SUMMARY
Insomnia disorder is a prevalent sleep disorder, which affects about 10% of general population. However, its neural mechanisms are poorly understood. Recently, several structural and functional neuroimaging studies have been conducted in patients with insomnia disorder, but these studies have yielded diverse findings. Here, we aimed to identify consistent patterns of abnormal brain alterations in insomnia disorder by performing a quantitative coordinate-based meta-analysis. Following the preferred reporting for systematic reviews and meta-analyses statement, we searched PubMed database and used reference tracking and finally retrieved 19 eligible studies (six task-based functional magnetic resonance imaging, eight resting-state functional magnetic resonance imaging, three voxel-based morphometry, and two positron emission tomography). We extracted peak coordinates from these studies and tested for convergence using the activation likelihood estimation method. Using this method, we found no significant convergent evidence for combination of structural atrophy and functional disturbances across previous studies (p = 0.914). Inconsistencies across these studies might be related to heterogonous clinical populations, the explorative nature of these studies in combination with small sample sizes, different experimental designs, and various preprocessing and statistical approaches. Future neuroimaging studies on insomnia disorder should include larger well-characterized samples, as well as standard imaging and analysis protocols.
Keywords: Insomnia disorder, ALE meta-analysis, fMRI, VBM, PET
Introduction
Insomnia disorder (ID) is the most common sleep disorder in adults with a prevalence range from about 4 to 22%, and an average of at least about 10% in the general population [1–3]. The Diagnostic and statistical manual of mental disorders-fifth edition (DSM-5) and the third edition of International classification of sleep disorders (ICSD-3) criteria [1,4] define ID as difficulties in falling and/or maintaining sleep or early morning awakening occurring for at least three times per week over a period of at least three months, not attributable to sleep-disrupting external conditions, and accompanied by subjective daytime complaints, for example fatigue, mood disturbance, poor concentration or memory impairment [5–7]. People with ID experience a lower quality of life, and may have an increased risk of road and motor vehicle accidents [5,6,8]. In addition, ID is a common comorbid condition in several psychiatric disorders including major depressive disorder (MDD), posttraumatic stress disorder (PTSD), anxiety, alcohol or drug abuse, bipolar disorder, eating disorder, obsessive compulsive disorder, and psychotic disorders [9–15]. Annual costs associated with ID-related absenteeism and productivity losses in Canada were estimated at $970.6 million and $5 billion, respectively [16]. ID has a strong association with days-out-of-role, which is independent from comorbidity [17]. Results from the America insomnia survey demonstrated that insomnia was associated with substantial workplace costs and lost work performance due to presenteeism [18]. Also, it accounts for 4.6% of injuries requiring medical attention [19]. Neurobiological and psychological studies have suggested various genetic, behavioral, cognitive, and emotional risk factors for the development and maintenance of insomnia symptoms [1]. Impaired daytime functioning due to insomnia is associated with cognitive deficits, interpersonal distress, low work productivity, and high sick leave [20–22]. However, despite the high socioeconomic burden [23], the neurobiology of ID remains poorly understood [1,24].
Over the last decade, a number of structural and functional neuroimaging studies, including voxel-based morphometry (VBM), positron emission tomography (PET), task-based functional magnetic resonance imaging (t-fMRI), and resting-state functional magnetic resonance imaging (rs-fMRI) have been conducted on patients with ID. For review see: [24–26]. Structural MRI studies demonstrated grey matter atrophy in the orbitofrontal cortex, temporal cortex, precuneus and hippocampus, as well as grey matter volume increase in the anterior cingulate cortex (ACC) in patients with ID compared to good sleepers [27–31]. Functional differences have also been reported in studies on ID. PET studies revealed an altered neural metabolism in the general arousal system including the ascending reticular activating system and hypothalamus, in the emotion-regulating system including the hippocampus, amygdala and ACC, and in the cognitive system including the prefrontal cortex [32,33]. Task-based fMRI studies suggested abnormal activation in various regions including the amygdala, temporal lobe, and frontostriatal networks including the caudate nucleus and inferior frontal gyrus [34–39]. Rs-fMRI studies reported abnormal connectivity patterns in ID [40–46] e.g., increased functional connectivity between the insula and salience network [47] and between hippocampi and the left middle frontal gyrus [48]. Thus, the available neuroimaging findings in ID are divergent. The variability of the findings has been suggested to be due to the explorative nature of the studies in combination with relatively small sample sizes and due to heterogeneous patient groups that differed in several key aspects including diagnostic criteria, duration and severity of disease, age, gender, imaging acquisition, preprocessing, and analysis methods [24,49–51], and a plea for subtyping insomnia to reduce heterogeneity has been made recently [8].
In order to find convergence in structure and function across the heterogeneity of patients, modalities and methods, activation likelihood estimation (ALE) has been proposed as an objective technique for coordinate-based meta-analyses (CBMA) to quantitatively gain a more unified investigation of neurobiological changes in neuropsychiatric disorders [52–54]. In particular, the CBMA method uses statistical inference by integrating neuroimaging findings to identify “where” in the brain the convergence between reported foci is more than expected by chance [55]. We here report a CBMA on published neuroimaging studies reporting functional and structural deviations in patients with ID.
