Imaging findings in 26 patients with spontaneous lateral sphenoid cephaloceles were studied. The authors were able to classify these lesions into those involving the lateral recess of the sphenoid sinus that typically manifested as CSF leaks and headaches, and a second type that involved the lateral sphenoidal wing without extension into the sinus and presented with a variety of findings including seizures, headaches, meningitis, or neuropathy, or were incidental. All patients showed sphenoid arachnoid pits and 61% had an empty or partially empty sella.
SUMMARY:
Spontaneous lateral sphenoid cephaloceles arise from bony defects in the lateral sphenoid, in the absence of predisposing factors such as trauma, surgery, mass, or congenital skull base malformation. We reviewed CT and MR imaging findings and clinical data of 26 patients with spontaneous lateral sphenoid cephaloceles to better understand anatomic contributions to pathogenesis, varying clinical and imaging manifestations, and descriptive terminology. Two types of spontaneous lateral sphenoid cephaloceles were identified. In 15 of 26 patients, a type 1 spontaneous lateral sphenoid cephalocele was noted, herniating into a pneumatized lateral recess of the sphenoid sinus, and typically presenting with CSF leak and/or headache. In 11 of 26 patients, a type 2 spontaneous lateral sphenoid cephalocele was noted, isolated to the greater sphenoid wing without extension into the sphenoid sinus, presenting with seizures, headaches, meningitis, cranial neuropathy, or detected incidentally. All patients had sphenoid arachnoid pits, and 61% of patients had an empty or partially empty sella, suggesting that altered CSF dynamics may play a role in their genesis.
Cephalocele is a hypernymous term describing a herniation of intracranial contents through a bony defect in the skull base or calvaria, such as meninges and CSF (meningocele), and sometimes brain tissue (meningoencephalocele or encephalocele). Cephaloceles may be congenital with failure of normal skull base development, or related to prior trauma, surgery, tumors, sphenoid dysplasia in neurofibromatosis type 2, or osteoradionecrosis. In the absence of the aforementioned predisposing factors, cephaloceles are referred to as spontaneous. Although spontaneous cephaloceles are believed to be rare, they are likely more common than previously reported1–4 and may have confusing imaging manifestations. A subset of spontaneous cephaloceles occurs off midline in the lateral sphenoid bone, and has been referred to as spontaneous lateral sphenoid cephaloceles (SLSCs). Left untreated, these lesions may pose a risk for ascending infection and meningitis1,5 and may be a cause of seizures and headaches.
The literature has focused on a subset of patients with SLSCs presenting with CSF leak, most likely because this is a common presentation, and patients with CSF leaks share a similar treatment approach. The etiopathogenesis of spontaneous lateral sphenoid CSF leaks was initially postulated to result from a persistence of a Sternberg canal.6–8 An interplay of physiologic and other anatomic factors is now more widely favored, based on CT and MR imaging observations of associations with pneumatization of the lateral recess of the sphenoid sinus (SS),3,9,10 arachnoid pits, and an empty or partially empty sella.1–3,9,11–17 Sharing not only similarities in associated imaging signs18 as well as common clinical and demographic features of female sex, middle age, and obesity, spontaneous skull base CSF leaks have been also postulated to represent a rare manifestation of idiopathic intracranial hypertension (IIH).1,3,13–18 Limiting analysis to the subset of patients with lesions presenting with CSF leaks, however, may result in an incomplete understanding of the etiopathogenesis of these lesions.
In this study, we retrospectively review CT and MR imaging findings and clinical presentation of patients with SLSCs. We specifically sought to better understand the anatomical factors contributing to pathogenesis, varying clinical presentations and imaging findings, and clarify the current descriptive terminology of these lesions.
Materials and Methods
A retrospective review of 4 university hospital imaging archives identified 29 patients with SLSCs who underwent imaging between 2000 and 2012. Search terms included sphenoid cephalocele, sphenoid meningocele, and sphenoid encephalocele. As a retrospective chart and imaging review, this study was exempted from informed patient consent by the institutional review board. Diagnosis was established by imaging findings and was confirmed with direct visualization in surgically repaired cases. Exclusion criteria included a history of head trauma, previous skull base or SS surgery, tumor, and known congenital malformation of the skull base. Three patients were excluded based on the presence of a pineal gland tumor, a history of previous head trauma, and multiple previous skull base surgeries.
