.749 (P = .005).
CONCLUSIONS:
The presence of fissured fibrous cap is associated with contrast plaque enhancement. Histologic analysis showed that the presence of fissured fibrous cap is associated with a larger contrast plaque enhancement compared with the contrast plaque enhancement of plaques without fissured fibrous cap.
The presence of fissured fibrous cap (FFC) in the carotid artery plaque is associated with an increased risk of cerebrovascular events, and therefore FFC is considered one element that makes a carotid plaque “vulnerable.”1–3 Identification of this condition is important to obtain a better stratification of stroke risk.1
The FFC has been studied by use of MR imaging, demonstrating the potential of MR imaging to detect the rupture of the fibrous cap,4,5 with or without the use of gadolinium.6,7 Recently, with the use of CTA and morphologic analysis of the carotid plaque, the FFC was documented.8 Even though the rupture of FFC has been shown to be associated with enhancement on MR imaging,7 this association has not yet been demonstrated by use of CT. The carotid plaque enhancement (CPE) on CTA is associated with plaque instability9–11; CPE is associated with microvessel attenuation, but the neovascularization alone cannot be the only factor because some plaques with high CPE do not show neovascularization.9
Our hypothesis is that the rupture of the FFC is an independent factor related to the CPE, and we aim to evaluate this association.
Materials and Methods
Study Design and Patient Population
We obtained institutional review board approval for this study. Forty-seven consecutive symptomatic patients (men = 25; women = 22; mean age = 66.8 ± 9 years) were prospectively analyzed from May 2010 to March 2012. The mean time interval between CTA and the clinical episode (stroke or TIA) was 4 days (range, 1–12 days). We considered as an inclusion criterion that the carotid endarterectomy (CEA) was performed within 10 days after the CTA. A subgroup of the current population (n = 29) was included in a previous study.9
CT Technique
CT was performed with the use of a 16-row multidetector CT system (Brilliance, Philips, Best, The Netherlands) with a previously described protocol.9 Briefly, the basal scan is performed, followed by the angiographic phase in the caudocranial direction. The volume of injected contrast is 80 mL (Ultravist 370; Bayer Schering Pharma, Berlin, Germany), with a flow rate of 5 mL/s. The precise timing of the injection is obtained with a bolus-tracking technique: the dynamic monitoring began 6 seconds after the beginning of the intravenous injection of contrast material. The delay between the acquisitions of each monitoring image was 1 second. When the threshold was reached (by using a threshold inside the region of interest set +90 Hounsfield units [HU] above the baseline as a trigger), the patient was instructed not to breathe; after an interval of 4 seconds, the CTA acquisition started in the caudocranial direction from the aortic arch to the circle of Willis, with a section thickness of 1.3 mm, increment 0.6 mm, matrix 512 × 512, FOV 16–19 cm, mAs 260–300, and kV 120–140. An intermediate reconstruction algorithm (C-filter) was used.
Carotid Plaque Enhancement Quantification
The CPE quantification was performed by use of a validated technique.9–11 Two radiologists performed all measurements of HU (L.S. and E.R.) by using as window/level settings W850:L300.12 Particular care was taken to obtain a correct registration between the basal phase and the enhanced phase (Fig 1A,-B). The HU quantification is obtained by use of a circular or elliptical region of interest (≥1 mm2). The threshold to consider the CPE as present was a variation of ≥10 HU.9
Fig 1.
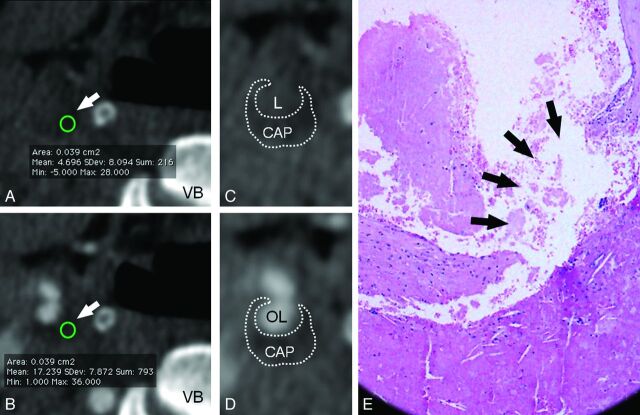
A 71-year-old male patient. In CT analysis, before (A) and after (B) contrast, the carotid artery plaque is visible (white arrows) and the CPE HU value is shown. The circles, indicated by the white arrows, represent the area of the plaque that was assessed (in this case, the area was 0.039 cm2); in the basal scan, the average HU value was 4.696, whereas after administration of contrast material it was 17.239 (VB indicates vertebral body). In C and D, the same images as A and B are shown with a zoom factor of 150% and demonstrate the contour of the carotid artery plaque (CAP). In C, the patent lumen of the carotid is visible (L indicates lumen), whereas in D, the opacified patent lumen is also indicated (OL indicates opacified lumen). In the histologic section (E), the rupture of the FFC is noted (black arrows).