Methods
Search strategies and study selection
We conducted our search in the PubMed database in December 2017 to identify studies that contain the following keywords: (“insomnia” OR “insomnia disorder”) AND (“functional magnetic resonance imaging” OR “fMRI” OR “Positron Emission Tomography” OR “PET” OR “Voxel-based morphometry” OR “VBM”). Following the Preferred reporting items for systematic reviews and metaanalyses statement [56], two researchers (K.N. & M.T.) independently screened the 313 retrieved abstracts and checked for their eligibility to be included. English peer-reviewed neuroimaging publications that compared a group of ID patients with healthy subjects were included, i.e., studies reporting within-group contrasts only or data from healthy subjects with insomnia symptoms were excluded. Diagnosis of ID patients in all included papers was based on ICSD or DSM-IV or DSM-IV-TR or DSM-5 criteria. We excluded editorial letters, case-reports, systematic reviews, metaanalyses, methodological studies, studies that did not report standard space coordinates, longitudinal/interventional studies, and studies with a sample size of less than seven subjects in each group as suggested previously [49,53,54]. Moreover, all included data came from voxel-wise whole brain analysis, because including heterogeneous region of-interest (ROI) analyses may lead to inflated significance for those ROIs [49]. In cases in which coordinates were not reported in the eligible studies, we contacted the authors to obtain the relevant information.
Data extraction
Our screening process yielded 19 studies consisting of six task-based fMRI, eight rs-fMRI, three VBM, and two PET publications comprising 404 patients with ID and 395 good sleepers [27,30–47] (Table 1, S1). Recorded data includes the first author’s name, year of publication, age, gender, number of subjects, scanning modality, and the peak coordinates (x,y,z) in Talairach [57] or Montreal neurological institute (MNI) [58] stereotactic space. Coordinates reported in Talairach space were transformed into MNI space for ALE analysis [59]. We used the extracted stereotactic coordinates for conducting the ALE meta-analysis. Of note, “study” reflects a single scientific publication and “experiment” represents an individual analysis or contrast of interest in a given study yielding localization information (i.e., ID > Controls or ID < Controls). Our dataset containing the coordinates of the extracted experiments was arranged based on the pooling approach [60]. In this approach, Turkeltaub and colleagues proposed that data should be organized by dependent subject groups rather than by individual experiments to minimize within group effects. This means that if several papers were published based on the same group of subjects and reported several experiments, we combined them [60].
Table 1.
Studies entered into the meta-analysis are listed based on the year of publication. VBM: voxel-based morphometry; fMRI: functional magnetic resonance imaging; Rs-fMRI: resting-state fMRI, FC: functional connectivity, ALFF: amplitude of low frequency fluctuation, GM: gray grey matter, WM: white matter, BDI: beck depression inventory.
| Author, year | Number of subjects (Insomnia/controls) | Number of males (Insomnia/controls) | Age of patients/controls (Mean ± SD) | Imaging modality | Covariates | |
|---|---|---|---|---|---|---|
| 1 | Son et al., 2017 [36] | 21/26 | 9/11 | 36.6 ± 9.8/33.2 ± 7.1 | t-fMRI | – |
| 2 | Kim et al., 2017 [39] | 14/18 | 4/4 | 49.0 ± 12.3/42.7 ± 12.3 | t-fMRI | Six motion parameters |
| 3 | Huang et al., 2017 [45] | 27/26 | 10/10 | 40.07 ± 11.62/41.19 ± 11.69 | rs-fMRI (whole-brain FC) |
Age and gender |
| 4 | Zhou et al., 2017 [41] | 27/27 | 17/17 | 42.59 ± 11.59/40.92 ± 11.46 | rs-fMRI (ALFF) | gender, age and the mean frame-wise displacement |
| 5 | Ran et al., 2017 [40] | 21/20 | 5/6 | 40.62 ± 7.52/38.65 ± 7.40 | rs-fMRI (ALFF) | Grey matter |
| 6 | Kay et al., 2016 [32] | 44/40 | 20/15 | 37/38 | PET | – |
| 7 | Li et al., 2016 [35] | 30/30 | 17/NS | 39.36 ± 8.53/36.15 ± 8.61 | t-fMRI | – |
