Abstract
There is considerable evidence that emotion dysregulation and self-control impairments lead to escalated aggression in populations with psychiatric disorders. However, convergent quantitative evidence on the neural network explaining how aggression arises is still lacking. To address this gap, peak activations extracted from extant functional magnetic resonance imaging (fMRI) studies were synthesized through coordinate-based meta-analyses. A systematic search in the PubMed database was conducted and 26 fMRI studies met the inclusion criteria. Three separate activation likelihood estimation (ALE) meta-analyses were performed on (1) individual differences in trait aggression (TA) studies, (2) individual differences in TA studies examining executive functioning, and (3) elicited aggression (EA) studies across fMRI behavioral paradigms. Ensuing clusters from ALE meta-analyses were further treated as seeds for follow-up investigations on consensus connectivity networks (CCN) delineated from meta-analytic connectivity modeling (MACM) and resting-state functional connectivity (RSFC) to further characterize their physiological functions. Finally, we obtained a data-driven functional characterization of the ensuing clusters and their networks. This approach offers a boarder view of the ensuing clusters using a boarder network perspective. In TA, aberrant brain activations were found only in the right precuneus. Follow-up analyses revealed that the precuneus seed was within the frontal-parietal network (FPN) associated with action inhibition, visuospatial processing and higher-level cognition. With further restricting to only experiments examining executive functioning, convergent evidence was found in the right rolandic operculum (RO), midcingulate cortex (MCC), precentral gyrus (PrG) and precuneus. Follow-up analyses suggested that RO, MCC and PrG may belong to a common cognitive control network, while the MCC seems to be the hub of this network. In EA, we only revealed a convergent region in the left postcentral gyrus. Follow-up CCN analyses and functional characterizations suggested that this region may also belong to the same cognitive control network found in the TA sub-analysis. Our results suggested that escalated aggression arises from abnormal precuneus activities within the FPN, disrupting the recruitment of other large-scale networks such as adaptive cognitive control network. Consequently, failure to recruit such a network results in an inability to generate adaptive responses, increasing the likelihood of acting aggressively.
Keywords: fMRI, ALE, MACM, Resting-state functional connectivity, Meta-analysis
Introduction
Aggression is in the behavioral repertoire of virtually all animals and serves an evolutionarily adaptive purpose in survival, where it is hostile when defending threats and instrumental in competition for limited resources and increasing mating opportunities (Buss and Shackelford 1997). Aggression, however, can become maladaptive and destructive when it results in violating the social norms that maintain the order of a society. Several subgroups of psychiatric populations are often reported to be more susceptible to escalated aggression, including individuals with intermittent explosive disorder (Coccaro et al. 1998; Olvera 2002), schizophrenia (Milton et al. 2001; Douglas et al. 2009), psychopathy (Glenn and Raine 2008; Blair 2010), and antisocial or borderline personality disorders (Dolan 2010; Soloff et al. 2017).
Escalated aggression has been conceptualized as reflecting increased negative affect (i.e., anger) in the context of under-regulation of aggressive impulses (Davidson et al. 2000). The brain systems implicated in aggression were qualitatively postulated in a wider perturbed frontolimbic network functionally associated with affective processing, self-regulation, and reinforcement-based decision making (Davidson et al. 2000; Siever 2008; Coccaro et al. 2011; Blair 2016), comprising the amygdala, hypothalamus, periaqueductal gray, cingulate cortex, ventrolateral and medial prefrontal cortex, supplementary motor area, anterior insular cortex and striatum. Notably, other regions like posterior medial cortices are rarely discussed in the aggression literature, but a recent large-scale structural MRI study (N = 556) showed that the thickness of the precuneus and its contiguous regions were positively correlated with aggressive behavior in girls (Thijssen et al. 2015).
Instead of distinct networks for numerous cognitive functions, we noticed that this wider frontolimbic network is overlapping with a superordinate cognitive control network subserving various executive functions involving flexibility, working memory, response initiation and inhibition (Miller 2000; Niendam et al. 2012), suggesting that an executive functioning neural network may play an cardinal role in modulating aggressive behavior. Indeed, psychological studies have established a link between executive functions and aggression in healthy individuals (Hoaken et al. 2003; Denson et al. 2012). In accordance, the general aggression model (Anderson and Bushman 2002; Allen et al. 2018) proposed that aggressive acts are built on the (re-)appraisal processes, while available cognitive resources play a key factor to bias the behavioral outcomes to either impulsive or thoughtful responses (Please note that the response can be either adaptive or maladaptive). Therefore, proper executive functioning signifies to ensure adaptive and non-aggressive behavior. More importantly, executive function impairment seems to be a core feature across psychopathology (Diamond 2013; Snyder et al. 2015). Recent neuroimaging meta-analyses have identified domain-nonspecific neural disruptions across psychiatric disorders in the salience (or anterior-cingulo-insular) network (Goodkind et al. 2015; McTeague et al. 2017). Evidence for transdiagnostic-impaired executive functioning leads to the question of whether there is a common disrupted cognitive control network in aggressive individuals with psychiatric diagnoses.
Previous functional magnetic resonance imaging (fMRI) studies of aggression focused primarily on cognitive processing including working memory (Kumari et al. 2006) and inhibition (Joyal et al. 2007), affective processes including perception of affective stimuli (Lee et al. 2009; Bobes et al. 2013) and emotional regulation (Kalnin et al. 2011; Schiffer et al. 2011) in aggressive individuals. These studies usually recruited participants with a history of violent incidents and heterogeneous psychiatric disorders who score high on measures of trait aggression (TA) by psychological scales. Other studies examined brain systems recruited when aggressive behavior is evoked. Such elicited aggression (EA) effects are studied through behavioral paradigms evoking aggression in a laboratory setting, for instance, the Taylor aggression paradigm (Taylor 1967), Point Subtraction Aggression Paradigm (Cherek et al. 1997) and violent video games (Mathiak and Weber 2006). These paradigms mimic aggressive responses of participants through provocation. These fMRI studies, however, use relatively small sample sizes, resulting in reduced statistical power and an increased likelihood of false positive results (Button et al. 2013). Furthermore, some studies abandon whole-brain inference spaces and adopt a region-of-interest approach to test a priori hypotheses. While this approach increases sensitivity for the chosen regions, it also entails the risk of limiting discovery.
Taken together, to identify a common disrupted neural network in trait aggressive individuals, we employed coordinate-based meta-analyses to quantitatively analyze the common aberrant brain activation pattern in aggressive individuals with psychiatric diagnoses. Specifically, we also investigate TA studies examining executive functioning (i.e., working memory and inhibition). To better understand the obtained clusters from trait aggressive individuals during the cognitive process, we also performed a meta-analysis on laboratory aggression paradigms. We hypothesized that both TA and EA studies should show convergent findings in the regions of the frontolimbic networks. Given that complex cognition and behavior, for example aggression, arise from dynamic interactions of distributed brain regions (Bressler and Menon 2010), a consensus connectivity network analysis was performed on each seed obtained from meta-analyses helps us estimating the coactivation pattern of a given seed region (i.e., how it connects with other brain regions) based on an independent healthy population and speculating dysfunctions in the aggressive population.
Method
Literature search and data acquisition
A systematic literature search was conducted on the PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/). We used the keywords “aggression”, “aggressive”, “violence”, “violent”, “intermittent explosive disorder” paired with “fMRI” or “functional magnetic resonance imaging” through June 2018. Further manuscripts were identified by reference tracing in the papers retrieved by the original search and textual reviews on the neurobiology of aggression (Hawkins and Trobst 2000; Anderson and Bushman 2002; Dolan 2010; Coccaro et al. 2011; Hoptman et al. 2011; Weiss 2012; Blair 2016). Studies were included if they (1) were peer-reviewed journal articles available in English, (2) reported whole-brain thresholded results; therefore, those results that restricted their analysis to a priori regions of interest (ROIs) were excluded, (3) reported coordinates in either the Montreal Neurological Institute (MNI) or Talairach standard stereotactic space, (4) for trait aggression (TA) studies, reported contrasts between aggressive psychiatric individuals with their non-aggressive counterparts; psychiatric individuals refer to a formal diagnosis of either Axis I or II disorders including schizophrenia, intermittent explosive disorder and personality disorders, (5) for elicited aggression (EA) studies, reported either contrasts between induced aggressive and non-aggressive events on a virtual or real human targets or a correlation analysis of aggressive response and BOLD signal change within the experimental group, (6) recruited participants older than 18. We were only interested in the general aberrant activation changes associated with aggression, so hyper- and hypo-activations were pooled for the current meta-analyses. Figure 1 illustrates the steps of systematic search based on PRISMA criteria (Moher et al. 2015).
Fig. 1.