Imaging was reviewed by a single reader (F.S.), a fellowship-trained neuroradiologist, who was blinded to clinical information at the time of the imaging review. CT and MR imaging of 26 patients were reviewed, with particular attention to SLSC location, presence of arachnoid pits, degree of pneumatization of the lateral recesses of the SS, and the presence of an empty or partially empty sella. Arachnoid pits were identified based on their appearance, as previously described and illustrated,3,9,10 which consists of focal lobulated or multilobulated outward concave bony areas along the inner table of the greater wing of the sphenoid on CT and/or MR imaging (Fig 1). On MR imaging, the contents of the arachnoid pits appear isointense to CSF. Pneumatization of the lateral recesses of the SS was defined as extension of the air-filled SSs inferolaterally into the sphenoid body/greater sphenoid wing junction, caudal to the foramen rotundum, anteroinferior to the foramen ovale, and often extending into the pterygoid plates.
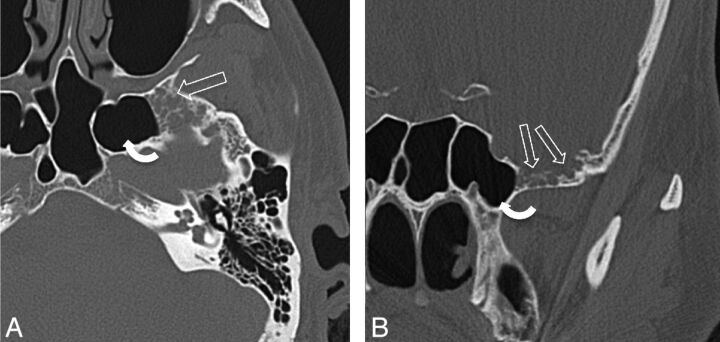
Fig 1.
A 43-year-old man presenting with headaches (patient 20). A, Axial bone CT image through the mid SS shows multiple ovoid bony defects in the greater wing of the sphenoid bone representing arachnoid pits due to aberrant arachnoid granulations (open arrows). This patient also has extensive pneumatization of the lateral recesses of the SS (curved arrow). B, Coronal CT image of the same patient as in panel A, again demonstrating multiple arachnoid pits in the GWS (open arrows). A cephalocele was seen on MR imaging (not shown here).
Imaging studies were acquired according to institutional protocols and presenting symptoms, and consisted of 1 or more of the following: routine head CT; multidetector CT of the sinuses acquired in the axial plane, and reconstructed in sagittal and coronal planes; MR imaging of the head at 1.5T or 3T, including axial and coronal T1-weighted with and without intravenous contrast material, and coronal T2-weighted fast spin-echo and steady-state free precession sequences, by use of a standard head coil. CT cisternography was also reviewed in cases where it was performed.
Clinical notes were reviewed for patient characteristics. The presenting symptom of CSF leak was confirmed by B2 transferrin and was visualized on CT cisternography in 3 patients.
Results
A total of 26 patients (16 women, 10 men) with 28 SLSCs were identified. The clinical and imaging findings are summarized in the accompanying Table.
Patient characteristics, clinical information, and imaging findings
| Patient | Age | Sex | Presenting Symptom(s) | Arachnoid Pits | Pneumatization of Lateral Recesses of SS | Site of Cephalocele | Side of Defect | Empty or PE Sella |
|---|---|---|---|---|---|---|---|---|
| 1 | 55 | M | CSF rhinorrhea | Yes | Yes | SS | R | Yes |
| 2 | 48 | M | Chronic sinusitis | Yes | Yes | SS | R | Yes |
| 3 | 49 | F | Seizures | Yes | Yes | SS | L | No |
| 4 | 54 | F | CSF rhinorrhea and headache | Yes | Yes | SS | R | Yes |
| 5 | 54 | F | CSF rhinorrhea and headache | Yes | Yes | SS | R | Yes |
| 6 | 59 | F | CSF rhinorrhea | Yes | Yes | SS | R | Yes |
| 7 | 55 | M | CSF rhinorrhea | Yes | Yes | SS | R | Yes |
| 8 | 75 | F | CSF rhinorrhea | Yes | Yes | SS | L | Yes |
| 9 | 76 | F | Headache | Yes | Yes | SS | L | No |
| 10 | 63 | F | CSF rhinorrhea and headache | Yes | Yes | SS | L | No |