| 8 | Li et al., 2016 [43] | 55/44 | 24/11 | 39.18 ± 10.34/39.91 ± 9.43 | rs-fMRI (ALFF) | sex, age and education level |
| 9 | Dai et al., 2016 [44] | 42/42 | 15/18 | 49.21 ± 10.96/49.14 ± 10.2 | rs-fMRI (ALFF) | age, sex, and years of education |
| 10 | Wang et al., 2015 [46] | 59/47 | 21/14 | 39.3 ± 10.7/40.0 ± 9.1 | rs-fMRI (ReHo) | head-motion measures |
| 11 | Dai et al., 2014 [42] | 24/24 | 7/12 | 54.8 ± 9.8/52.5 ± 6.6 | rs-fMRI (ReHo) | age, years of education, and/or sex |
| 12 | Baglioni et al., 2014 [34] | 22/38 | 7/17 | 40.7 ± 12.6/39.6 ± 8.9 | t-fMRI | six motion estimates, BDI scores |
| 13 | Chen et al., 2014 [47] | 17/17 | 0/0 | 27.16 ± 6.67/27.56 ± 6.83 | rs-fMRI (ICA) | – |
| 14 | Stoffers et al., 2014 [37] | 24/13 | 7/4 | 60.3 ± 6.0/60.1 ± 8.3 | t-fMRI | caudate region of interest baseline perfusion |
| 15 | Joo et al., 2014 [30] | 27/30 | 2/2 | 51.2 ± 9.6/50.4 ± 7.1 | Surface-based-analysis | BDI-II |
| 16 | Joo et al., 2013 [31] | 27/27 | 2/4 | 52.3 ± 7.8/51.7 ± 5.4 | VBM | age and sex and intracranial volume |
| 17 | Altena et al., 2010 [27] | 24/13 | 7/4 | 60.3 ± 6.0/60.2 ± 8.4 | VBM | Total GM or WM and age |
| 18 | Altena et al., 2008 [38] | 21/12 | 4/3 | 61 ± 6.2/60 ± 8.2 | t-fMRI | – |
| 19 | Nofzinger et al., 2004 [33] | 7/20 | 3/7 | 34.2 ± 8.9/32.6 ± 8.4 | PET | Global metabolism and age |
Activation likelihood estimation
CBMA was performed with the revised version of ALE [61]. ALE estimates convergence and compares this with a random topography. ALE employs three steps: 1) extracted coordinates are modeled as center of three-dimensional Gaussian probability distribution confirming uncertainty results from between-subject variations and technical differences; 2) creation of a modeled activation map (MA map) from combination of the probability distributions of all foci in the considered experiment. Then, incorporation of MA maps yields the final ALE map that reflects the likelihood convergence of results across all experiments; 3) statistically comparing the ALE map with a random distribution to discern random convergence from true convergence between experiments [52,61,62]. Significant statistical threshold set at p < 0.05 family-wise error in cluster level (cFWE) [49].
Confirmatory ROI-based analysis
In addition to the whole brain ALE approach, we performed ROI analysis using the LONI Probabilistic Brain Atlas (LPBA40) [63] in order to indicate potential regions in which there is above-chance convergence. Here, we compared the sum of all ALE values in the particular region to their distribution order null, which tests for the maximum convergence for the average across the entire ROI. Afterwards, we corrected the results for multiple comparisons using FWE threshold in cluster level to avoid false-positive results [49,64].
Results
From 313 retrieved papers, 19 fulfilled the criteria (Fig. 1), including 10 “patients < controls” contrasts and nine “patients > controls” contrasts resulting in 115 peak foci (Fig. 2 A). Included studies were consisting of eight rs-fMRI studies, which applied various methods. For example, one study performed whole brain functional connectivity, one study applied independent components analysis (ICA), two studies performed regional homogeneity (ReHo), and four studies applied the amplitude of low frequency fluctuations (ALFF) method. We also included six task-fMRI studies including letter fluency, category fluency, working memory, tower of London, and emotional tasks. In addition, two PET experiments were included in our analysis.
Fig. 1.
Study selection strategy flow chart.
Fig. 2.
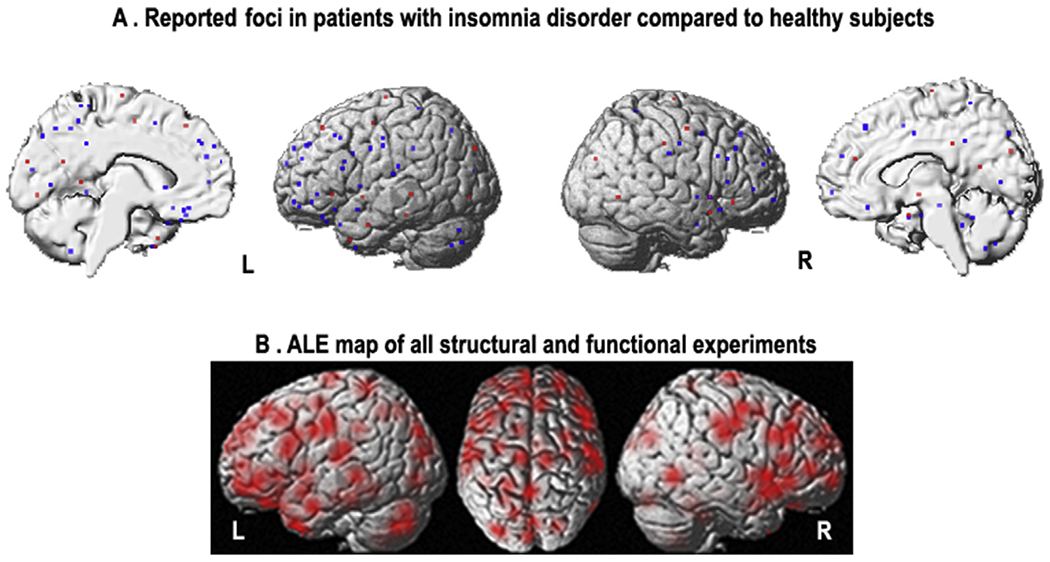
A) Reported coordinates reflecting structural/functional brain alterations in patients with insomnia disorder compared to healthy subjects (red = increased, blue = decreased); B) ALE maps relevant to meta-analysis, reflecting spatial uncertainty associated with each peak coordinate by modeling Gaussian probability distributions around each peak coordinate (p = 0.914, family-wise error in cluster level). (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article.)