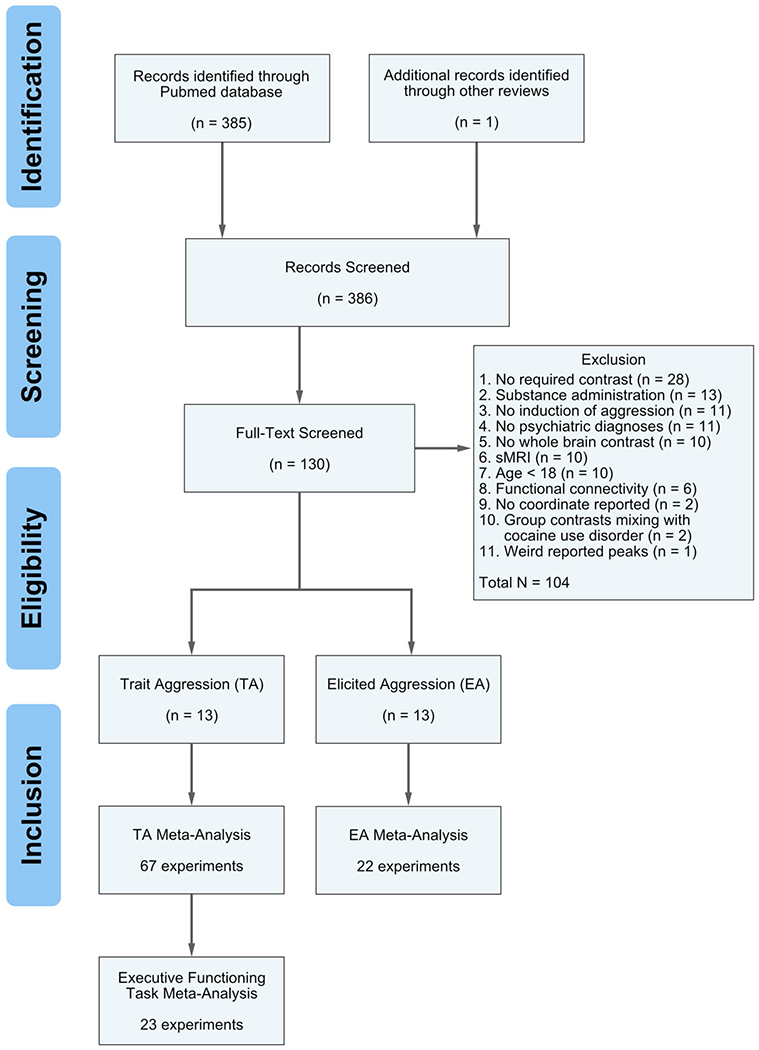
The PRISMA flowchart of the steps conducted in the meta-analyses
In the context of ALE, the term “study” refers to a scientific publication, while the term “experiment” refers to any single contrast analysis within a study (Laird et al. 2011). A total of 26 studies met our inclusion criteria (see Table 1 for details of the studies). Based on the original contrasts reported by the studies, we assigned them into two groups. Studies enrolling individuals with psychiatric diagnoses and a history of violence, e.g., violent schizophrenia patients compared to their non-aggressive counterparts, were categorized as studies on trait aggression (TA; N = 13). Studies using a behavioral paradigm to compare BOLD responses during induced aggression were categorized as studies of elicited aggression (EA; N = 13). A follow-up analysis on fMRI tasks related to executive functioning was performed. A total of 569 participants with 67 experiments (23 executive functioning experiments) from TA studies and a total of 393 with 22 experiments from EA studies were included. All activation foci reported in a Talairach space were linearly transformed into a MNI space (Lancaster et al. 2007).
Table 1.
Study samples and fMRI tasks
| Studies | N1 (aggression) | Age 1 (M ± SD) | N2 (control) | Age 2 (M ± SD) | fMRI taskd |
|---|---|---|---|---|---|
| Trait aggression | |||||
| Barkataki et al. (2008) | 14 (APD) + 12 (SZV) | 34.11 ± 7.93 | 12 (SZ) + 14 (HC) | 33.42 ± 7.68 | Go/NoGo task |
| Coccaro et al. (2007) | 10 (IED) | 34.30 ± 7.30 | 10 (HC) | 30.90 ± 5.60 | Emotion face viewing |
| Gan et al. (2016) | 9 (IED) | 34.40 ± 7.50 | 9 (HC) | 31.80 ± 6.50 | Point subtraction aggression paradigm |
| Gregory et al. (2015) | 32 (VO with APD)a | 38.04 ± 8.08 | 18 (HC) | 34.80 ± 8.80 | Probabilistic response reversal task |
| Joyal et al. (2007) | 12 (SZV + APD + SUD) | NA (aged 28 to 54) | 12 (SZ), 12 (HC) | NA (aged 28 to 54) | Go/NoGo task |
| Kumari et al. (2006) | 13 (SZV) + 10 (APD) | 32.83 ± 6.27 | 12 (SZ), 13 (HC) | 33.57 ± 7.19 | Modified N-back task |
| Kumari et al. (2009) | 13 (SZV) + 13 (APD) | 33.66 ± 7.75 | 14 (SZ), 13 (HC) | 33.75 ± 6.96 | Fear conditioning |
| McCloskey et al. (2016) | 20 (IED) | 33.20 ± NA | 20 (HC) | 32.80 ± NA | Emotion face viewing |
| Prehn et al. (2013a) | 23 (VO with APD)b | 27.65 ± 8.02 | 13 (HC) | 26.62 ± 8.40 | Behavioral investment allocation strategy |
| Prehn et al. (2013b) | 15 (VO with APD + BPD) | 27.87 ± 9.86 | 17 (HC) | 28.88 ± 9.49 | N-back task |
| Schiffer et al. (2014) | 21 (VO with APD) | 35.20 ± 8.20 | 23 (HC) | 34.10 ± 8.90 | Stroop task |
| Schiffer et al. (2017) | 45 (VO)c | 36.12 ± 8.22 | 18 (SZ) + 18 (HC) | 37.05 ± 9.05 | Theory of mind |
| Tikàsz et al. (2016) | 20 (VSZ) | 30.00 ± 1.60 | 19 (SZ) + 21 (HC) | 31.14 ± 1.70 | Emotion picture viewing |
| Elicited aggression | |||||
| Beyer et al. (2014) | – | – | 40 (healthy) | 22.50 ± NA | Taylor aggression paradigm |
| Buades-Rotger et al. (2017) | – | – | 36 (healthy) | 22.00 ± 4.00 | Modified Taylor aggression paradigm |
| Chester and DeWall (2016) | – | – | 69 (healthy) | 18.70 ± 0.93 | Taylor aggression paradigm |
| Chester and DeWall (2018a) | – | – | 24 (healthy) | 23.04 ± 2.46 | Taylor aggression paradigm |
| Chester and DeWall (2018b) | – | – | 61 (healthy) | 18.61 ± 0.84 | Taylor aggression paradigm |
| Dambacher et al. (2013) | – | – | 18 (healthy) | 22.33 ± 2.35 | Taylor aggression paradigm |
| Emmerling et al. (2016) | – | – | 15 (healthy) | 22.33 ± 2.35 | Taylor aggression paradigm |
| Krämer et al. (2007) | – | – | 15 (healthy) | 22.90 ± 2.20 | Taylor aggression paradigm |
| Krämer et al. (2011) | – | – | 36 (healthy) | 24.80 ± 3.10 | Taylor aggression paradigm |
| Mathiak and Weber (2006) | – | – | 13 (healthy) | NA (aged 18–26) | Violent first-person shooter gaming |
| Mathiak et al. (2011) | – | – | 13 (healthy) | 22.70 ± 2.00 | Violent first-person shooter gaming |
| Repple et al. (2017) | – | – | 33 (healthy) | 23.60 ± 3.20 | Modified Taylor aggression paradigm |
| Skibsted et al. (2017) | – | – | 20 (healthy) | 24.60 ± 2.90 | Point subtraction aggression paradigm |
APD antisocial personality disorder with a violent history, BPD borderline personality disorder, HC healthy controls, IED intermittent explosive disorder, SZ schizophrenia without a violent history, SZV schizophrenia with a violent history, SUD substance use disorder, VO violent offenders
12 VO with APD and psychopathy and 18 VO with APD but not psychopathy
23 VO with APD consists of 11 emotion hypo-reactivity and 13 emotion hyper-reactivity
13 VO with SZ plus CD/APD, 16 VO with SZ and 16 VO with CD/APD
Underlined tasks were considered as executive functioning tasks
Coordinate-based meta-analysis: ALE
The latest revised version of activation likelihood estimation (ALE) algorithm (Turkeltaub et al. 2002; Eickhoff et al. 2009, 2012) was implemented in MATLAB to perform our coordinate-based meta-analysis. Through modeling activation peak maxima that were identified from the existing literature as three-dimensional probability distributions centered at the given coordinate rather than one-dimensional points, the algorithm aims at quantitatively identifying topographic convergence across studies while accommodating spatial uncertainty. ALE is a well-established method adopted in multiple studies (Kohn et al. 2014; Nickl-Jockschat et al. 2015; Müller et al. 2017) and involves three main steps. First, to compensate for the spatial uncertainty due to a between-template and between-subject variance in most neuroimaging studies (Eickhoff et al. 2009), all the foci are modeled as centers of three-dimensional Gaussian probability distributions. Assuming a larger sample size that is more likely to provide a reliable approximation of a “true” localization, studies are further weighted by the number of participants per study. Thus, a larger sample size leads to a smaller Gaussian distribution. Second, a modeled activation (MA) map is generated through combining the probabilities of all activation foci in a single experiment for each voxel (Turkeltaub et al. 2012). A voxel-wise ALE score is determined by calculating the union of these MA maps. Finally, to test significance, the yielded scores are compared with an empirical random spatial null distribution among all MA maps yielding a p value which was thresholded at p < 0.05 with a cluster-level family-wise error (cFWE) correction (cluster-forming threshold at voxel level: p < 0.001) which provides the best compromise between sensitivity and specificity (Hopkins et al. 2016). Neuroanatomical labeling of the ensuing clusters was derived from the SPM anatomy toolbox (Eickhoff et al. 2005).