| 11 | 32 | F | CSF rhinorrhea | Yes | Yes | SS | L | No |
| 12 | 57 | F | CSF rhinorrhea | Yes | Yes | SS | R | No |
| 13 | 57 | F | CSF rhinorrhea | Yes | Yes | SS | L | Yes |
| 14 | 52 | M | CSF rhinorrhea | Yes | Yes | SS | R | Yes |
| 15 | 29 | M | Seizures | Yes | No | GWS | R | No |
| 16 | 53 | F | Nasal fullness | Yes | Yes | GWS | R | No |
| 17 | 50 | M | Seizures | Yes | No | GWS | R | No |
| 18 | 20 | M | Seizures | Yes | No | GWS | L | No |
| 19 | 34 | M | Meningitis, seizures, and CSF rhinorrhea | Yes | Yes | SS | R | Yes |
| 20 | 43 | M | Headaches | Yes | Yes | GWS | B | Yes |
| 21 | 27 | M | Seizures | Yes | No | GWS | B | NK |
| 22 | 67 | F | Seizures | Yes | Yes | GWS | L | Yes |
| 23 | 65 | F | Incidental (imaging for decreased vision in right eye) | Yes | Yes | GWS | L | No |
| 24 | 26 | F | Headaches | Yes | No | GWS and clivus | L | NK |
| 25 | 26 | F | Proptosis | Yes | No | GWS | L | Yes |
| 26 | 45 | F | Headache and infraorbital nerve symptoms | Yes | No | GWS | L | NK |
Note:—B indicates bilateral; L, left; NK, not known; PE, partially empty; R, right.
Clinical Findings
Patients ranged in age from 20–76 years with an average age of 49 ± 15 years. Of the 26 patients, the most common clinical presentations were CSF leak in 12 (46%), headache in 7 (27%), and seizures in 7 (27%). One patient with headaches and CSF rhinorrhea also had meningitis at presentation. One patient each presented with cranial neuropathy, nasal fullness, and proptosis, and in a single patient, an SLSC was an incidental imaging finding (Table).
Imaging Findings
Nineteen patients had both MR imaging and CT available for review, 4 patients had only CT imaging performed, and 3 patients had only MR imaging. Five patients underwent additional imaging with CT cisternography. The following imaging observations were made with reference to the patient group: the presence of arachnoid pits, SLSCs, pneumatization of the lateral recess of the SSs, and an empty or partially empty sella. These characteristics are described in detail below.
Presence of Arachnoid Pits
Arachnoid pits were present in all 26 patients with SLSCs, and all patients had 1 or more arachnoid pits that were noncontiguous with the SLSC, either on the same or contralateral side (Figs 1–3). Size varied from 1–2 mm to > 1 cm in size. The arachnoid pits involved the inner table of the medial greater wing of the sphenoid (GWS) in the middle cranial fossa, lateral to the foramen rotundum and anterior to the foramen ovale. In some cases, arachnoid pits extended laterally along the inner table of the squamosal temporal bone.
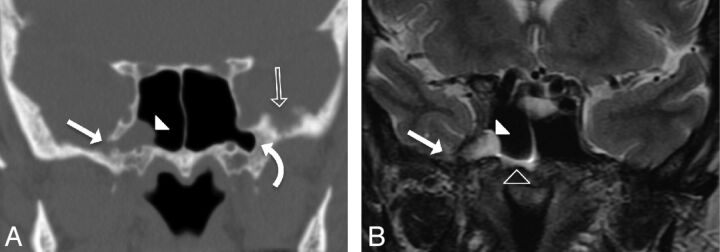
Fig 2.
A 59-year-old woman presenting with CSF rhinorrhea (patient 6). A, Coronal CT image in bone windows showing focal bony dehiscence of the lateral wall of the right SS (solid arrow), with soft tissue attenuation herniating into the SS through the defect (arrowhead). Note the presence of arachnoid pits along the inner table of the contralateral GWS (open arrow) and bilateral pneumatization of the lateral sphenoid recesses (curved arrow). B, Coronal T2-weighted image demonstrating the contents of the herniation from panel A as a cephalocele composed of meninges, CSF, and a portion of the right mesial temporal lobe (solid arrowhead). Note the CSF fluid level within the right SS (open arrowhead). This type 1 SLSC best illustrates the ability of these lesions to simulate a mucous retention cyst.
