Abstract
Objective
The study aimed to validate the original Caprini score and its modifications considering coronavirus disease (COVID-19) as a severe prothrombotic condition in patients admitted to the hospital.
Methods
The relevant data were extracted from the electronic medical records with an implemented Caprini score and were retrospectively evaluated. The score was calculated twice: by the physician on admission and by the investigator at discharge (death). The final assessment considered additional risk factors that occurred during inpatient treatment. Besides the original Caprini score (a version of 2005), the modified version added the elevation of D-dimer and specific scores for COVID-19 as follows: two points for asymptomatic, three points for symptomatic, and five points for symptomatic infection with positive D-dimer. Cases were evaluated retrospectively. The primary end point was symptomatic venous thromboembolism (VTE) detected during inpatient treatment and confirmed by appropriate imaging testing or autopsy. The secondary end points included those observed during hospitalization (admission to the intensive care unit, a requirement for invasive mechanical ventilation, death, bleeding), and those assessed at 6-month follow-up (symptomatic VTE, bleeding, death). The association of eight different versions of the Caprini score with VTE events was evaluated.
Results
A total of 168 patients (83 males and 85 females at the age of 58.3 ± 12.7 years) were admitted to the hospital between April 30 and May 29, 2020, and were discharged or died to the time of data analysis. The original Caprini score varied between 2 and 12 (5.4 ± 1.8) at the admission and between 2 and 15 (5.9 ± 2.5) at discharge or death. The maximal score was observed with modification including specific COVID-19 points of 5 to 20 (10.0 ± 3.0). Patients received prophylactic (enoxaparin 40 mg once daily: 2.4%), intermediate (enoxaparin 80 mg once daily: 76.8%), or therapeutic (enoxaparin 1 mg/kg twice daily: 20.8%) anticoagulation. Despite this, symptomatic VTE was detected in 11 (6.5%) inpatients. Of the 168 individuals, 28 (16.7%) admitted to the intensive care unit, 8 (4.8%) required invasive mechanical ventilation, and 8 (4.8%) died. Clinically relevant nonmajor bleeding was detected in two (1.2%) cases. The Caprini score of all eight versions demonstrated a significant association with inpatient VTE frequency. The highest predictability was observed for the original scale when assessed at discharge (death). Only symptomatic VTE was reported after discharge with a cumulative incidence of 7.1%. This did not affect the predictability of the Caprini score. Extended antithrombotic treatment was prescribed to 49 (29%) patients with a cumulative incidence of bleeding of 1.8% at 6 months.
Conclusions
The study identified a significant correlation between the Caprini score and the risk of VTE in patients with COVID-19. All models including specific COVID-19 scores showed equally high predictability, and use of the original Caprini score is appropriate for patients with COVID-19.
Keywords: COVID-19, Venous thromboembolism, Risk assessment, Caprini score, Prophylaxis
Article Highlights.
-
•
Type of Research: A single-center retrospective analysis of prospectively collected data
-
•
Key Findings: The original version of the Caprini score and its modifications considering the elevation of D-dimer and specific COVID-19 points demonstrated a significant association with venous thromboembolism (VTE) in 168 hospitalized patients with COVID-19 of whom 6.5% developed symptomatic VTE during inpatient treatment with a cumulative incidence of 7.1% at 6 months of follow-up.
-
•
Take Home Message: The Caprini score allows stratification of inpatients with COVID-19 according to their VTE risk and identification of subjects at the highest risk.