Carotid Endarterectomy
CEA was performed by 2 vascular surgeons (R.M. and Robert Sanfilippo) by use of the en bloc technique (to reduce the manipulation of the carotid plaque and avoid the potential rupture/fragmentation of the plaque), by scoring the adventitia and outer media with a scalpel and removing the plaque as an intact tube. The criteria to perform carotid endarterectomy were based on the recommendations by the NASCET and European Carotid Surgery Trial studies for symptomatic patients.13–15
Histologic Analysis of the Plaque
Histologic examination was performed by 2 observers (L.S. and E.R.) blinded to clinical-radiologic data. The plaque specimen was immediately fixed in formalin, and it was taken to the laboratory directly after the surgical procedure. None of the carotid endarterectomy specimens showed disruption of the luminal surface of the plaque. Carotid plaque was decalcified and embedded in paraffin wax. The portion of the specimen showing the carotid plaque was divided transversely in sections at 3-mm intervals that were air-dried at 60°C for 45 minutes. After this phase, paraffin was removed by xylol and the sections were hydrated. Finally, endogenous peroxidase activity was blocked by 2% H2O2. The 5-μm transverse sections were subjected to histologic examination to identify the plaque components, the fibrous cap, and its rupture (Fig 1C). The fibrous cap components such as attenuated and loose (proteoglycan) matrix, hemorrhage, neovasculature, calcification, and inflammatory cell infiltrate were analyzed, and the condition of FFC was identified by the loss of anatomic continuity (integrity) of the fibrous cap.6 In addition, the neovascularization was assessed by analyzing the microvessel attenuation according to the methodology indicated by Saba et al.9
Calculation of the Radiation Dose
For each examination, the CT dose index (CTDI), dose-length product (DLP), and length of the scans in centimeters was collected. The effective dose (measured in mSv) was also calculated by converting the DLP by use of the following conversion equation for CT of the neck: mSv = 0.0059 * DLP.16
Statistical Analysis
Continuous quantitative variables were expressed as mean ± standard deviations. The plaque HU values were averaged between the 2 radiologists, and the Bland-Altman statistic was performed. The Mann-Whitney test was used to test the differences between the 2 groups of patients, with and without FFC, and the nonparametric McNemar test was used to test the difference between the prevalence of FFC in carotid arteries with and without CPE. Receiver operating characteristic (ROC) curve analysis was also performed to test the association between the presence of FFC and CPE; the area under the curve was also calculated. Multiple logistic regression analysis was also performed to assess the effect of the FFC versus the microvessel attenuation. R software (www.r-project.org) was used for statistical analyses.
Results
General Results
Demographic and CPE characteristics of the studied patients are shown in Table 1. In the 47 carotid arteries studied, 12 cases with FFC were found. In 92% (11/12) of the plaques with FFC we found CPE, whereas in only 69% (24/35) of the plaques without FFC, was CPE found and the McNemar test confirmed that there was a statistically significant difference (P = .0001) Bland-Altman analysis demonstrated very good concordance between readers, with a mean difference between them of 4%.
Table 1:
Patient characteristics
| Parameter | FFC | Non-FFC | P Value |
|---|---|---|---|
| Patients, n | 12 (25.5%) | 35 (74.5%) | NC |
| CPE, HU | 22.6 ± 10.9 | 12.9 ± 9.4 | .001 |
| CPE, 95% CI, HU | 12.8–33.1 | 10.2–20.8 | NC |
| Age, y | 67 ± 8 | 65 ± 9 | .495 |
| Sex, male | 8 (67%) | 17 (49%) | .278 |
| Smoker | 6 (55%) | 19 (54%) | .938 |
| Hypertension | 7 (58%) | 22 (63%) | .781 |
| CAD | 8 (67%) | 19 (54%) | .454 |
| Diabetes | 3 (25%) | 3 (9%) | .141 |
| Dyslipidemia | 7 (58%) | 14 (40%) | .271 |
| Statins and other drugsa | 6 (50%) | 14 (40%) | .545 |
Note:—CAD indicates coronary artery disease; NC, not calculable.