Analyses across all experiments including both grey matter atrophy and functional abnormalities revealed no significant convergence between different neuroimaging studies performed in ID patients compared to healthy controls (p = 0.914, cFWE) (Fig. 2B).
Repeating analysis with a more liberal statistical threshold (i.e., threshold-free enhancement (TFCE) demonstrated no significant findings as well (p = 0.807). Separate analyses for different imaging modality or increased versus decreased structural/functional alterations were not possible because they would each require at least 17 independent contrasts to achieve reasonable power for statistical analysis [49,65]. Analysis of the ALE integral within the brain ROIs within the LPBA40 atlas demonstrated clusters within the right amygdala and hippocampus (p = 0.008), left inferior frontal gyrus (IFG) (p = 0.012), right IFG (p = 0.032), left orbitofrontal cortex (OFC) (p = 0.019), and right cingulate cortex (p = 0.030). However, none of these findings survived after corrections for multiple comparisons.
Discussion
The current meta-analysis provided no significant convergent evidence for structural atrophy and functional impairment across 19 published ID studies. The number of experiments using each neuroimaging modality was not high enough to perform analyses for fMRI, PET and VBM separately as at least 17 experiments are necessary to achieve 80% power for moderate effects [65]. Our inconsistent findings might be related to heterogonous clinical populations, the explorative nature of the studies in combination with small sample sizes of participants, and various experimental design, preprocessing and statistical approaches. It has been demonstrated that low statistical power of individual studies decreases the chance of detecting true effects in neuroscience research [50]. The heterogeneity in experimental designs across task-based fMRI experiments might be another of the potential reasons of inconsistent findings. Paradigm diversity of tasks performed in fMRI experiments in ID include a working memory task, a spatial working memory, letter or category fluency task, a tower of London task, emotional task, and passive viewing tasks (emotional, sleep-related and neutral pictures) [34–39]. The number of experiments with similar tasks was not sufficient to conduct sub-analyses for specific cognitive domains. Moreover, applied rs-fMRI methods in ID were also diverse e.g., independent component analysis (ICA), amplitude of low-frequency fluctuations (ALFF), regional homogeneity, and whole brain functional connectivity [40–47]. Similarly, although there is no experimental variability in the included structural MRI [27,30,31] and PET studies [32,33], analytical heterogeneity such as various statistical thresholds or spatial transformation of the brain images is an important issue in such studies.
CBMA using the ALE approach is an unbiased method to integrate multiple neuroimaging studies. ALE quantifies consistency across studies and thus aims to overcome limitations of individual studies with respect to generalizability and robustness [49,55,61]. ALE establish consensus to identify consistent brain regions in various cognitive and emotional domains [66,67] or to indicate the spatial locations of functional and/or structural disruptions in neuropsychiatric disorders [53,54,68,69]. In the present study, we followed the recently suggested standard protocols of neuroimaging meta-analysis [49]. Firstly, our dataset was organized based on the pooling approach introduced by Turkeltaub and colleagues to minimize within-group effects [60]. Here, the foci have been organized according to groups of participants rather than experiments to optimize the degree to which ALE values represent concordance of results [60]. Secondly, we performed cluster-level FWE correction, which is superior to false discovery rate (FDR) correction to provide maximum statistical rigor [65,70]. In addition, previous experiments based on the ROI analyses were excluded to avoid inflating significance for those particular regions [49]. We also repeated the analyses with TFCE as a more liberal statistical threshold and found no significant consistent region again, which support the observed null findings using cluster-level FWE correction.
Beside the whole-brain ALE approach, the regional ALE analysis showed small effects (uncorrected p-values) in the right amygdala and hippocampus, bilateral IFG, left OFC and right cingulate cortex. These small effects might be due to low number of included experiments. The accumulative findings from previous reviews suggest that ID-related structural and functional disruptions are distributed across various intrinsic brain networks e.g., the salience network and default mode network (DMN) [24,71]. The salience network detects emotional stimuli and mediates the switching between activation of the central executive network and the DMN to guide appropriate responses to salient stimuli [72]. Chen and colleagues found higher insula coactivation with salience networks in patients with ID [47]. Patients with ID also demonstrated higher functional connectivity between the dorsal attention and frontoparietal control networks and lower functional connectivity between the anterior and posterior parts of DMN [73]. Recently, we also highlighted the key role of the salience network in hyperarousal and affective symptoms in ID [71]. Moreover, Nie and colleagues found impaired region-to-region functional connectivity between the main hubs of DMN in ID [74]. Disrupted structural covariance between the anterior and posterior parts of DMN was observed in the ID patients, which could be related to abnormal transition from wake to sleep [75].
Recent Genom wide association studies (GWAS) on ID have suggested a few cortical and subcortical areas where gene expression profiles show above-chance resemblance to the genetic risk profile of ID [76–78]. Identified areas included the caudate nucleus and Brodmann areas nine and 24. The GWAS studies also found enriched insomnia risk gene expression in specific types of neurons that are located in the striatum, claustrum and hypothalamus [76–78]. Seed-based analyses, as well as fMRI employing tasks that specifically targeting these areas, could be used to evaluate whether the gene variants that add to the risk of insomnia, also lead to changes that are detectable with our current methods for structural and functional MRI. We did not find any of the mentioned regions in the whole-brain ALE analysis. Moreover, our ROI analysis was also unable to find significant regions after corrections for multiple comparisons. Taken together, the effects of ID may be distributed across the whole brain and heterogeneity of available experiments did not allow us to find consistent abnormal brain regions in ID. Hence, further neuroimaging studies with larger sample size, homogenous clinical presentation of participants, and using standard preprocessing and analysis protocols are needed. Moreover, future human or animal studies could focus on cellular or molecular mechanism of insomnia.