Consensus connectivity networks (CCNs): conjunction analysis
Unlike simple physiological process such as primary sensory perception, complex cognition and behavior, such as aggression, arise from dynamic interactions of distributed brain regions (Bressler and Menon 2010). Knowing the connectivity profile of a given brain region in healthy subjects helps to delineate the physiological functions of that region and allows to formulate data-driven hypotheses regarding dysfunctions, e.g., in population with escalated aggression. To identify the functional connectivity patterns that are consistently engaged across two different states of brain functions (i.e., task-dependent and task-independent), we performed a conjunction analysis across coactivation maps obtained from meta-analytic connectivity modeling (MACM) and resting-state functional connectivity (RSFC) using the strict minimum statistics with an individual seed (Amft et al. 2015). As the brain regions identified by this approach exhibit connectivity with the seed in two independent modalities of connectivity simultaneously, the ensuing networks can be regarded as especially robust (Nickl-Jockschat et al. 2015). The methodological details of MACM and RSFC are described later in this section. Surviving clusters from this conjunction analysis were, thus, functionally associated with both task-dependent and task-independent brain states. To reduce the likelihood of false positive findings, ensuing clusters smaller than 50 voxels were discarded. The CCN approach allows for inference on the involvement of clusters ensuing from our ALE meta-analyses in larger-scale cortical networks with reference to healthy populations. As stated above, our basic aim was the identification of neural networks in which the seed regions involve in further characterizing their physiological function.
Task-dependent functional connectivity: MACM
To characterize the functional connectivity among ensuing clusters from our original ALE meta-analyses, we determined the coactivation profile based on meta-analytic connectivity modeling (MACM). MACM was performed to examine the functional connectivity of a specific seed region (i.e., regions from our meta-analysis results) through investigating patterns of coactivation across large-scale neuroimaging experiments on the BrainMap Database (Fox et al. 2014). All experiments that activate at least one focus within the seed volume were retrieved. Subsequently, meta-analytic modeling using the ALE algorithm was employed to test for convergence across the foci reported in these experiments. With this approach, we aimed at a general neurobiological task-based coactivation profile for our ROIs. A cFWE-corrected threshold for multiple comparisons at p < 0.05 (cluster-forming threshold at voxel level: p < 0.001) was adopted.
Task-independent functional connectivity: RSFC
The same seeds obtained from our original ALE meta-analyses were also used for a resting-state functional connectivity (RSFC) analysis to assess a task-independent modality of functional connectivity (Bzdok et al. 2015). Resting-state data were obtained from an open online database: the enhanced Nathan Kline Institute Rockland sample (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html). A total of 124 healthy subjects without a history of psychiatric or neurological disorders aged between 20 and 75 years (mean age 46.56 ± 17.56 years; 40 males, 84 females) were included. 404 echo-planar imaging (EPI) images of each subject were acquired on a Siemens TrioTim 3T scanner using blood-oxygen-level-dependent (BOLD) contrast [multiband EPI sequence with acceleration factor 4, repetition time (TR) = 1.4 s, echo time (TE) = 30 ms, flip angle = 65°, in-plane resolution = 2.0 × 2.0 mm, 64 axial slices with 2.0 mm thickness, covering the whole brain]. To allow for magnetic field saturation, the first four scans of each subject were discarded; the remaining 400 images were processed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). First, images were corrected for head movements in a two-pass procedure, following by alignment to the initial volume and subsequently the mean of all volumes. Spatial normalization to a non-linear MNI152 template was applied to the mean image using the “unified segmentation” approach. The ensuing deformation was then applied to the individual EPI image. Finally, images were smoothed by a 5 mm FWHM Gaussian kernel and a band-pass filter between 0.01 and 0.08 Hz was applied to data. Time courses of all voxels of a given seed of each individual subject and the time course of the entire seed were extracted as their first eigenvariate. Pearson correlation coefficients between the time series of the seeds and all other gray matter voxels across the whole brain were then computed to assess the RSFC. These correlation coefficients were transformed into Fisher’s Z scores and entered as dependent measures in a second level analysis of covariance, co-varying with age, gender and handedness. The RSFC results, consistent with MACM analysis, were thresholded at a cFWE corrected p < 0.05 (cluster-forming threshold at voxel level: p < 0.001).
Functional characterization through a meta-data-driven approach
Functional properties of the ensuing clusters and their CCNs were further assessed, using the “Behavioral Domain” and “Paradigm Class” meta-data categories assigned for each neuroimaging study stored in the BrainMap database (Laird et al. 2011). BrainMap taxonomy for “Behavioral Domain” codes mental processes into five major categories: action, cognition, emotion, interoception and perception; each category is further subdivided into sub-categories. “Paradigm Class” describes the categorization of the employed behavioral task (e.g., reward, Stroop or go/nogo, etc.) of a single study. Details about the taxonomy can be retrieved from the BrainMap website (http://www.brainmap.org/taxonomy/). In this study, we were interested in the precise functional associations of the ensuing clusters and their CCNs, while a “Paradigm Class” may involve various “Behavioral domains”; thus, only functional categorization on “Behavioral Domains” was conducted. Both forward inferences and reverse inferences on the functional categorization using binomial test (FDR corrected p < 0.05) were performed (Eickhoff et al. 2011). The former, P(Activation|Domain), indicates the probability of activation in a neuroanatomical seed given a psychological process or behavioral task; the latter, P(Domain|Activation), tests the probability of observing a psychological process or behavioral task given a brain activation.
Results
Overall analyses on trait and elicited aggression
Trait aggression
Across 67 experiments in TA individuals, our ALE meta-analysis revealed a convergent cluster in the right precuneus (peak MNI: 4/– 64/48; k = 116 voxels; see Fig. 2a). Functional characterization showed that the ensuing cluster was significantly associated with behavioral domains of action inhibition, reasoning, working memory, sexuality interoception, and motion vision perception. The functional coactivation pattern of the ensuing cluster was examined with MACM and RSFC. For more detail see Table S1 and Figure S1 in the Supplementary Materials. The CCN of the precuneus seed (see Table 2A and Fig. 3a) comprised the bilateral precuneus, bilateral middle occipital gyrus, bilateral middle frontal gyrus with the right clusters extending into the superior frontal gyrus. Functional characterization documented that the ensuing consensus network was associated with a wide range of the cognition domain including space, attention, explicit memory, working memory and reasoning plus other behavioral domains including interoception of sleep, action inhibition, visual perception of motion and shape (see Fig. 4a for forward inference and reverse inference).
Fig. 2.