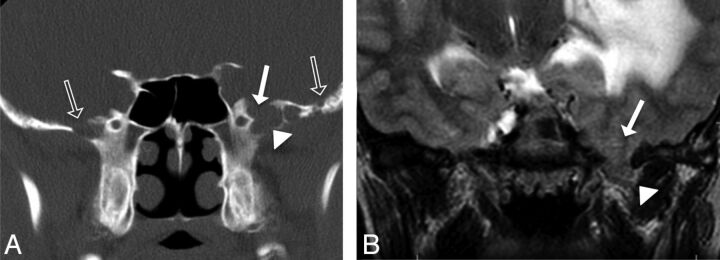
Fig 3.
A 27-year-old man presenting with seizures (patient 21). A, Coronal CT image shows absence of pneumatization of the lateral sphenoid recesses, but focal dehiscence of inner table of the left greater sphenoid wing (solid arrow) with soft tissue attenuation herniating into the defect (solid arrowhead). Note the presence of arachnoid pits along the inner table of both the ipsilateral and contralateral GWS (open arrows). B, Coronal T2-weighted image demonstrating brain parenchyma (solid arrowhead) herniating through the bony defect in the GWS (solid arrow). Also, note the T2 hyperintensity and mass effect within the left temporal lobe. This patient was found to also have a left occipital dural capillary hemangioma, which, in addition to the white matter T2 abnormality, may have increased intracranial CSF pressure causing enlargement of arachnoid pits bilaterally and subsequent development of a type 2 SLSC seen here.
Spontaneous Lateral Sphenoid Cephaloceles
CT and MR imaging depicted 28 SLSCs in 26 patients: 12 on the left side, 12 on the right, and bilaterally in 2 patients. On CT, a SLSC appears as a focal dehiscence of bone (Figs 2 and 3) with soft tissue or fluid attenuation herniating into the defect, often mimicking a mucous retention cyst. The contents of the cephalocele were better demonstrated on MR imaging or CT cisternography (Figs 2B and 3B). Meninges were seen herniating through the defect as a thin dark line on T2 imaging. CSF was characteristically T2 hyperintense or filled with intrathecal contrast on CT cisternography. The soft tissue herniating through the defect was contiguous with brain parenchyma, which allowed for a specific diagnosis of a cephalocele. The herniated brain typically followed T1 and T2 signal characteristics of normal brain parenchyma. In some cases, white matter T2 hyperintensity was seen within the involved temporal lobe, suggesting edema and/or gliosis (Fig 3B).
Of 28 SLSCs, 15 (54%) involved a bony defect of the lateral wall of the SS, resulting in herniation of the cephalocele into the SS. In 13 of 28 SLSCs, the cephalocele herniated through a bony defect of the GWS that did not involve the wall of the SS (Fig 3). The CT appearance often mimicked an aggressive lytic lesion of the sphenoid bone. As with SLSCs extending into the SS, MR imaging signal characteristics of these cephaloceles followed those of CSF and brain tissue, which allowed for a specific diagnosis.
Degree of Pneumatization of the Lateral Recess of the Sphenoid Sinuses
Pneumatization of the lateral recesses of the SS was present in 19 (73%) of 26 patients. The lateral recess of the SS was the site of the cephalocele in 15 (54%) of 28 cephaloceles, with all 15 of these demonstrating pneumatization of the lateral recess of the SS (Table and Fig 2). The other 13 cephaloceles involved the GWS lateral to the SS (Fig 3).
Empty or Partially Empty Sella
Imaging through the sella was available in 23 (88%) of 26 patients. An empty or partially empty sella was noted in 14 (61%) of these 23 patients (Table).
Discussion
The clinical presentation of SLSCs is varied. The literature has focused on a subset of patients presenting with CSF leak, most likely because this is a common presentation, and patients with CSF leaks share similar treatment approaches. In our study, however, only 46% of patients with SLSCs presented with a CSF leak. This study illustrates that the clinical presentation of a SLSC largely depends on the location of the bony defect, and that SLSCs can be divided into 2 subtypes. Type 1 SLSC herniates into a pneumatized lateral recess of the SS. Patients with this subtype typically present with a CSF leak and/or headache. Type 2 SLSC herniates into the GWS and not into the SS. In this subtype, the SS lateral recess is usually not pneumatized. Type 2 SLSCs most commonly present with seizures and/or headaches, but they can also be found incidentally and may result in diagnostic confusion (Table).