ID is clinically described as a heterogeneous disorder, which includes different subtypes of pathophysiology in terms of cognitions, mood, traits, history of life events and family history and not necessarily due to sleep complaints only [8]. Previous meta-analyses and systematic reviews have reported ID as a heterogeneous disorder with both sleep-related and non-sleep symptoms including poor cognitive performance [79], and emotional deficits [5]. ID is also associated with mood disturbance and depression [9,80], attention-deficit/hyperactivity symptoms [81], personality traits [82], mental illness [83]. These studies suggest that ID triggers several functional domains and therefore various brain regions are involved. Interestingly, it has been shown that depression risk and differential behavioral profiles of patients with ID are linked to the objective sleep duration phenotype [84]. This wide range of symptoms and limited consistency might be also due to different subtypes of ID with specific multivariate profile of characteristics. Unaccounted for heterogeneity may thus be involved in discrepancies and failure of replication in neuroimaging studies on discerning characteristics of ID.
Conclusion
To the best of our knowledge, this study is the first neuroimaging meta-analysis of structural and functional alterations in patients with ID and we observed no significant convergent regional alterations. This heterogeneity might be due to differences in clinical populations, experimental design and statistical inference procedures. Of note, inconsistency across neuroimaging studies is not limited to ID, and has e.g., also been observed in depression [68]. Whereas the current findings only summarize the studies on people with a diagnosis of ID, it could prove useful to run a similar analysis on the more extensive literature on brain correlates of insomnia complaints. Here, quality assessment of studies and pre-registration of analysis protocol were not performed, which should be considered in future meta-analyses.
Improving reproducibility in neuroimaging studies should be considered as a high priority to deliver valid findings [50]. Hence, we suggest focusing on replication studies in ID using well-characterized, subtype-homogeneous samples and including moreover a comprehensive description of recruitment strategy, comorbidities, gender, age, and medication status of patients. Moreover, standard preprocessing and statistical analysis should be followed. Our results might inspire future individual neuroimaging studies, as well as mega-analyses and emphasis on standardization of designs, analysis and reporting of the results.
Supplementary Material
Practice Points.
We found no significant convergent structural and functional alterations in patients with insomnia disorder compared to healthy subjects across previous publications.
The observed heterogeneity might be due to heterogeneity within small samples of participants, various experimental design, preprocessing and statistical approaches.
Recent works highlighted that insomnia disorder is clinically described as a heterogeneous disorder, which includes different subtypes. This is an important point to recruit homogeneous samples in future studies.
Research Agenda.
Future neuroimaging individual studies, as well as mega-analyses on insomnia disorder should use larger number of participants, well-characterized, and subtype-homogeneous samples.
Standard preprocessing and statistical analysis should be followed in individual neuroimaging studies of insomnia disorder.
By expansion of insomnia literature, further neuro-imaging meta-analysis is needed following the suggested standard protocols of neuroimaging meta-analysis in terms of in-/exclusion criteria, data organization, and statistical inference.
Acknowledgment
This study was supported by Kermanshah University of Medical Sciences (No. 94526). Simon B. Eickhoff is supported by the Deutsche Forschungsgemeinschaft (EI 816/11-1), the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain” and the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 7202070 (HBP SGA1) and under Grant Agreement No. 785907 (HBP SGA2). Eus Van Someren is supported by the European Research Council (ERC-ADG-2014-671084-INSOMNIA).
Abbreviations
- ACC
anterior cingulate cortex
- ALE
activation likelihood estimation
- ALFF
amplitude of low frequency fluctuations
- CBMA
coordinate-based meta-analysis
- DMN
default mode network
- DSM-IV
diagnostic and statistical manual of mental disorders-fourth edition
- DSM-5
diagnostic and statistical manual of mental disorders-fifth edition
- FC
functional connectivity
- FEW
family wise error
- GWAS
genome wide association studies
- ICA
independent components analysis
- ICSD-3
international classification of sleep disorders
- ID
insomnia disorder
- IFG
inferior frontal gyrus
- LPBA40
LONI probabilistic brain atlas
- MA
modeled activation
- MDD
major depressive disorder
- MNI
Montreal neurological institute
- OFC
orbitofrontal cortex
- PET
positron emission tomography
- PTSD
posttraumatic stress disorder
- ReHo
regional homogeneity
- ROI
region of interest
- rs-fMRI
resting-state functional magnetic resonance imaging
- TFCE
threshold-free cluster enhancement
- t-fMRI
task-based functional magnetic resonance imaging
- VBM
voxel-based morphometry
Footnotes
Conflicts of interest
The authors do not have any conflicts of interest to disclose.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.smrv.2018.07.004.