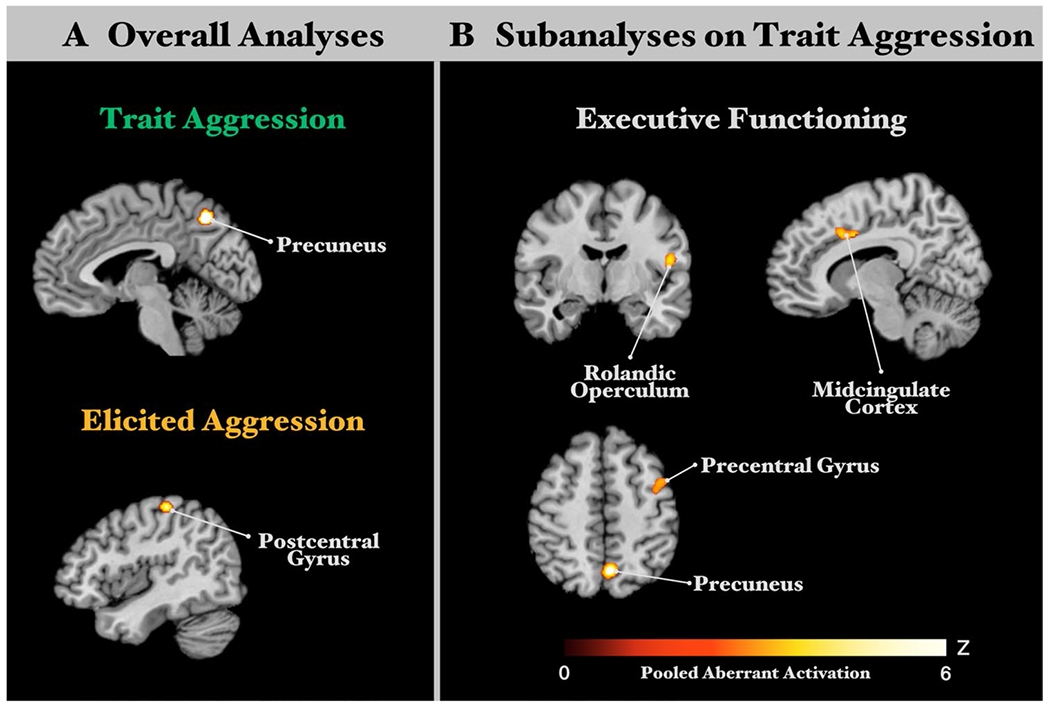
Convergent aberrant activation clusters from overall ALE analyses and subanalyses. a Overall ALE analyses revealed significant clusters of convergent aberrant activation from all experiments in trait aggression (TA) studies and all experiments in elicited aggression (EA) studies. b Subanalyses focusing on executive functioning experiments in TA studies revealed significant clusters of convergent aberrant activation. All results survived a cluster-level FWE corrected threshold for multiple comparisons of p < 0.05 (cluster-forming threshold at voxel level, p < 0.001)
Table 2.
Peak activations of the consensus connectivity networks (MACM ∩ RSFC)
| Cluster | k (voxels) | Hemisphere | x | y | z | Cluster breakdowns |
|---|---|---|---|---|---|---|
| (A) Overall analyses | ||||||
| Trait aggression: precuneus seed | ||||||
| 1 | 788 | R | 9 | −63 | 50 | R precuneus; R superior parietal lobule |
| 2 | 596 | L | −7 | −65 | 51 | L precuneus; L superior parietal lobule |
| 3 | 281 | R | 37 | −64 | 42 | R angular gyrus; R middle occipital gyrus; R inferior parietal lobule; R superior parietal lobule |
| 4 | 231 | R | 28 | 10 | 53 | R middle frontal gyrus; R superior frontal gyrus |
| 5 | 99 | R | 34 | 33 | 39 | R middle frontal gyrus |
| 6 | 87 | R | 28 | 58 | 3 | R middle frontal gyrus; R superior frontal gyrus |
| 7 | 57 | L | −25 | 9 | 53 | L middle frontal gyrus |
| Elicited aggression: postcentral gyrus | ||||||
| 1 | 3205 | L | −38 | −26 | 53 | L postcentral gyrus; L supramarginal gyrus; L superior parietal lobule; L inferior parietal lobule; L superior temporal gyrus |
| 2 | 790 | R | 40 | −25 | 56 | R inferior parietal lobule; R postcentral gyrus; R precentral gyrus; R supramarginal gyrus; R superior frontal gyrus |
| 3 | 648 | L | −7 | −8 | 54 | L posterior-medial frontal; L midcingulate cortex |
| 4 | 289 | R | 20 | −52 | −21 | R cerebellum |
| 5 | 181 | L | −57 | 4 | 30 | L precentral gyrus |
| 6 | 164 | R | 7 | −2 | 54 | R posterior-medial frontal; R midcingulate cortex |
| 7 | 81 | L | −47 | −3 | 10 | L insula lobe; L rolandic operculum |
| 8 | 64 | R | 58 | 8 | 24 | R precentral gyrus |
| (B) Subanalyses on executive functioning in trait aggression | ||||||
| Rolandic operculum seed | ||||||
| 1 | 1325 | R | 53 | −11 | 16 | R rolandic operculum; R Heschl’s gyrus; R superior temporal gyrus; R insula lobe; R postcentral gyrus |
| 2 | 1132 | L | −49 | −13 | 15 | L superior temporal gyrus; L insula lobe; L postcentral gyrus |
| 3 | 114 | R | 6 | −1 | 54 | R posterior-medial frontal; R midcingulate cortex |
| 4 | 77 | L | −4 | 7 | 49 | L posterior-medial frontal; L midcingulate cortex |
| Midcingulate cortex seed | ||||||
| 1 | 2159 | L | −40 | 5 | 23 | L insula lobe; L inferior frontal gyrus; L precentral gyrus; L middle frontal gyrus; L Rolandic operculum; L temporal pole |
| 2 | 962 | L | −7 | 7 | 45 | L midcingulate cortex; L posterior-medial frontal; L anterior cingulate cortex |
| 3 | 902 | R | 37 | 17 | 2 | R insula lobe; R putamen; R pallidum; R Rolandic operculum |
| 4 | 689 | R | 8 | 14 | 43 | R midcingulate cortex; R posterior-medial frontal |
| 5 | 316 | R | 53 | −1 | 18 | R postcentral gyrus; R precentral gyrus; R inferior frontal gyrus |
| 6 | 216 | L | −49 | −46 | 23 | L Rolandic operculum |
| 7 | 194 | L | −10 | −17 | 6 | L thalamus |
| 8 | 193 | R | 10 | −16 | 6 | R thalamus |
| 9 | 143 | R | 55 | −23 | 23 | R supramarginal gyrus |
| Precentral gyrus seed | ||||||
| 1 | 3304 | R | 41 | 16 | 31 | R precentral gyrus; R insula lobe; R middle frontal gyrus; R inferior frontal gyrus |
| 2 | 1232 | L | −42 | 3 | 42 | L precentral gyrus; L inferior frontal gyrus |
| 3 | 1165 | R | 46 | −49 | 38 | R inferior parietal lobule; R superior temporal gyrus; R supramarginal gyrus; R middle occipital gyrus; R middle temporal gyrus |
| 4 | 830 | R | 8 | 14 | 52 | R posterior-medial frontal; R midcingulate cortex |
| 5 | 336 | L | −5 | 9 | 54 | L posterior-medial frontal |
| 6 | 142 | L | −34 | −52 | 46 | L inferior parietal lobule; L superior parietal lobule |
| 7 | 121 | L | −36 | 20 | 4 | L insula lobe |
| Precuneus seed | ||||||
| 1 | 1276 | R | 18 | −63 | 48 | R precuneus; R angular gyrus; R inferior parietal lobule; R middle occipital gyrus; R superior parietal lobule; R middle occipital gyrus |
| 2 | 639 | L | −8 | −64 | 52 | L precuneus; L superior parietal lobule |
| 3 | 264 | R | 28 | 9 | 53 | R middle frontal gyrus; R superior frontal gyrus |
| 4 | 127 | R | 36 | 33 | 39 | R middle frontal gyrus |
| 5 | 113 | L | −27 | 9 | 55 | L middle frontal gyrus |
| 6 | 100 | R | 29 | 58 | 4 | R middle frontal gyrus |
Clusters smaller than 50 voxels were excluded. Coordinates are reported in a Montreal Neuroimaging Institute (MNI) stereotaxic space. Macroanatomical locations are labeled by SPM Anatomy Toolbox (Eickhoff et al. 2005)
Fig. 3.