The development of a spontaneous lateral sphenoid CSF leak was initially postulated to be related to a congenital bony defect in the lateral wall of the SS first described by Sternberg in 1888.6 Although the Sternberg canal was reported to persist to adulthood in 4% of patients in an anatomic study,6 this canal underlies the cavernous sinus, whereas SLSCs occur lateral to the foramen rotundum,10 a finding confirmed in this study. Moreover, a congenital cause is unlikely, because SS pneumatization occurs in later childhood. SLSCs are not accompanied by brain malformations as with true congenital meningocele/meningoencephaloceles,9 SLSCs are smaller in size and occasionally multiple and bilateral,9 and patients present during adulthood.3,9,10
The etiopathogenesis of lateral sphenoid CSF leaks is now more widely thought to result from a combination of physiologic and anatomic factors, based on CT and MR imaging observations in these patients of associations with arachnoid pits, an empty or partially empty sella, and pneumatization of the lateral recess of the SS. The presence of arachnoid pits in all patients with SLSC in our study, as well as an association with an empty or partially empty sella and with pneumatization of the lateral recess of the SS, is consistent with prior studies on subsets of patients with SLSCs presenting with CSF leaks.3,9,10
The most important mechanism underlying the development of SLSCs is likely related to altered CSF dynamics in aberrant arachnoid granulations.14 Arachnoid granulations are found along the dura and are commonly seen projecting into the dural venous sinuses. They contain arachnoid villi, which are responsible for CSF reabsorption. Less commonly, they can be seen along the calvaria or skull base outside of a dural venous sinus, for instance, along the middle cranial fossa. Outside the dural venous sinuses, they have been termed aberrant arachnoid granulations (AbAGs). AbAGs result in small concave pits in the inner table of the calvaria (Fig 1), best seen on thin-section CT coronal reformats, giving rise to the descriptive term arachnoid pit.9 On MR imaging, the arachnoid pits follow CSF intensity, and arachnoid strands may occasionally be visible. No postcontrast enhancement is typically seen. The incidence of arachnoid pits in 1000 patients without CSF leak undergoing sinus CT was reported to be 23%.10 In most cases, arachnoid pits are asymptomatic and incidental; however, in the setting of persistently elevated or large-fluctuation CSF pressure, egress of CSF from the AbAGs may be impaired by their location outside a dural venous sinus. This may lead to progressive enlargement and scalloping of the underlying bone.9 Arachnoid pits may appear unilocular or multilocular and may mimic a multicystic bone lesion on CT (Figs 1–3), though their characteristic location along the middle cranial fossa is a helpful distinguishing feature. Further enlargement may eventually result in a bony dehiscence, especially if the underlying sphenoid bony substrate is inherently thin to begin with.
The association of SLSCs with pneumatization of the lateral recesses of the SS may be related to the thinning of the SS wall caused by the pneumatization, in addition to the tendency of arachnoid pits to occur along the inner table of the greater sphenoid wing (Figs 1 and 2). The extent of normal pneumatization of the SS is variable in the general population. Pneumatization of the lateral recess of the SS is seen in 23%–43% of healthy adults.3,9,10,13,16 In this study, pneumatization of the lateral recess of the SS was present in 19 (74%) of 26 patients, and in all 12 patients presenting with CSF rhinorrhea. Previous studies of spontaneous lateral sphenoid CSF leaks found pneumatization of the lateral recess of the SS in 91%–100%.9,10 However, SLSC herniation into the SS may not always necessarily result in CSF leak at presentation, as seen in 3 patients in our study (Table).
In addition to arachnoid pits, further suggestive evidence of altered CSF hydrodynamics in patients with SLSCs is an association with an empty or partially empty sella.1–3,9,11–17 In our study, the prevalence of an empty or partially empty sella was 61%, in keeping with prior studies of patients with spontaneous SS CSF leaks.3,9,17 In contrast, the prevalence of an empty sella in the general population is estimated to be 5%–6%.19 Sharing not only similarities in associated imaging signs18 as well as common clinical and demographic features of female sex, middle age, and obesity, spontaneous skull base CSF leaks and cephaloceles have been postulated to represent a rare manifestation of idiopathic intracranial hypertension in retrospective cohort studies.1,3,13–18,20 Furthermore, patients with spontaneous CSF leaks have historically had a significantly lower repair success rate and a 25%–87% recurrence rate compared with other types of CSF leaks.17,21,22 Both elevated intracranial pressure and predisposing osseous thinning at the skull base have also been implicated in the formation of spontaneous cephaloceles and CSF leaks involving the anterior cranial fossa and temporal bone. Although arachnoid pits have not been commonly described in association with ethmoid/cribriform plate cephaloceles and CSF leaks,3 the predisposition may be related to the inherently decreased thickness of the cribriform plate. Spontaneous cephaloceles and/or CSF otorrhea involving the tegmen tympani, roof of the Eustachian tube, jugular foramen, and the posterior plate of the temporal bone between the sigmoid sinus and the bony labyrinth have also been reported, with arachnoid pits causing progressive scalloping of the temporal bone postulated as a cause.1–3,5,12–16,23–36 These skull base regions must also be assessed any patient presenting with an SLSC, as there may be multiple cephaloceles at presentation, and on follow-up imaging, especially in patients with persistently elevated CSF pressure or IIH.