References
* The most important references are denoted by an asterisk
- *[1].Morin CM, Drake CL, Harvey AG, Krystal AD, Manber R, Riemann D, et al. Insomnia disorder. Nat Rev Dis Primers 2015;1:15026. [DOI] [PubMed] [Google Scholar]
- [2].Ohayon MM, Reynolds CF 3rd. Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD). Sleep Med 2009;10(9):952—60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med 2006;7(2):123—30. [DOI] [PubMed] [Google Scholar]
- [4].Chung KF, Yeung WF, Ho FY, Yung KP, Yu YM, Kwok CW. Cross-cultural and comparative epidemiology of insomnia: the Diagnostic and statistical manual (DSM), International classification of diseases (ICD) and International classification of sleep disorders (ICSD). Sleep Med 2015;16(4):477–82. [DOI] [PubMed] [Google Scholar]
- [5].Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep Med Rev 2010;14(4):227–38. [DOI] [PubMed] [Google Scholar]
- *[6].Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol 2015;14(5):547–58. [DOI] [PubMed] [Google Scholar]; * The most important references are denoted by an asterisk
- [7].Rezaie L, Fobian AD, McCall WV, Khazaie H. Paradoxical insomnia and subjective-objective sleep discrepancy: a review. Sleep Med Rev 2018. August;40:196–202. 10.1016/j.smrv.2018.01.002. [DOI] [PubMed] [Google Scholar]
- *[8].Benjamins JS, Migliorati F, Dekker K, Wassing R, Moens S, Blanken TF, et al. Insomnia heterogeneity: characteristics to consider for data-driven multi-variate subtyping. Sleep Med Rev 2017;36:71–81. [DOI] [PubMed] [Google Scholar]; * The most important references are denoted by an asterisk
- [9].Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord 2011;135(1–3):10–9. [DOI] [PubMed] [Google Scholar]
- [10].Insomnia Riemann D. and comorbid psychiatric disorders. Sleep Med 2007;8(Suppl 4):S15–20. [DOI] [PubMed] [Google Scholar]
- [11].Sarsour K, Morin CM, Foley K, Kalsekar A, Walsh JK. Association of insomnia severity and comorbid medical and psychiatric disorders in a health plan-based sample: insomnia severity and comorbidities. Sleep Med 2010;11(1):69–74. [DOI] [PubMed] [Google Scholar]
- [12].Roth T Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med JCSM: Off Publ Am Acad Sleep Med 2007;3(5 Suppl):S7–10. [PMC free article] [PubMed] [Google Scholar]
- [13].Tahmasian M, Jamalabadi H, Abedini M, Ghadami MR, Sepehry AA, Knight DC, et al. Differentiation chronic post traumatic stress disorder patients from healthy subjects using objective and subjective sleep-related parameters. Neurosci Lett 2017;650:174–9. [DOI] [PubMed] [Google Scholar]
- [14].Khazaie H, Ghadami MR, Knight DC, Emamian F, Tahmasian M. Insomnia treatment in the third trimester of pregnancy reduces postpartum depression symptoms: a randomized clinical trial. Psychiatry Res 2013;210(3):901–5. [DOI] [PubMed] [Google Scholar]
- [15].Tahmasian M, Khazaie H, Golshani S, Avis KT. Clinical application of actigraphy in psychotic disorders: a systematic review. Curr Psychiatry Rep 2013;15(6):359. [DOI] [PubMed] [Google Scholar]
- [16].Daley M, Morin CM, LeBlanc M, Gregoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep 2009;32(1):55–64. [PMC free article] [PubMed] [Google Scholar]
- [17].Hajak G, Petukhova M, Lakoma MD, Coulouvrat C, Roth T, Sampson NA, et al. Days-out-of-role associated with insomnia and comorbid conditions in the America insomnia survey. Biol Psychiatry 2011;70(11):1063–73. [DOI] [PubMed] [Google Scholar]
- [18].Kessler RC, Berglund PA, Coulouvrat C, Hajak G, Roth T, Shahly V, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep 2011;34(9):1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kessler RC, Berglund PA, Coulouvrat C, Fitzgerald T, Hajak G, Roth T, et al. Insomnia, comorbidity, and risk of injury among insured Americans: results from the America insomnia survey. Sleep 2012;35(6):825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shekleton JA, Rogers NL, Rajaratnam SM. Searching for the daytime impairments of primary insomnia. Sleep Med Rev 2010;14(1):47–60. [DOI] [PubMed] [Google Scholar]
- [21].Wade AG. The societal costs of insomnia. Neuropsychiatr Dis Treat 2010;7:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gunn HE, Troxel WM, Hall MH, Buysse DJ. Interpersonal distress is associated with sleep and arousal in insomnia and good sleepers. J Psychosom Res 2014;76(3):242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Metlaine A, Leger D, Choudat D. Socioeconomic impact of insomnia in working populations. Ind Health 2005;43(1):11–9. [DOI] [PubMed] [Google Scholar]
- *[24].Spiegelhalder K, Regen W, Baglioni C, Nissen C, Riemann D, Kyle SD. Neuroimaging insights into insomnia. Curr Neurol Neurosci Rep 2015;15(3):9. [DOI] [PubMed] [Google Scholar]
- [25].O’Byrne J, Rosa MB, Gouin J- P, Dang-Vu T. Neuroimaging findings in primary insomnia. Pathol Biol 2014;62(5):262–9. [DOI] [PubMed] [Google Scholar]
- [26].Desseilles M, Dang-Vu T, Schabus M, Sterpenich V, Maquet P, Schwartz S. Neuroimaging insights into the pathophysiology of sleep disorders. Sleep 2008;31(6):777–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry 2010;67(2):182–5. [DOI] [PubMed] [Google Scholar]