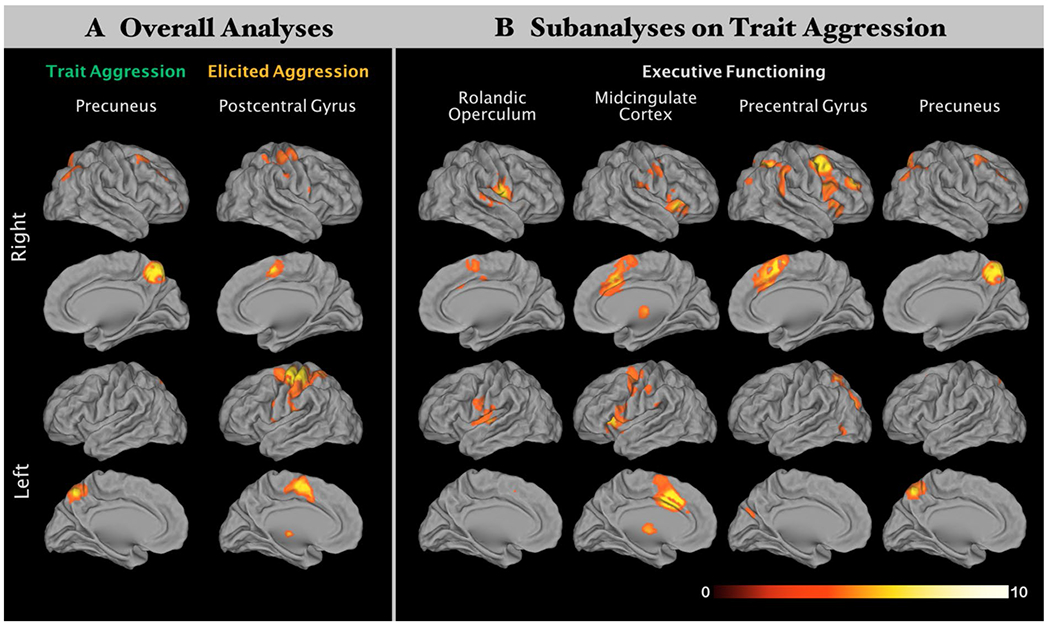
Consensus connectivity networks (CCNs) for each ensuing cluster from ALE analyses. Overlapping functional connectivity patterns between meta-analytic connectivity modeling (MACM) and resting-state functional connectivity (RSFC) of each ensuing cluster from ALE analyses. Please refer to Table 2 for peak activations of each cluster. All results survived at a cluster-level FWE-corrected threshold for multiple comparisons of p < 0.05 (cluster-forming threshold at voxel level: p < 0.001). The right bottom color bar represents Z values
Fig. 4.

Forward and reverse inferences on behavioral domains in the consensus connectivity networks (CCNs) based on the BrainMap database. All results were significantly associated with the consensus connectivity networks (CCNs) with an FDR-corrected threshold for multiple comparisons of p < 0.05
Elicited aggression
Across 22 experiments in TA individuals, our ALE meta-analysis revealed a convergent cluster in the left postcentral gyrus (peak MNI: – 40/– 28/58; k = 99 voxels; see Fig. 2a). This result does not change after restricting only studies using the Taylor aggression paradigm. Functional characterization showed that the ensuing cluster was significantly and exclusively associated with action execution. The functional connectivity network of the ensuing cluster was examined with MACM and RSFC. For more detail see Table S1 and Figure S1 in the Supplementary Materials. The CCN of the postcentral gyrus seed (see Table 2A for abbreviations and Fig. 3a) comprised the bilateral postcentral gyrus extending into bilateral midcingulate cortex, bilateral precentral gyrus, right cerebellum, left insula, and left thalamus. Functional characterization documented that the ensuing consensus network was associated with pain perception as well as execution, preparation and imagination of actions (see Fig. 4a for forward inference and reverse inference).
Subanalyses on executive functioning in trait aggression
Activation patterns during executive functioning
Across 23 experiments in TA studies during performing executive functions, our ALE meta-analysis revealed convergent clusters in the right precuneus (peak MNI: 5/– 63/49; k = 171 voxels), the left midcingulate cortex (peak MNI: – 9/10/37; k = 110 voxels), the rolandic operculum (peak MNI: 51/– 9/14; k = 95 voxels), and the precentral gyrus (peak MNI: 42/4/50; k = 91 voxels). The functional connectivity network of the ensuing cluster was examined with MACM and RSFC. For more detail see Table S1 and Figure S2 in the Supplementary Materials.
The CCN of the right rolandic operculum seed (see Table 2B for abbreviations and Fig. 3b) comprised the right rolandic operculum and Heschl’s gyrus plus the bilateral insular cortices, superior temporal gyri, postcentral gyri, midcingulate cortices, and posterior-medial frontal gyri. Functional characterization documented that the ensuing consensus network was associated with interoception, action execution, motor learning, perception in pain and audition (see Fig. 4b for forward inference and reverse inference).
The CCN of the right midcingulate cortex seed (see Table 2B for abbreviations and Fig. 3b) comprised the bilateral midcingulate cortices, rolandic operculum, insula, thalamus, precentral gyri, inferior frontal gyri, and posterior-medial frontal gyri. The left hemisphere clusters also included the middle frontal gyrus, temporal pole and anterior cingulate cortex and the right hemisphere clusters also included the putamen, pallidum, postcentral gyrus and supramarginal gurus. Functional characterization documented that the ensuing consensus network was associated with action execution, language cognition, perception in pain and gustation (see Fig. 4b for forward inference and reverse inference).
The CCN of the right precentral gyrus seed (see Table 2B for abbreviations and Fig. 3b) comprised the bilateral precentral gyri, insula lobes, inferior frontal gyri, posterior-medial frontal gyri, and inferior parietal lobules. The right hemisphere clusters also included the middle frontal gyrus, superior temporal gyrus, supramarginal gyrus, middle occipital gyrus, middle temporal gyrus, midcingulate cortex and the right hemisphere clusters also included the superior parietal lobule. Functional characterization documented that the ensuing consensus network was associated with space and language cognition (see Fig. 4b for forward inference and reverse inference).
Like the ALE seed from the overall result, the CCN of the precuneus seed in the sub-analysis (see Table 2B and Fig. 3b) comprised the bilateral precuneus, bilateral middle occipital gyrus, bilateral middle frontal gyrus with the right clusters extending into the superior frontal gyrus. Functional characterization documented that the ensuing consensus network was associated with a wide range of the cognition domain including space, attention, explicit memory, working memory and reasoning plus other behavioral domains including interoception of sleep, action inhibition, visual perception of motion and shape (see Fig. 4 for forward inference and reverse inference).
Discussion
Extant fMRI literature in aggression can be broadly divided into two directions: (1) examining neural mechanisms in violent or aggressive samples with high trait aggression (TA) and (2) examining neural mechanisms in elicited aggression (EA) through in a laboratory setting in a healthy sample. With the aid of data-driven coactivation analyses and functional characterization, the present coordinate-based meta-analysis quantitatively summarized the reported peak coordinates from these two directions to probe a generic functional neuroanatomy of aggression. We revealed that aggressive individuals, comparing to their non-aggressive counterparts, demonstrated aberrant activation changes in the right precuneus across different fMRI paradigms. By further restricting to experiments that required executive functions, our results displayed that disrupted activations associated with a cognitive control network in aggressive individuals comprised the right rolandic operculum (RO), midcingulate cortex (MCC), precentral gyrus (PrG) and precuneus. In provoked healthy subjects across EA experiments, the left postcentral gyrus was consistently involved.
The disrupted precuneus-related networks in trait-aggressive individuals
Surprisingly, aggressive individuals from TA studies were not characterized by significant abnormalities in limbic activation, but rather in the precuneus, which is part of the regulatory system and is implicated in self-consciousness and self-referential processes (Cavanna and Trimble 2006). Suppressing aggressive proneness initiated by external stimuli requires top-down cognitive control, with failure leading to escalated aggression (Davidson et al. 2000). Neuroanatomically, the precuneus is widely interconnected within the frontoparietal network (FPN) associated with elaborating highly integrated and associative information (Cavanna and Trimble 2006). Functionally, our CCN analysis showed the precuneus seed was strongly coactivated with posterior parietal and lateral frontal cortices. The FPN has been shown to be involved in initiating and adjusting control according to feedback (Dosenbach et al. 2007, 2008; Zhang and Li 2012b). Recent evidence suggested the FPN may serve as a flexible hub facilitating adaptive task performance (Cole et al. 2013). Supporting the notion that abnormal precuneus activities are linked to aberrant activations in a wider part of the control network, our results demonstrated that the precuneus and its ensuing coactivation network were functionally associated with various cognitive domains related to executive functioning and higher-order cognition.
Arguably, the aberrant precuneus activations from our ALE analysis could reflect disruptions in default mode network (DMN) linking to aggression. For example, attenuated deactivation in the posterior-medial cortex (PMC) of the DMN was documented in the high-psychopathy group during a task state (Freeman et al. 2015). The PMC, comprising the precuneus, posterior cingulate cortex and retrosplenial cortex, consume the highest level of glucose for brain energy metabolism during a resting state (Gusnard and Raichle 2001; Gur et al. 2009); when behavior becomes goal-directed, its consummation of glucose attenuates. Typically, the DMN comprises the bilateral temporal parietal junction, posterior cingulate cortex, middle temporal gyrus, precuneus and dorsomedial prefrontal cortex (dmPFC), while precuneus is considered as a functional core of the DMN (Utevsky et al. 2014). Notably, controversy on whether the precuneus is part of the DMN arose in the literature, and few recent connectivity-based parcellation studies suggest that perhaps not the entire precuneus belong to the DMN (Zhang and Li 2012a; Bzdok et al. 2015).