Prompt repair of a SLSC defect in patients with CSF leaks is important, as delay in treatment may result in ascending infection (meningitis, encephalitis, or abscess),1,5 with an annual and long-term risk for meningitis of 10% and 40%, respectively.11 If the SLSC extends into the SS (type 1), endoscopic repair is usually preferred because it affords good visualization while being less traumatic and has a superior success rate to transcranial approaches.12,37,38 In this study, seizure was found to be a more common presentation of type 2 SLSCs (5/7 patients presenting with seizures had a type 2 SLSC) and only 1 of these 7 patients presented with a CSF leak. The cause of increased seizure incidence in type 2 SLSCs is unknown; however, this may be related to relative delay in diagnosis or the lack of decompression of herniated brain tissue into the SS. Patients with an untreated type 2 SLSC presenting with seizures may be at risk for continued seizure activity. Type 2 SLSCs may be treated medically or surgically repaired by use of a transcranial approach, but only after clinical confirmation of the SLSC as a distinct seizure focus, source of headaches, or other symptoms, because some SLSCs may be detected incidentally on imaging (Table).
Our study had several important limitations. As a retrospective study, a selection bias may have been present in the patients included in this study. Endoscopic or pathologic confirmation of lesions suspected to represent cephaloceles on imaging was not available for all patients included in this study. Indirect confirmation was available, however, for the 12 patients with documented CSF rhinorrhea. In others, MR imaging appearance was pathognomonic. Because of limited follow-up data, clinical confirmation of the cause of symptoms other than CSF rhinorrhea, such as seizures or headaches, was also limited. In addition, inference on the role of altered CSF hydrodynamics and/or IIH in the etiopathogenesis of SLSCs in this study was limited because of incomplete clinical data relating to patient body habitus, obesity, papilledema, history of IIH, or CSF opening pressure measurements. The association of SLSCs with clinical as well as imaging signs of increased intracranial pressure, such as papilledema, optic nerve-sheath complex enlargement, and narrowing of the dural venous sinuses, in addition to an empty or partially empty sella, should be addressed in a prospective study.
Conclusions
We propose a classification of SLSCs into 2 types determined by their location. A type 1 SLSC herniates into a pneumatized SS lateral recess and may simulate a mucous retention cyst on CT. This subtype typically presents with CSF leak and/or headache and may be amenable to transphenoidal endoscopic repair. A type 2 SLSC herniates into or through the greater wing of the sphenoid with GWS scalloping or defect, and may have a confusing imaging appearance. Patients with this subtype most frequently present with seizure or headache, and surgical repair requires a transcranial approach. The presence of arachnoid pits in all patients and an association of both types of SLSC with an empty or partially empty sella suggest that altered CSF flow dynamics may play an important role in the pathogenesis.
ABBREVIATIONS:
- AbAGs
aberrant arachnoid granulations
- GWS
greater wing of the sphenoid
- IIH
idiopathic intracranial hypertension
- SLSC
spontaneous lateral sphenoid cephalocele
- SS
sphenoid sinus
Footnotes
Disclosures: Michelle Michel—UNRELATED: Consultancy: Amirsys; Payment for Lectures (including service on speaker bureaus): iiCME; Royalties: Amirsys; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: iiCMHE. Christine M. Glastonbury—UNRELATED: Royalties: Amirsys, Comments: Royalties from books; Payment for Development of Educational Presentations: Amirsys, Comments: StatDx material; Stock/Stock Options: Amirsys, Comments: Family trust.
Paper previously presented at: 45th Annual Meeting of the American Society of Head and Neck Radiology, September 7–11th, 2011; San Diego, California.