Coronavirus disease (COVID-19) is a highly infectious disease caused by the SARS-CoV-2 virus leading to severe pneumonia and acute respiratory distress syndrome as primary clinical symptoms. The infection first appeared in Wuhan, Hubei Province, China, in December of 2019 and then spread globally.1 A high prevalence of venous thromboembolism (VTE) including deep vein thrombosis (DVT), pulmonary embolism (PE), and pulmonary artery (PA) thrombosis among inpatients and critically ill patients has been reported since the beginning of the pandemic.2 Duplex ultrasound scan (DUS) detects the presence of DVT in 46% of the patients on the general ward and up to 79% of the patients in the intensive care unit (ICU).3 , 4 Proven PE using contrast computed tomography (CT) scan is observed in 30% of all patients with COVID-19.5 Evidence of small and mid-sized PA thrombosis and microthrombi in alveolar capillaries was found in most of the deceased patients in parallel with occlusion of proximal or large branches of the PA in 9% to 33% of dissections.6, 7, 8, 9, 10 Considering the high incidence of thrombotic complications in COVID-19, most guidelines suggest routine pharmacoprophylaxis using low-molecular-weight heparin (LMWH) or unfractionated heparin for patients admitted to the hospital.11, 12, 13, 14, 15 Some publications support the escalation of anticoagulation (intermediate to therapeutic doses of heparin) in subjects at individually highest risk for VTE including those critically ill, obese, or with high levels of D-dimer.11 , 13 , 14
The tools mentioned in these publications for VTE risk assessment include the Caprini score, Padua score, and IMPROVE VTE score.12, 13, 14 The Padua score was assessed without a clear correlation with VTE events.16, 17, 18 To the best of our knowledge, the IMPROVE VTE scores have not yet been tested in patients with COVID-19. Despite the Caprini score being the most validated risk assessment tool for VTE and being evaluated in approximately 5 million medical and surgical patients worldwide, it has not yet been tested in COVID-19.19, 20, 21 Rising evidence suggests that hypercoagulation markers such as increased D-dimer are a predictor for VTE, severe disease, and death.22 , 23 Increased D-dimer may be considered an acquired thrombophilia within the original Caprini model, and coronavirus infection may be a prothrombotic state by itself. Thus, there is no clear recommendation on using the Caprini score in this setting of novel infection.
The primary aim of this study was to validate the original Caprini score and its modified versions considering elevated D-dimer and COVID-19 as a severe prothrombotic condition in patients admitted to the hospital with SARS-CoV-2 infection. The secondary aim was to identify individuals at the highest risk for VTE via different versions of the Caprini score while selecting the most accurate version.
Methods
This study is a single-center retrospective analysis of prospectively collected data obtained from medical records of patients with COVID-19 admitted at the Clinical Hospital No. 1 (Volynskaya) of the President's Administration of the Russian Federation (Moscow, Russian Federation). The hospital stopped standard medical care and was reoriented for COVID-19 on April 30, 2020. Two years before, the Caprini score (a version of 2005 in Russian translation24) was integrated into the electronic medical records (EMRs) and became mandatory for assessment. All physicians were educated in the proper identification and collection of all relevant risk factors. The EMRs were extracted on May 30, 2020, by the time all patients had been discharged. The resulting database contained appropriate clinical data on every admitted patient focused on VTE. Patients were interviewed by phone with a standard questionnaire (Supplementary Table I, online only) 6 months after discharge. The interview was completed, and the data were updated by November 30, 2020.
The initial Caprini score was calculated by the physician at the time of admission. It did not consider risk factors related to inpatient treatment (bed rest for >72 hours, central venous catheter, acquired thrombophilia, etc.). Standard coagulation testing including the level of D-dimer, prothrombin time, and activated partial thromboplastin time was performed in all admitted patients but was not considered within the primary assessment of the Caprini score. The reason was that the blood test results were not known by the initial examiner at the time of admission. In Russia, the indication for inpatient treatment for adults at the time of observation was moderate-to-severe disease with a respiratory rate >22 per minute or SpO2 <93% with room air, mild illness in subjects over 65 years old with significant comorbidities (chronic heart failure, diabetes mellitus, chronic obstructive pulmonary disease), and pregnancy. We decided that all patients with moderate-to-severe illness and/or representing specific signs of pneumonia on a chest CT scan should receive one Caprini point for pneumonia at admission as well as one additional point for medical illness with bed rest (Table I ).
Table I.