- [28].Riemann D, Voderholzer U, Spiegelhalder K, Hornyak M, Buysse DJ, Nissen C, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep 2007;30(8):955–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Winkelman JW, Plante DT, Schoerning L, Benson K, Buxton OM, O’Connor SP, et al. Increased rostral anterior cingulate cortex volume in chronic primary insomnia. Sleep 2013;36(7):991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Joo EY, Kim H, Suh S, Hong SB. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep 2014;37(7):1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Joo EY, Noh HJ, Kim JS, Koo DL, Kim D, Hwang KJ, et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep 2013;36(7):999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kay DB, Karim HT, Soehner AM, Hasler BP, Wilckens KA, James JA, et al. Sleep-wake differences in relative regional cerebral metabolic rate for glucose among patients with insomnia compared with good sleepers. Sleep 2016;39(10):1779–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry 2004;161(11):2126–8. [DOI] [PubMed] [Google Scholar]
- [34].Baglioni C, Spiegelhalder K, Regen W, Feige B, Nissen C, Lombardo C, et al. Insomnia disorder is associated with increased amygdala reactivity to insomnia-related stimuli. Sleep 2014;37(12):1907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li Y, Liu L, Wang E, Zhang H, Dou S, Tong L, et al. Abnormal neural network of primary insomnia: evidence from spatial working memory task fMRI. Eur Neurol 2016;75(1–2):48–57. [DOI] [PubMed] [Google Scholar]
- [36].Son YD, Kang JM, Cho SJ, Lee JS, Hwang HY, Kang SG. fMRI brain activation in patients with insomnia disorder during a working memory task. Sleep Breath 2018. May;22(2):487–93. 10.1007/s11325-017-1575-5. [DOI] [PubMed] [Google Scholar]
- [37].Stoffers D, Altena E, van der Werf YD, Sanz-Arigita EJ, Voorn TA, Astill RG, et al. The caudate: a key node in the neuronal network imbalance of insomnia? Brain 2014;137(Pt 2):610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Altena E, Van Der Werf YD, Sanz-Arigita EJ, Voorn TA, Rombouts SA, Kuijer JP, et al. Prefrontal hypoactivation and recovery in insomnia. Sleep 2008;31(9):1271–6. [PMC free article] [PubMed] [Google Scholar]
- [39].Kim SJ, Lee YJ, Kim N, Kim S, Choi JW, Park J, et al. Exploration of changes in the brain response to sleep-related pictures after cognitive-behavioral therapy for psychophysiological insomnia. Sci Rep 2017;7(1):12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ran Q, Chen J, Li C, Wen L, Yue F, Shu T, et al. Abnormal amplitude of low-frequency fluctuations associated with rapid-eye movement in chronic primary insomnia patients. Oncotarget 2017;8(49):84877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhou F, Huang S, Zhuang Y, Gao L, Gong H. Frequency-dependent changes in local intrinsic oscillations in chronic primary insomnia: a study of the amplitude of low-frequency fluctuations in the resting state. Neuroimage Clin 2017;15:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dai XJ, Peng DC, Gong HH, Wan AL, Nie X, Li HJ, et al. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat 2014;10:2163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Li C, Ma X, Dong M, Yin Y, Hua K, Li M, et al. Abnormal spontaneous regional brain activity in primary insomnia: a resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat 2016;12:1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dai XJ, Nie X, Liu X, Pei L, Jiang J, Peng DC, et al. Gender differences in regional brain activity in patients with chronic primary insomnia: evidence from a resting-state fMRI study. J Clin Sleep Med 2016;12(3):363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Huang S, Zhou F, Jiang J, Huang M, Zeng X, Ding S, et al. Regional impairment of intrinsic functional connectivity strength in patients with chronic primary insomnia. Neuropsychiatr Dis Treat 2017;13:1449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang T, Li S, Jiang G, Lin C, Li M, Ma X, et al. Regional homogeneity changes in patients with primary insomnia. Eur Radiol 2016;26(5):1292–300. [DOI] [PubMed] [Google Scholar]
- [47].Chen MC, Chang C, Glover GH, Gotlib IH. Increased insula coactivation with salience networks in insomnia. Biol Psychol 2014;97:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Leerssen J, Wassing R, Ramautar JR, Stoffers D, Lakbila-Kamal O, Perrier J, et al. Increased hippocampal-prefrontal functional connectivity in insomnia. Neurobiol Learn Mem 2018. February 12. 10.1016/j.nlm.2018.02.006. pii: S1074-7427(18)30019-4. [DOI] [PubMed] [Google Scholar]
- *[49].Muller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, et al. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev 2018;84:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The most important references are denoted by an asterisk
- [50].Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013;14(5):365–76. [DOI] [PubMed] [Google Scholar]
- [51].Open Science C Psychology. Estimating the reproducibility of psychological science. Science 2015;349(6251):aac4716. [DOI] [PubMed] [Google Scholar]
- [52].Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009;30(9):2907–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tahmasian M, Eickhoff SB, Giehl K, Schwartz F, Herz DM, Drzezga A, et al. Resting-state functional reorganization in Parkinson’s disease: an activation likelihood estimation meta-analysis. Cortex 2017;92:119–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tahmasian M, Rosenzweig I, Eickhoff SB, Sepehry AA, Laird AR, Fox PT, et al. Structural and functional neural adaptations in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Neurosci Biobehav Rev 2016. June;65:142–56. https://doi.org/1016/j.neubiorev.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[55].Eickhoff S, Bzdok D. Meta-analyses in basic and clinical neuroscience: state of the art and perspective. In: Ulmer S, Jansen O, editors. fMRI. Springer Berlin Heidelberg; 2013. p. 77–87. [Google Scholar]; * The most important references are denoted by an asterisk