Other lipid-lowering drugs.
Mann-Whitney Test
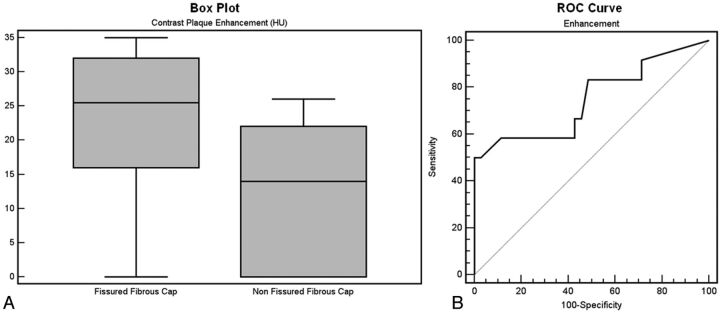
The Mann-Whitney test showed a statistically significant difference between the amount of CPE in plaques with FFC (HU = 22.6 ± 10.9) and without FFC (HU = 12.9 ± 9.4) (P = .011) (Fig 2A).
Fig 2.
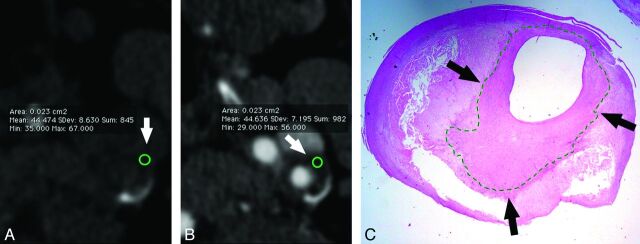
A 68-year-old male patient. In CT analysis, before (A) and after (B) contrast, the carotid artery plaque is visible (white arrows) and the CPE HU value is shown. The circles, indicated by the white arrows, represent the area of the plaque that was assessed; in the basal scan, the average HU value was 44.474, whereas after administration of contrast material it was 44.636. In this case, there was no contrast plaque enhancement. In the histologic section (C), the thick fibrous cap is visible (black arrows).
ROC Curve Analysis
The ROC curve analysis (Fig 2B) confirmed an association between FFC and CPE, with an area under the curve of 0.749 (95% confidence interval = 0.601–0.864; P = .005; standard error = 0.089).
Multiple Logistic Regression Analysis
The multiple logistic regression analysis was performed to assess the effect of the FFC on the CPE by avoiding the confounding effect of the neovascularization (Table 2). We found that both FFC and neovascularization had a statistically significant association with the presence of CPE (P = .0366 and .0001, respectively).
Table 2:
Multiple logistic regression analysis
| Independent Variables | Coefficient | Standard Error | t | P Value | r |
|---|---|---|---|---|---|
| (Constant) | 0.23552 | ||||
| FFC | 0.25401 | 0.1178 | 2.156 | .0366 | 0.208 |
| Neovascularization | 0.19714 | 0.04244 | 4.645 | <.0001 | 0.539 |
Radiation Dose Analysis
The radiation dose analysis was performed in our cohort of patients, and the results are summarized in Table 3.
Table 3:
Radiation dose parameters of the population for the basal or contrast-enhanced phase
| n | Mean | 95% CI | SD | Minimum | Maximum | 2.5–97.5 Percentiles | |
|---|---|---|---|---|---|---|---|
| CTDI, mGy | 47 | 11.657 | 11.510–11.805 | 0.5021 | 10.9 | 13 | 11.035–12.933 |
| DLP, mGy*cm | 47 | 348.113 | 341.502–354.725 | 22.5172 | 306.23 | 415 | 307.425–400.353 |
| Effective dose, mSv | 47 | 2.054 | 2.015–2.093 | 0.1329 | 1.807 | 2.449 | 1.814–2.362 |
| Length | 47 | 29.866 | 29.416–30.316 | 1.5339 | 27.1 | 34.2 | 27.370–33.525 |
Discussion
CTA is widely used for the imaging of carotid arteries; this technique allows for the study of plaque morphology and for the quantification and characterization of plaque composition with excellent detail. In CTA, carotid artery plaques may demonstrate the presence of contrast enhancement,9–11 which is associated with the presence of cerebrovascular symptoms10,11; the reasons underlying the enhancement are otherwise unclear.