The distribution of the individual risk factors for venous thromboembolism (VTE) according to the Caprini score
| Risk factor | Score | No. of patients (%) |
|---|---|---|
| Age 41-60 years | 1 | 75 (45) |
| Swollen legs | 1 | 32 (19) |
| Varicose veins | 1 | 32 (19) |
| Obesity (BMI >25) | 1 | 131 (78) |
| Minor surgery | 1 | 0 (0) |
| Sepsis (<1 month) | 1 | 72 (43) |
| Acute miocardial infarction | 1 | 0 (0) |
| Congestive heart failure (<1 month) | 1 | 24 (14) |
| Medical patient currently at bed rest | 1 | 168 (100) |
| History of inflammatory bowel disease | 1 | 4 (2) |
| History of prior major surgery (<1 month) | 1 | 0 (0.0) |
| Abnormal pulmonary function (COPD) | 1 | 8 (5) |
| Serious ling disease including pneumonia (<1 month) | 1 | 168 (100) |
| Oral contraceptives or hormone replacement therapy | 1 | 0 (0) |
| Pregnancy or postpartum (<1 month) | 1 | 0 (0) |
| History of unexplained stillborn infant, recurrent spontaneous abortion (≥3), premature birth with toxemia or growth-restricted infant | 1 | 1 (0.6) |
| Age 61-74 years | 2 | 61 (36) |
| Central venous accessa | 2 | 22 (13) |
| Arthroscopic surgery | 2 | 0 (0) |
| Major surgery (>45 minutes) | 2 | 0 (0) |
| Malignancy (present or previous) | 2 | 0 (0) |
| Laparoscopic surgery (>45 minutes) | 2 | 0 (0) |
| Patient confined to bed (>72 hours)a | 2 | 14 (8) |
| Immobilizing plaster cast (<1 month) | 2 | 0 (0) |
| COVID-19 asymptomaticb | 2 | 0 (0) |
| Age 75 years or older | 3 | 19 (11) |
| History of DVT/PE | 3 | 6 (4) |
| Family history of thrombosis | 3 | 0 (0) |
| Positive factor V Leiden | 3 | 0 (0) |
| Positive prothrombin 20210A | 3 | 0 (0) |
| Elevated serum homocysteine | 3 | 0 (0) |
| Heparin-induced thrombocytopenia | 3 | 0 (0) |
| Positive lupus anticoagulant | 3 | 0 (0) |
| Elevated anticardiolipin antibodies | 3 | 0 (0) |
| Other congenital or acquired thrombophilia: D-dimer >ULNb | 3 | 102 (61) |
| Other congenital or acquired thrombophilia: D-dimer >3 ULNb | 3 | 15 (9) |
| COVID-19 symptomaticb | 3 | 66 (39) |
| Stroke (<1 month) | 5 | 0 (0) |
| Multiple trauma (<1 month) | 5 | 0 (0) |
| Elective major lower extremity arthroplasty | 5 | 0 (0) |
| Hip, pelvis or leg fracture (<1 month) | 5 | 0 (0) |
| Acute spinal cord injury, paralysis (<1 month) | 5 | 0 (0) |
| COVID-19 symptomatic with positive D-dimerb | 5 | 102 (61) |
BMI, Body mass index; COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; PE, pulmonary embolism; ULN, upper limit or normal.
Evaluated at discharge or after death.
Evaluated within the modified versions of the Caprini score.
The final score was assessed by two investigators via EMR after patients' discharge or death. The additional risk factors related to inpatient treatment were also evaluated. Elevated D-dimer at admission was assessed as acquired thrombophilia from the section of three scores. We used a liberal or strict approach in D-dimer's assessment and achieved two different versions of the Caprini score. In the liberal version, we added an additional three points for any patient who had elevated D-dimer over the upper limit of normal (ULN: >0.55 mg/L) at the admission. Within the strict version, only patients with D-dimer >3 times ULN (>1.5 mg/L) received an additional three points. Because of the coronavirus pandemic, one of the authors (J.A.C.) proposed using a modified score for patients with COVID-19. It was developed empirically according to the published epidemiological data and was not supported by robust statistical calculations. This modification adds two points for COVID-19 patients with asymptomatic disease, three points for those with symptomatic illness, and five points for symptomatic patients with a positive D-dimer. This modification was also used for the retrospective analysis.