References
- 1. Lloyd KM, DelGaudio JM, Hudgins PA. Imaging of skull base cerebrospinal fluid leaks in adults. Radiology 2008;248:725–36 [DOI] [PubMed] [Google Scholar]
- 2. Lopatin AS, Kapitanov DN, Potapov AA. Endonasal endoscopic repair of spontaneous cerebrospinal fluid leaks. Arch Otolaryngol Head Neck Surg 2003;129:859–63 [DOI] [PubMed] [Google Scholar]
- 3. Schuknecht B, Simmen D, Briner HR, et al. Nontraumatic skull base defects with spontaneous CSF rhinorrhea and arachnoid herniation: imaging findings and correlation with endoscopic sinus surgery in 27 patients. AJNR Am J Neuroradiol 2008;29:542–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alonso RC, de la Peña MJ, Caicoya AG, et al. Spontaneous skull base meningoencephaloceles and cerebrospinal fluid fistulas. Radiographics 2013;33:553–70 [DOI] [PubMed] [Google Scholar]
- 5. Meco C, Oberascher G. Comprehensive algorithm for skull base dural lesion and cerebrospinal fluid fistula diagnosis. Laryngoscope 2004;114:991–99 [DOI] [PubMed] [Google Scholar]
- 6. Sternberg M. Ein bisher nicht beschriebener Kanal im Keilbein des Menschen. Anat Anz 1888;23:784–86 [Google Scholar]
- 7. Schick B, Brors D, Prescher A. Sternberg's canal–cause of congenital sphenoidal meningocele. Eur Arch Otorhinolaryngol 2000;257:430–32 [DOI] [PubMed] [Google Scholar]
- 8. Castelnuovo P, Dallan I, Pistochini A, et al. Endonasal endoscopic repair of Sternberg's canal cerebrospinal fluid leaks. Laryngoscope 2007;117:345–49 [DOI] [PubMed] [Google Scholar]
- 9. Shetty PG, Shroff MM, Fatterpekar GM, et al. A retrospective analysis of spontaneous sphenoid sinus fistula: MR and CT findings. AJNR Am J Neuroradiol 2000;21:337–42 [PMC free article] [PubMed] [Google Scholar]
- 10. Barañano CF, Curé J, Palmer JN, et al. Sternberg's canal: fact or fiction? Am J Rhinol Allergy 2009;23:167–71 [DOI] [PubMed] [Google Scholar]
- 11. La Fata V, McLean N, Wise SK, et al. CSF leaks: correlation of high-resolution CT and multiplanar reformations with intraoperative endoscopic findings. AJNR Am J Neuroradiol 2008;29:536–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mirza S, Thaper A, McClelland L, et al. Sinonasal cerebrospinal fluid leaks: management of 97 patients over 10 years. Laryngoscope 2005;115:1774–77 [DOI] [PubMed] [Google Scholar]
- 13. Woodworth BA, Palmer JN. Spontaneous cerebrospinal fluid leaks. Curr Opin Otolaryngol Head Neck Surg 2009;17:59–65 [DOI] [PubMed] [Google Scholar]
- 14. Schlosser RJ, Woodworth BA, Wilensky EM, et al. Spontaneous cerebrospinal fluid leaks: a variant of benign intracranial hypertension. Ann Otol Rhinol Laryngol 2006;115:495–500 [DOI] [PubMed] [Google Scholar]
- 15. Silver RI, Moonis G, Schlosser RJ, et al. Radiographic signs of elevated intracranial pressure in idiopathic cerebrospinal fluid leaks: a possible presentation of idiopathic intracranial hypertension. Am J Rhinol 2007;21:257–61 [DOI] [PubMed] [Google Scholar]
- 16. Woodworth BA, Prince A, Chiu AG, et al. Spontaneous CSF leaks: a paradigm for definitive repair and management of intracranial hypertension. Otolaryngol Head Neck Surg 2008;138:715–20 [DOI] [PubMed] [Google Scholar]
- 17. Schlosser RJ, Bolger WE. Significance of empty sella in cerebrospinal fluid leaks. Otolaryngol Head Neck Surg 2003;128:32–38 [DOI] [PubMed] [Google Scholar]
- 18. Prichard CN, Isaacson B, Oghalai JS, et al. Adult spontaneous CSF otorrhea: correlation with radiographic empty sella. Otolaryngol Head Neck Surg 2006;134:767–71 [DOI] [PubMed] [Google Scholar]