- [56].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain : 3-dimensional proportional system : an approach to cerebral imaging. Stuttgart; New York: Georg Thieme; 1988. p. 122. [Google Scholar]
- [58].Evans Dlc AC, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical models from 305 MRI volumes. Nuclear science symposium and medical imaging conference. IEEE Conference Record; 1993. p. 1813–7. [Google Scholar]
- [59].Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, ZilleS K, et al. Bias between MNI and talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 2007;28(11):1194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[60].Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp 2012;33(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The most important references are denoted by an asterisk
- *[61].Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012;59(3):2349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The most important references are denoted by an asterisk
- [62].Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 2002;16(3 Pt 1):765–80. [DOI] [PubMed] [Google Scholar]
- [63].Shattuck DW, Mirza M, Adisetiyo V, Hojatkashani C, Salamon G, Narr KL, et al. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage 2008;39(3):1064–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016;113(28):7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Eickhoff SB, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, et al. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 2016;137:70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rottschy C, Caspers S, Roski C, Reetz K, Dogan I, Schulz JB, et al. Differentiated parietal connectivity of frontal regions for “what” and “where” memory. Brain Struct Funct 2013;218(6):1551–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, et al. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One 2012;7(2):e30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Muller VI, Cieslik EC, Serbanescu I, Laird AR, Fox PT, Eickhoff SB Altered brain activity in unipolar depression revisited: meta-analyses of neuroimaging studies. JAMA Psychiatry 2017;74(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 2015;72(4):305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Eickhoff SB, Laird AR, Fox PM, Lancaster JL, Fox PT. Implementation errors in the GingerALE software: description and recommendations. Hum Brain Mapp 2017;38(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[71].Khazaie H, Veronese M, Noori K, Emamian F, Zarei M, Ashkan K, et al. Functional reorganization in obstructive sleep apnoea and insomnia: a systematic review of the resting-state fMRI. Neurosci Biobehav Rev 2017;77:219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The most important references are denoted by an asterisk
- [72].Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage 2014;99:180–90. [DOI] [PubMed] [Google Scholar]
- [73].Dong X, Qin H, Wu T, Hu H, Liao K, Cheng F, et al. Rest but busy: aberrant resting-state functional connectivity of triple network model in insomnia. Brain Behav 2018;8(2):e00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nie X, Shao Y, Liu SY, Li HJ, Wan AL, Nie S, et al. Functional connectivity of paired default mode network subregions in primary insomnia. Neuropsychiatr Dis Treat 2015;11:3085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Suh S, Kim H, Dang-Vu TT, Joo E, Shin C. Cortical thinning and altered corticocortical structural covariance of the default mode network in patients with persistent insomnia symptoms. Sleep 2016;39(1):161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hammerschlag AR, Stringer S, de Leeuw CA, Sniekers S, Taskesen E, Watanabe K, et al. Genome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Nat Genet 2017;49(11):1584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Stein MB, McCarthy MJ, Chen CY, Jain S, Gelernter J, He F, et al. Genome-wide analysis of insomnia disorder. Mol Psychiatry 2018. March 8. 10.1038/s41380-018-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jansen PR, Watanabe K, Stringer S, Skene N, Bryois J, Hammerschlag AR, et al. Genome-wide analysis of insomnia (N=1,331,010) identifies novel loci and functional pathways. bioRxiv 2018:214973. 10.1101/214973. [DOI] [PubMed] [Google Scholar]
- [79].Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev 2012;16(1):83–94. [DOI] [PubMed] [Google Scholar]
- [80].Li L, Wu C, Gan Y, Qu X, Lu Z. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC Psychiatry 2016;16(1):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Psychiatry 2009;48(9):894–908. [DOI] [PubMed] [Google Scholar]
- [82].van de Laar M, Verbeek I, Pevernagie D, Aldenkamp A, Overeem S. The role of personality traits in insomnia. Sleep Med Rev 2010;14(1):61–8. [DOI] [PubMed] [Google Scholar]
- [83].Baglioni C, Nanovska S, Regen W, Spiegelhalder K, Feige B, Nissen C, et al. Sleep and mental disorders: a meta-analysis of polysomnographic research. Psychol Bull 2016;142(9):969–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fernandez-Mendoza J, Calhoun SL, Vgontzas AN, Li Y, Gaines J, Liao D, et al. Insomnia phenotypes based on objective sleep duration in adolescents: depression risk and differential behavioral profiles. Brain Sci 2016;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