Cognitive control network in aggression
Diverse executive function domains may be implicated in a common superordinate fronto–cingulo–parietal network (Niendam et al. 2012). With a more detailed analysis of experiments examining executive functioning in TA studies, we further identified aberrant activations in the MCC, RO, and PrG as well as the precuneus. The follow-up CCN analyses showed that RO, PrG and MCC belonged to a common network in which the MCC seemed to be the core hub of the network. Evidence showed that the MCC and its network not only integrates domains including negative affect, pain and cognitive control (Vogt 2005; Shackman et al. 2011), but also represents an important hub for regulating emotional reactivity (Kohn et al. 2014) as well as intentional motor control (Hoffstaedter et al. 2014). In accordance, our results showed that the MCC associated CCN were functionally associated with action execution and pain perception. Aggression is often prompted by underlying motives (Bushman and Anderson 2001). This MCC network leverages socioemotional information (i.e., pain, negative affect and cognitive control) for translating intentions into adaptive motor expressions (Paus 2001; Shackman et al. 2011; Shenhav et al. 2013). Thus, abnormal MCC activation in aggressive individuals may lead to failure of modulating inappropriate and aggressive reactions. Evaluating gains and losses could therefore promote the choice of a socially appropriate response guided by different motives (Shackman et al. 2011; Hillman 2013). A recent meta-analysis on youths with disruptive behavior, a population that often displays a wide range of phenotypical aspects of aggression such as hostile thoughts and violating social norms, showed that hypoactivation of the bilateral anterior MCC extending into SMA, rACC and medial prefrontal cortex with bilateral ventral caudate was found converging across studies (Alegria et al. 2016). Another study recruiting healthy youth revealed that the gray matter density of the anterior MCC was positively associated with hostile behavior (Nakagawa et al. 2017). Taken together, our findings provided evidence of a disrupted adaptive control network in aggressive psychiatric individuals, suggesting they are less likely to generate adaptive responses.
It is noteworthy that the derived CCN of the MCC was functionally associated with aspects of cognition related to language, suggesting that language might be engaged as a mechanism to mediate conflict and frustration in social situation thereby reducing risky elicited aggressive acts. Indeed, there is evidence in children that language impairment contributes to aggression because of frustrating communication (Girard et al. 2014).
Elicited aggression in healthy samples: issues of paradigm design
To study neural mechanisms of aggression, paradigms including the Taylor aggression paradigm, point subtraction aggression paradigm and violent video games are adopted in a laboratory setting. Surprisingly, the right postcentral gyrus (PoG) was the only region identified in the meta-analysis across these aggression paradigms. In our CCN analyses, this region showed a similar coactivation pattern as the MCC, RO and PrG seeds, suggesting that it might be involved in a common cognitive control network, while the functional characterization showed that the CCN of the PoG was associated with action preparation. Critically, our results suggested that the laboratory aggression paradigm might not capture completely what the paradigms intend to study. For example, the Taylor aggression paradigm aims at provoking a negative affect (e.g., anger) in participants and instigating aggressive acts for others’ punishment. It was expected that activation in limbic and prefrontal systems related to emotion and emotion regulation should take part during provocation. Such an inability is possibly caused by numerous inadequacies in these paradigms, such as external validity and experimental realism (Tedeschi and Quigley 1996). More recently, its methodological and analytic standardization were further criticized (Chester et al. 2018). These flexibilities eventually lead to inflation of false positive findings.
We would like to emphasize that none of the paradigms used in the elicited aggression studies elicited an actual act of violence. The need for standardized, often reductionist, paradigms that meet the technical requirements for functional imaging has often been discussed critically, especially when it comes to the study of complex behaviors, such as “aggression”. This is a pertinent problem in neuroimaging. Of note, our approach to synthesize findings across distinct paradigms yields a great advantage to adjust for effects that are associated with a specific paradigm rather than the process or domain that is aimed to be studied. Despite this advantage, we would like to point out that these factors need to be considered in the interpretation of original studies as well as meta-analyses.
The common neural signature in aggression
Generally, aggression can be further categorized into either a reactive (also impulsive or hostile) and proactive (also premediated or instrumental) subtype (e.g., Dodge and Coie 1987; Barratt et al. 1999; Liu 2004). The former is described as an impulsive response driven by a strong emotional arousal under a perceived threat or provocation, while the latter is described as a planned response driven by anticipation of reward. Real life aggression, however, tends to fall into a gray zone of this dichotomy. For example, a potential mass shooter plans for his/her revenge on the next day after he/she gets humiliated by peers. This example comprises elements of hostile emotions (anger out of humiliation) and deliberation which can be categorized as either subtypes of aggression. In fact, the dichotomy between reactive and proactive aggression was questioned by Bushman and Anderson (2001); they argued that a similar dichotomy, namely the automatic-controlled information processing dichotomy, can better describe the phenomenon with the emergence of modern cognitive theory. According to the General Aggression Model (Anderson and Bushman 2002; Allen et al. 2018), the (re)appraisal processes determine these subtypes of aggression. In other words, varying degrees of individual control can over their motivation and initiation of an action would result in largely different behavioral outcomes. The present meta-analysis aimed at examining the neural mechanism underlying a generic concept of aggression (i.e., above reactive or proactive aggression) and indeed there was a common disrupted adaptive cognitive control network across aggressive individuals with psychiatric diagnoses. Our findings offer potential to develop novel treatments to ameliorate aggressive symptoms in various psychiatric populations showing mixed forms of reactive and proactive aggression. Undeniably, aggression subtypes may involve domain-specific neural networks, i.e., emotion-related processing in reactive aggression and reward-related processing in proactive aggression. However, our literature search did not yield a clear boundary that helps us to categorize studies into various subtypes of aggression. Future studies could focus on the domain-specific neural mechanisms in different subtypes of aggression.
Methodological limitations
Our study has several limitations. First, while we tried our best to minimize heterogeneity across fMRI studies with strict inclusion criteria, the studies still varied on numerous variables including smoothing kernels, and analysis packages. These variables cannot be addressed separately due to methodological limitations; nevertheless ALE algorithm does estimate a spatial uncertainty per individual experiment to account for between-studies variability (Eickhoff et al. 2009). Second, included trait aggressive individuals (i.e., violent individuals with psychiatric diagnoses) varied on the demographical and medical background. However, we are looking for a least common denominator across heterogeneous individuals. Third, comparable to other meta-analyses, our study is limited by the quality and scope of the available literature and the publication bias preferring positive results. Particularly, the small samples in each neuroimaging study might fail to randomize inter-subject variability such as personality traits (Simon et al. 2010).
Conclusion
The present coordinate-based meta-analysis identified not only the convergent brain regions associated with aggression but also their derived neural networks from a data-driven approach based on healthy participants. We argue that escalated aggression arises from abnormal precuneus activities within the FPN and/or DMN. This abnormality may further disrupt the recruitment of other large-scale networks such as adaptive cognitive control network comprising MCC, RO, PrG and PoG, resulting in inabilities to generate adaptive responses when needed. Generally, these results support the importance of the (re-)appraisal process in general aggression model, while this abnormality may be linked to both reactive and proactive aggression.
Supplementary Material
Acknowledgements
This study is supported by Deutsche Forschun-gsgemeinschaft (DFG, International Research Training Group IRTG2150). SBE is supported by the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain” and the European Union’s Horizon 2020 Research and Innovation Program under Grant Agreement Bi. 7202070 (HBP SGA1).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00429-018-1765-3) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no existing conflict of interest.