In our study, we found that the plaques with FFC enhance more often than do the plaques without FFC: in 92% of the plaques with FFC we found CPE, whereas in only 69% of the plaques without FFC, was CPE found (P = .0001). These results are also concordant with histologic and fluid-dynamic analyses that have clarified that the rupture of the FFC creates a breach into the lipid-rich necrotic core with blood flow that actively enters into the plaque by determining pro-thrombotic effects.17,18 In the only case of FFC without CPE, the histologic sections were re-analyzed and an absence of lipid-rich necrotic core was detected. This fact suggests to us that the configuration of the core of the plaque may play a role: in these cases with a weak necrotic core, the rupture of the plaque may be associated with a blood invasion into the plaque, whereas in the case of more robust configuration, the rupture of the fibrous cap cannot be associated with intraplaque hemorrhage.
Moreover, we also found that the amount of CPE is larger in the plaques with FFC (22.6 versus 12.9; P = .011). This can be explained by the fact that the rupture of the fibrous cap allows the blood to massively enter into the plaque. ROC curve analysis confirmed an association between FFC and CPE (Fig 3). These results suggest that FFC is one of the factors that determine the enhancement of the plaque. It was demonstrated that the neovascularization of the plaque is associated with the presence of CPE, and this parameter may represent a confounding factor in the assessment of the effect of the FFC in CPE. Therefore, a multiple logistic regression analysis was performed, and this confirmed that both FFC and neovascularization had a statistically significant association with the presence of CPE (P = .0366 and .0001, respectively) and that they independently can determine the presence of CPE. These results may explain why in previous analysis,9 plaques with low levels of neovascularization had a large CPE. In our study, notably, the 2 groups of patients, with and without rupture of the FFC, were similar with regard to risk factors for carotid artery disease (Table 1). A similar result has been described with the use of MR imaging,7 but the description of this finding by use of CT has obvious consequences on the usefulness of this evaluation in clinical practice. The results are also concordant with histologic and fluid-dynamic analyses that have clarified that the rupture of the FFC creates a breach into the lipid-rich necrotic core with the blood flow that actively enters into the plaque by determining pro-thrombotic effects.17,18
Fig 3.
Boxplot analysis shows the difference in CPE between the group of patients with FFC and the patients without FFC (A); the ROC curve shows the association between FFC and CPE (B).
In the past years, several investigations have demonstrated the potentialy of MR in the assessment of FFC status.1,2,6,7 However in several institutions, CTA is widely used in the assessment of the carotid artery status, in particular in emergency settings19 or for those subjects with contraindications to MR imaging such as claustrophobic patients or patients with pacemakers. Some advantages of CT are the short time of the procedure (the CT acquisition requires only few seconds) and the excellent spatial resolution that can reach 0.3 mm isotropic voxels. In recent years, several investigations have demonstrated the potential of CT in the assessment of carotid artery plaque composition20,21 by suggesting that some parameters, easily detectable with CT, are important for stroke risk stratification. With the current study, we showed that the presence of FFC is associated with contrast plaque enhancement and that this should be considered a further useful element for the stratification of stroke risk. It is not our opinion that the CTA should be performed to merely test the presence or absence of the CPE; once the CTA is performed, we think that this element should be assessed.
The 2 main disadvantages of CTA are the nephrotoxicity and radiation dose. To reduce the nephrotoxicity, we are currently using the “bolus technique,” with a reduced volume of contrast material (80 mL) injected at a high flow rate. In the future, it is likely that the volume of the injected contrast material will be further reduced, thanks to the potential and velocity of the newer CT scanners. The radiation dose delivered to the patient is the second main issue related to the use of CT. In our cohort of patients, we had a mean CTDI of 11.657 mGy, which is a value similar to another recent publication where the single-source CT was used22 (12.5 mGy); the effective dose for each acquisition is similar. It is important to underline that there is an evolution with the new CT scanners, in particular the dual-energy systems that allow performing examinations with similar image quality and lower radiation dose compared with single-source CT.22
In this study, there are some limitations: the first is the number of analyzed patients (n = 47), which does not allow us to obtain a strong statistical analysis with tight confidence intervals; for this reason, the current results should be considered as preliminary results that must be confirmed in a larger population. The second limitation of this study is the potential plaque rupture during the surgeon's manipulation of the carotid arteries while performing the carotid endarterectomy. However, it is our hypothesis that this can be considered as a minor limitation because our surgeons used particular care in the CEA procedure; to reduce this effect, an ex vivo CEA should be performed.
Conclusions
The results of this preliminary study indicated that the presence of FFC is associated with CPE. Histologic analysis showed that the presence of FFC is associated with a larger CPE compared with the CPE of plaques without FFC.