- 19. Garcĩa-Uria J, Ley L, Parajõn A, et al. Spontaneous cerebrospinal fluid fistulae associated with empty sellae: surgical treatment and long-term results. Neurosurgery 1999;45:766–73; discussion 773–74 [DOI] [PubMed] [Google Scholar]
- 20. Wang EW, Vandergrift WA, 3rd, Schlosser RJ. Spontaneous CSF leaks. Otolaryngol Clin North Am 2011;44:845–56, vii [DOI] [PubMed] [Google Scholar]
- 21. Schlosser RJ, Wilensky EM, Grady MS, et al. Cerebrospinal fluid pressure monitoring after repair of cerebrospinal fluid leaks. Otolaryngol Head Neck Surg 2004;130:443–48 [DOI] [PubMed] [Google Scholar]
- 22. Schlosser RJ, Wilensky EM, Grady MS, et al. Elevated intracranial pressures in spontaneous cerebrospinal fluid leaks. Am J Rhinol 2003;17:191–95 [PubMed] [Google Scholar]
- 23. Connor SE. Imaging of skull-base cephalocoeles and cerebrospinal fluid leaks. Clin Radiol 2010;65:832–41 [DOI] [PubMed] [Google Scholar]
- 24. Gacek RR, Gacek MR, Tart R. Adult spontaneous cerebrospinal fluid otorrhea: diagnosis and management. Am J Otol 1999;20:770–76 [PubMed] [Google Scholar]
- 25. Goddard JC, Meyer T, Nguyen S, et al. New considerations in the cause of spontaneous cerebrospinal fluid otorrhea. Otol Neurotol 2010;31:940–45 [DOI] [PubMed] [Google Scholar]
- 26. Wind JJ, Caputy AJ, Roberti F. Spontaneous encephaloceles of the temporal lobe. Neurosurg Focus 2008;25:E11. [DOI] [PubMed] [Google Scholar]
- 27. Arai A, Mizukawa K, Nishihara M, et al. Spontaneous cerebrospinal fluid rhinorrhea associated with a far lateral temporal encephalocele–case report. Neurol Med Chir (Tokyo) 2010;50:243–45 [DOI] [PubMed] [Google Scholar]
- 28. May JS, Mikus JL, Matthews BL, et al. Spontaneous cerebrospinal fluid otorrhea from defects of the temporal bone: a rare entity? Am J Otol 1995;16:765–71 [PubMed] [Google Scholar]
- 29. Papanikolaou V, Bibas A, Ferekidis E, et al. Idiopathic temporal bone encephalocele. Skull Base 2007;17:311–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shetty PG, Shroff MM, Sahani DV, et al. Evaluation of high-resolution CT and MR cisternography in the diagnosis of cerebrospinal fluid fistula. AJNR Am J Neuroradiol 1998;19:633–39 [PMC free article] [PubMed] [Google Scholar]
- 31. Stone JA, Castillo M, Neelon B, et al. Evaluation of CSF leaks: high-resolution CT compared with contrast-enhanced CT and radionuclide cisternography. AJNR Am J Neuroradiol 1999;20:706–12 [PMC free article] [PubMed] [Google Scholar]
- 32. Sillers MJ, Morgan CE, el Gammal T. Magnetic resonance cisternography and thin coronal computerized tomography in the evaluation of cerebrospinal fluid rhinorrhea. Am J Rhinol 1997;11:387–92 [DOI] [PubMed] [Google Scholar]
- 33. Ommaya AK, Di Chiro G, Baldwin M, et al. Non-traumatic cerebrospinal fluid rhinorrhoea. J Neurol Neurosurg Psychiatry 1968;31:214–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hooper AC. Sphenoidal defects–a possible cause of cerebrospinal fluid rhinorrhoea. J Neurol Neurosurg Psychiatry 1971;34:739–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lang J. Clinical Anatomy of the Nose, Nasal Cavity and Paranasal Sinuses. New York: Thieme Stuttgart;1989:121 [Google Scholar]
- 36. Gacek RR. Arachnoid granulation cerebrospinal fluid otorrhea. Ann Otol Rhinol Laryngol 1990;99:854–62 [DOI] [PubMed] [Google Scholar]
- 37. Castelnuovo P, Dallan I, Battaglia P, et al. Endoscopic endonasal skull base surgery: past, present and future. Eur Arch Otorhinolaryngol 2010;267:649–63 [DOI] [PubMed] [Google Scholar]
- 38. Nyquist GG, Anand VK, Mehra S, et al. Endoscopic endonasal repair of anterior skull base non-traumatic cerebrospinal fluid leaks, meningoceles, and encephaloceles. J Neurosurg 2010;113:961–66 [DOI] [PubMed] [Google Scholar]