References
- Alegria AA, Radua J, Rubia K (2016) Meta-analysis of fMRI studies of disruptive behavior disorders. Am J Psychiatry 173:1119–1130. 10.1176/appi.ajp.2016.15081089 [DOI] [PubMed] [Google Scholar]
- Allen JJ, Anderson CA, Bushman BJ (2018) The general aggression model. Curr Opin Psychol 19:75–80. 10.1016/j.copsyc.2017.03.034 [DOI] [PubMed] [Google Scholar]
- Amft M, Bzdok D, Laird AR et al. (2015) Definition and characterization of an extended social-affective default network. Brain Struct Funct 220:1031–1049. 10.1007/s00429-013-0698-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CA, Bushman BJ (2002) Human aggression. Annu Rev Psychol 53:27–51. 10.1146/annurev.psych.53.100901.135231 [DOI] [PubMed] [Google Scholar]
- Barratt ES, Stanford MS, Dowdy L et al. (1999) Impulsive and premeditated aggression: a factor analysis of self-reported acts. Psychiatry Res 86:163–173. 10.1016/S0165-1781(99)00024-4 [DOI] [PubMed] [Google Scholar]
- Blair RJR (2010) Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. Br J Psychol 101:383–399. 10.1348/000712609X418480 [DOI] [PubMed] [Google Scholar]
- Blair RJR (2016) The neurobiology of impulsive aggression. J Child Adolesc Psychopharmacol 26:4–9. 10.1089/cap.2015.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobes MA, Ostrosky F, Diaz K et al. (2013) Linkage of functional and structural anomalies in the left amygdala of reactive-aggressive men. Soc Cogn Affect Neurosci 8:928–936. 10.1093/scan/nss101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V (2010) Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 14:277–290. 10.1016/j.tics.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Bushman BJ, Anderson CA (2001) Is it time to pull the plug on the hostile versus instrumental aggression dichotomy? Psychol Rev 108:273–279. 10.1037///0033-295X.108.1.273 [DOI] [PubMed] [Google Scholar]
- Buss DM, Shackelford TK (1997) Human aggression in evolutionary psychological perspective. Clin Psychol Rev 17:605–619. 10.1016/S0272-7358(97)00037-8 [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C et al. (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14:365–376. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Bzdok D, Heeger A, Langner R et al. (2015) Subspecialization in the human posterior medial cortex. Neuroimage 106:55–71. 10.1016/j.neuroimage.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Cherek DR, Moeller FG, Dougherty DM, Rhoades H (1997) Studies of violent and nonviolent male parolees: I. Laboratory and psychometric measurements of aggression. Biol Psychiatry 41:514–522. 10.1016/S0006-3223(96)00059-5 [DOI] [PubMed] [Google Scholar]
- Chester DS, Lasko EN, Chester DS, House T (2018) Validating a standardized approach to the Taylor aggression paradigm. Soc Psychol Personal Sci. 10.1177/1948550618775408 [DOI] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Berman ME, Lish JD (1998) Intermittent explosive disorder-revised: development, reliability, and validity of research criteria. Compr Psychiatry 39:368–376. 10.1016/S0010-440X(98)90050-5 [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Sripada CS, Yanowitch RN, Phan KL (2011) Corticolimbic function in impulsive aggressive behavior. Biol Psychiatry 69:1153–1159. 10.1016/j.biopsych.2011.02.032 [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD et al. (2013) Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16:1348–1355. 10.1038/nn.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL (2000) Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science (80-) 289:591–594. 10.1126/science.289.5479.591 [DOI] [PubMed] [Google Scholar]
- Denson TF, DeWall CN, Finkel EJ (2012) Self-control and aggression. Curr Dir Psychol Sci 21:20–25. 10.1177/0963721411429451 [DOI] [Google Scholar]
- Diamond A (2013) Executive Functions. Annu Rev Psychol 64:135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Coie JD (1987) Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Personal Soc Psychol 53:1146–1158. 10.1037/0022-3514.53.6.1146 [DOI] [PubMed] [Google Scholar]
- Dolan MC (2010) What imaging tells us about violence in antisocial men. Crim Behav Ment Health 20:199–214. 10.1002/cbm.771 [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL et al. (2008) A dual-networks architecture of top–down control. Trends Cogn Sci 12:99–105. 10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM et al. (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci 104:11073–11078. 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas KS, Guy LS, Hart SD (2009) Psychosis as a risk factor for violence to others: a meta-analysis. Psychol Bull 135:679–706. 10.1037/a0016311 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR et al. (2012) Activation likelihood estimation meta-analysis revisited. Neuroimage 59:2349–2361. 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR et al. (2011) Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57:938–949. 10.1016/j.neuroimage.2011.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C et al. (2009) Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. 10.1002/hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H et al. (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. 10.1016/j.neuroimage.2004.12.034 [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL, Laird AR, Eickhoff SB (2014) Meta-analysis in human neuroimaging: computational modeling of large-scale databases. Annu Rev Neurosci 37:409–434. 10.1146/annurev-neuro-062012-170320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Clewett DV, Bennett CM et al. (2015) The posteromedial region of the default mode network shows attenuated task-induced deactivation in psychopathic prisoners. Neuropsychology 29:493–500. 10.1037/neu0000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard LC, Pingault JB, Falissard B et al. (2014) Physical aggression and language ability from 17 to 72 months: cross-lagged effects in a population sample. PLoS One. 10.1371/journal.pone.0112185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn AL, Raine A (2008) The neurobiology of psychopathy. Psychiatr Clin N Am 31:463–475. 10.1016/j.psc.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ et al. (2015) Identification of a common neurobiological substrate for mental Illness. JAMA Psychiatry 72:305–315. 10.1001/jamapsychiatry.2014.2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Reivich M et al. (2009) Regional differences in the coupling between resting cerebral blood flow and metabolism may indicate action preparedness as a default state. Cereb Cortex 19:375–382. 10.1093/cercor/bhn087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694. 10.1038/35094500 [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Trobst KK (2000) Frontal lobe dysfunction and aggression: conceptual issues and research findings. Aggress Violent Behav 5:147–157. 10.1016/S1359-1789(98)00033-0 [DOI] [Google Scholar]
- Hillman KL (2013) Cost-benefit analysis: the first real rule of fight club? Front Neurosci 7:248. 10.3389/fnins.2013.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoaken PNS, Shaughnessy VK, Pihl RO (2003) Executive cognitive functioning and aggression: is it an issue of impulsivity? Aggress Behav 29:15–30. 10.1002/ab.10023 [DOI] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S et al. (2014) The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp 35:2741–2753. 10.1002/hbm.22363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J, Cleveland WS, Mcgill R et al. (2016) Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 137:70–85. 10.1038/d41586-017-07522-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Antonius D, Kline N (2011) Neuroimaging correlates of aggression in schizophrenia: an update. Curr Opin Psychiatry 24:100–106. 10.1097/YCO.0b013e328342c8e0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal CC, Putkonen A, Mancini-Marïe A et al. (2007a) Violent persons with schizophrenia and comorbid disorders: a functional magnetic resonance imaging study. Schizophr Res 91:97–102. 10.1016/j.schres.2006.12.014 [DOI] [PubMed] [Google Scholar]
- Kalnin AJ, Edwards CR, Wang Y et al. (2011) The interacting role of media violence exposure and aggressive-disruptive behavior in adolescent brain activation during an emotional Stroop task. Psychiatry Res Neuroimaging 192:12–19. 10.1016/j.pscychresns.2010.11.005 [DOI] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M et al. (2014) Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. Neuroimage 87:345–355. 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Taylor P et al. (2006a) Neural dysfunction and violence in schizophrenia: an fMRI investigation. Schizophr Res 84:144–164. 10.1016/j.schres.2006.02.017 [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM et al. (2011) The Brain Map strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes 4:349. 10.1186/1756-0500-4-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M et al. (2007) Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 28:1194–1205. 10.1002/hbm.20345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TMC, Chan SC, Raine A (2009) Hyperresponsivity to threat stimuli in domestic violence offenders: a functional magnetic resonance imaging study. J Clin Psychiatry 70:36–45. 10.4088/JCP.08m04143 [DOI] [PubMed] [Google Scholar]
- Liu J (2004) Concept analysis: aggression. Issues Ment Health Nurs 25:693–714. 10.1080/01612840490486755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak K, Weber R (2006a) Toward brain correlates of natural behavior: fMRI during violent video games. Hum Brain Mapp 27:948–956. 10.1002/hbm.20234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM et al. (2017) Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry 174:676–685. 10.1176/appi.ajp.2017.16040400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK (2000) The prefrontal cortex and cognitive control. Nat Rev Neurosci 1:59–65. 10.1038/35036228 [DOI] [PubMed] [Google Scholar]