ABBREVIATIONS:
- CEA
carotid endarterectomy
- CPE
carotid plaque enhancement
- CTDI
CT dose index
- DLP
dose-length product
- FFC
fissured fibrous cap
- ROC
receiver operating characteristic
- HU
Hounsfield Units
References
- 1. Hatsukami TS, Yuan C. MRI in the early identification and classification of high-risk atherosclerotic carotid plaques. Imaging Med 2010;2:63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yuan C, Zhang SX, Polissar NL, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation 2002;105:181–85 [DOI] [PubMed] [Google Scholar]
- 3. Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI: initial results. Stroke 2006;37:818–23 [DOI] [PubMed] [Google Scholar]
- 4. Saba L, Potters F, van der Lugt A, et al. Imaging of the fibrous cap in atherosclerotic carotid plaque. Cardiovasc Intervent Radiol 2010;33:681–89 [DOI] [PubMed] [Google Scholar]
- 5. Watanabe Y, Nagayama M. MR plaque imaging of the carotid artery. Neuroradiology 2010;52:253–74 [DOI] [PubMed] [Google Scholar]
- 6. Hatsukami TS, Ross R, Polissar NL, et al. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000;102:959–64 [DOI] [PubMed] [Google Scholar]
- 7. Cai J, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005;112:3437–44 [DOI] [PubMed] [Google Scholar]
- 8. Saba L, Mallarini G. Fissured fibrous cap of vulnerable carotid plaques and symptomaticity: are they correlated? Preliminary results by using multi-detector-row CT angiography. Cerebrovasc Dis 2009;27:322–27 [DOI] [PubMed] [Google Scholar]
- 9. Saba L, Lai ML, Montisci R, et al. Association between carotid plaque enhancement shown by multidetector CT angiography and histologically validated microvessel density. Eur Radiol 2012;22:2237–45 [DOI] [PubMed] [Google Scholar]
- 10. Saba L, Piga M, Raz E, et al. Carotid artery plaque classification: does contrast enhancement play a significant role? AJNR Am J Neuroradiol 2012;33:1814–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saba L, Mallarini G. Carotid plaque enhancement and symptom correlations: an evaluation by using multidetector row CT angiography. AJNR Am J Neuroradiol 2011;32:1919–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saba L, Mallarini G. MDCTA in the study of carotid plaque stenosis degree: evaluation of inter-observer agreement. AJR Am J Roentgenol 2008;190:W41–46 [DOI] [PubMed] [Google Scholar]
- 13. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade stenosis. N Engl J Med 1991;325:445–53 [DOI] [PubMed] [Google Scholar]
- 14. Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415–25 [DOI] [PubMed] [Google Scholar]
- 15. Rothwell PM, Eliasziw M, Gutnikov SA. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107–16 [DOI] [PubMed] [Google Scholar]
- 16. Bongartz G, Golding SJ, Jurik AG. CT quality criteria. Luxembourg:European Commission; 2004 [Google Scholar]
- 17. Redgrave JN, Gallagher P, Lovett JK, et al. Critical cap thickness and rupture in symptomatic carotid plaques: the Oxford Plaque Study. Stroke 2008;39:1722–9 [DOI] [PubMed] [Google Scholar]
- 18. Cicha I, Wörner A, Urschel K, et al. Carotid plaque vulnerability: a positive feedback between hemodynamic and biochemical mechanisms. Stroke 2011;42:3502–10 [DOI] [PubMed] [Google Scholar]
- 19. Magge R, Lau BC, Soares BP, et al. Clinical risk factors and CT imaging features of carotid atherosclerotic plaques as predictors of new incident carotid ischemic stroke: a retrospective cohort study. AJNR Am J Neuroradiol 2013;34:402–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saba L, Sanfilippo R, Sannia S, et al. Association between carotid artery plaque volume, composition, and ulceration: a retrospective assessment with MDCT. AJR Am J Roentgenol 2012;199:151–56 [DOI] [PubMed] [Google Scholar]
- 21. van Gils MJ, Vukadinovic D, van Dijk AC, et al. Carotid atherosclerotic plaque progression and change in plaque composition over time: a 5-year follow-up study using serial CT angiography. AJNR Am J Neuroradiol 2012;33:1267–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paul J, Mbalisike EC, Nour-Eldin NE, et al. Dual-source 128-slice MDCT neck: radiation dose and image quality estimation of three different protocols. Eur J Radiol 2013;82:787–96 [DOI] [PubMed] [Google Scholar]