- Milton J, Amin S, Singh SP et al. (2001) Aggressive incidents in first-episode psychosis. Br J Psychiatry 178:433–440. 10.1192/bjp.178.5.433 [DOI] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M et al. (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1–9. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Serbanescu I et al. (2017) Altered brain activity in unipolar depression revisited. JAMA Psychiatry 74:47–55. 10.1001/jamapsychiatry.2016.2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Takeuchi H, Taki Y et al. (2017) The anterior midcingulate cortex as a neural node underlying hostility in young adults. Brain Struct Funct 222:61–70. 10.1007/s00429-016-1200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Rottschy C, Thommes J et al. (2015) Neural networks related to dysfunctional face processing in autism spectrum disorder. Brain Struct Funct 220:2355–2371. 10.1007/s00429-014-0791-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL et al. (2012) Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12:241–268. 10.3758/s13415-011-0083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera RL (2002) Intermittent explosive disorder: epidemiology, diagnosis and management. CNS Drugs 16:517–526. 10.2165/00023210-200216080-00002 [DOI] [PubMed] [Google Scholar]
- Paus T (2001) Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2:417–424. 10.1038/35077500 [DOI] [PubMed] [Google Scholar]
- Schiffer B, Müller BW, Scherbaum N et al. (2011) Disentangling structural brain alterations associated with violent behavior from those associated with substance use disorders. Arch Gen Psychiatry 68:1039–1049. 10.1001/archgenpsychiatry.2011.61 [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA et al. (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12:154–167. 10.1038/nrn2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick M, Cohen J (2013) The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79:217–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ (2008) Neurobiology of aggression and violence. Am J Psychiatry 165:429–442. 10.1176/appi.ajp.2008.07111774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ et al. (2010) Neural reward processing is modulated by approach- and avoidance-related personality traits. Neuroimage 49:1868–1874. 10.1016/j.neuroimage.2009.09.016 [DOI] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, Hankin BL (2015) Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol 6:328. 10.3389/fpsyg.2015.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Abraham K, Burgess A et al. (2017) Impulsivity and aggression mediate regional brain responses in borderline personality disorder: an fMRI study. Psychiatry Res Neuroimaging 260:76–85. 10.1016/j.pscychresns.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SP (1967) Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. J Personal 35:297–310. 10.1111/j.1467-6494.1967.tb01430.x [DOI] [PubMed] [Google Scholar]
- Tedeschi JT, Quigley BM (1996) Limitations of laboratory paradigms for studying aggression. Aggress Violent Behav 1:163–177. 10.1016/1359-1789(95)00014-3 [DOI] [Google Scholar]
- Thijssen S, Ringoot AP, Wildeboer A et al. (2015) Brain morphology of childhood aggressive behavior: a multi-informant study in school-age children. Cogn Affect Behav Neurosci 15:564–577. 10.3758/s13415-015-0344-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002) Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16:765–780. 10.1006/nimg.2002.1131 [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR et al. (2012) Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp 33:1–13. 10.1002/hbm.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA (2014) Precuneus is a functional core of the default-mode network. J Neurosci 34:932–940. 10.1523/JNEUROSCI.4227-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6:533–544. 10.1038/nrn1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EM (2012) Neuroimaging and neurocognitive correlates of aggression and violence in schizophrenia. Scientifica (Cairo) 2012:1–12. 10.6064/2012/158646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CR (2012a) Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage 59:3548–3562. 10.1016/j.neuroimage.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CR (2012b) Functional networks for cognitive control in a stop signal task: independent component analysis. Hum Brain Mapp 33:89–104. 10.1002/hbm.21197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Studies on Trait Aggression (TA)
- Barkataki I, Kumari V, Das M, Sumich A, Taylor P, Sharma T (2008) Neural correlates of deficient response inhibition in mentally disordered violent individuals. Behav Sci Law 26:51–64 [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL (2007) Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry 62:168–178 [DOI] [PubMed] [Google Scholar]
- Gan G, Preston-Campbell RN, Moeller SJ, Steinberg JL, Lane SD, Maloney T et al. (2016) Reward vs. retaliation-the role of the mesocorticolimbic salience network in human reactive aggression. Front Behav Neurosci 10:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S, Blair RJ, Ffytche D, Simmons A, Kumari V, Hodgins S, Blackwood N (2015) Punishment and psychopathy: a case-control functional MRI investigation of reinforcement learning in violent antisocial personality disordered men. Lancet Psychiatry 2:153–160 [DOI] [PubMed] [Google Scholar]
- Joyal CC, Putkonen A, Mancini-Mari’e A, Hodgins S, Kononen M, Boulay L et al. (2007b) Violent persons with schizophrenia and comorbid disorders: a functional magnetic resonance imaging study. Schizophr Res 91:97–102 [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Taylor P, Ffytche DH, Das M, Barkataki I et al. (2006b) Neural dysfunction and violence in schizophrenia: an fMRI investigation. Schizophr Res 84:144–164 [DOI] [PubMed] [Google Scholar]
- Kumari V, Das M, Taylor PJ, Barkataki I, Andrew C, Sumich A et al. (2009) Neural and behavioural responses to threat in men with a history of serious violence and schizophrenia or antisocial personality disorder. Schizophr Res 110:47–58 [DOI] [PubMed] [Google Scholar]
- McCloskey MS, Phan KL, Angstadt M, Fettich KC, Keedy S, Coccaro EF (2016) Amygdala hyperactivation to angry faces in intermittent explosive disorder. J Psychiatr Res 79:34–41 [DOI] [PubMed] [Google Scholar]
- Prehn K, Schlagenhauf F, Schulze L, Berger C, Vohs K, Fleischer M et al. (2013a) Neural correlates of risk taking in violent criminal offenders characterized by emotional hypo- and hyper-reactivity. Soc Neurosci 8:136–147 [DOI] [PubMed] [Google Scholar]
- Prehn K, Schulze L, Rossmann S, Berger C, Vohs K, Fleischer M et al. (2013b) Effects of emotional stimuli on working memory processes in male criminal offenders with borderline and antisocial personality disorder. World J Biol Psychiatry 14:71–78 [DOI] [PubMed] [Google Scholar]
- Schiffer B, Pawliczek C, Muruller B, Forsting M, Gizewski E, Leygraf N, Hodgins S (2014) Neural mechanisms underlying cognitive control of men with lifelong antisocial behavior. Psychiatry Res Neuroimaging 222:43–51 [DOI] [PubMed] [Google Scholar]
- Schiffer B, Pawliczek C, Müller BW, Wiltfang J, Brüne M, Forsting M et al. (2017) Neural mechanisms underlying affective theory of mind in violent antisocial personality disorder and/or schizophrenia. Schizophr Bull 43:1229–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikàsz A, Potvin S, Lungu O, Joyal CC, Hodgins S, Mendrek A, Dumais A (2016) Anterior cingulate hyperactivations during negative emotion processing among men with schizophrenia and a history of violent behavior. Neuropsychiatr Dis Treat 12:1397–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
Studies on Elicited Aggression (EA)
- Beyer F, Münte TF, Krämer UM (2014) Increased neural reactivity to socio-emotional stimuli links social exclusion and aggression. Biol Psychol 96:102–110 [DOI] [PubMed] [Google Scholar]
- Buades-Rotger M, Beyer F, Krämer UM (2017) Avoidant responses to interpersonal provocation are associated with increased amygdala and decreased mentalizing network activity. eNeuro 4:e0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester DS, DeWall CN (2016) The pleasure of revenge: retaliatory aggression arises from a neural imbalance toward reward. Soc Cogn Affect Neurosci 11:1173–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester DS, DeWall CN (2018a) Aggression is associated with greater subsequent alcohol consumption: a shared neural basis in the ventral striatum. Aggress Behav 44:285–293 [DOI] [PubMed] [Google Scholar]
- Chester DS, Dewall CN (2018b) Intimate partner violence perpetration corresponds to a dorsal-ventral gradient in medial PFC reactivity to interpersonal provocation. Soc Neurosci. 10.1080/17470919.2018.1430613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugman S, Schuhmann T (2013) Out of control: evidence for anterior insula involvement in motor impulsivity and reactive aggression. Soc Cogn Affect Neurosci 10:508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling F, Schuhmann T, Lobbestael J, Arntz A, Brugman S, Sack AT (2016) The role of the insular cortex in retaliation. PLoS One 11:e0152000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer UM, Jansma H, Tempelmann C, Munte TF (2007) Tit-for-tat: the neural basis of reactive aggression. Neuroimage 38:203–211 [DOI] [PubMed] [Google Scholar]
- Krämer UM, Riba J, Richter S, Münte TF (2011) An fMRI study on the role of serotonin in reactive agggression. PLoS One 6:e27668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak K, Weber R (2006b) Toward brain correlates of natural behavior: fMRI during violent video games. Hum Brain Mapp 27:948–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak KA, Klasen M, Weber R, Ackermann H, Shergill SS, Mathiak K (2011) Reward system and temporal pole contributions to affective evaluation during a first person shooter video game. BMC Neurosci 12:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repple J, Pawliczek CM, Voss B, Siegel S, Schneider F, Kohn N, Habel U (2017) From provocation to aggression: the neural network. BMC Neurosci 18:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibsted AP, da Cunha-Bang S, Carré JM, Hansen AE, Beliveau V, Knudsen GM, Fisher PM (2017) Aggression-related brain function assessed with the point subtraction aggression paradigm in fMRI. Aggress Behav 43:601–610 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