We calculated four different Caprini scores obtained at the admission and the discharge or death. We achieved eight versions of the scale: Caprini[orig:adm]—original score calculated at admission; Caprini[orig:fin]—original score calculated at discharge or death; Caprini[Dd>ULN:adm]—score with liberal D-dimer calculated at admission; Caprini[Dd>ULN:fin]—score with liberal D-dimer calculated at discharge or death; Caprini[Dd>3ULN:adm]—score with strict D-dimer calculated at admission; Caprini[Dd>3ULN:fin]—score with strict D-dimer calculated at discharge or death; Caprini[COVID-19:adm]—modified score calculated at admission; and Caprini[COVID-19:fin]—modified score calculated at discharge or death.
The standard prevention of VTE included prophylactic (enoxaparin 40 mg once daily) or intermediate (enoxaparin 80 mg once daily) doses of LMWH. The local protocol required intermediate doses of LMWH for all patients without increased bleeding risk. This intervention was suggested due to rising evidence of the high prevalence of VTE and PA thrombosis in patients with COVID-19. Although not all international guidelines support this approach, it was not contradictory to the interim Russian national recommendations. Only patients at high risk of bleeding were managed with the standard prophylactic doses of LMWH. Therapeutic anticoagulation (enoxaparin 1 mg/kg twice daily) was prescribed for subjects without confirmed VTE in case of a critical level of D-dimer (>6 times the ULN) or suspected thrombi in PA (eg, hypoxemia disproportionate to known respiratory pathologies or acute unexplained right ventricular dysfunction). The local protocol suggested empiric initiation of therapeutic anticoagulation in presumptive VTE with mandatory further verification by appropriate imaging tests before discharge. A DUS was used to assess superficial and deep veins of the lower and upper extremities as well as computed tomography pulmonary angiogram to exclude thrombi in the PA. If the patient died, then autopsy with an evaluation of lung vessels and veins of lower limbs was obligatory for the final VTE verification. No additional mechanical prophylaxis for VTE was required.
The primary end point was symptomatic VTE during inpatient treatment confirmed via appropriate imaging testing or autopsy. The secondary end points obtained in the hospital included admission to the ICU, a requirement for invasive mechanical ventilation, death, and a major or clinically relevant nonmajor (CRNM) bleeding as defined by the International Society of Thrombosis and Hemostasis.25 , 26 The secondary end points obtained after discharge included symptomatic VTE, major or CRNM bleeding, and overall mortality at 6 months' follow-up. All inpatient events were assessed in a retrospective manner by three investigators experienced in vascular surgery according to the data extracted from EMRs. After discharge, telephone interviews evaluated the outcomes with a standard questionnaire (Supplementary Table I, online only) by one investigator. The preliminary (calculated at admission) and final (calculated at discharge or death) Caprini scores were tested for correlation with inpatient VTE, and only the final score was used to test the association with VTE after discharge.
There was no need for ethical approval or a waiver of informed consent because the retrospective character of the analysis in light of the urgency of the pandemic was confirmed by the Institutional Review Board of Clinical Hospital No. 1 of the President's Administration of the Russian Federation.
Statistical analysis
All absolute values are represented by mean with standard deviation in the normal distribution or by a median with interquartile range (IQR) 25 to 75 percentile in the non-normal distribution assessed by the Kolmogorov-Smirnov test. The relative values are represented as percent with a 95% confidence interval (CI) calculated by Wilson. The data were compared using the paired t-test or analysis of variance (normal distribution) and the Mann-Whitney U test (non-normal distribution) or the χ 2 test (categorical variables). The correlation between the Caprini score and the incidence of VTE was evaluated by Cramer's V test. The predictability was assessed by a single-factor univariate logistic regression analysis represented by odds ratio and evaluation of receiver operating characteristic (ROC) curves with their areas under the curve (AUC). The cutoff for the Caprini score with current sensitivity and specificity was extracted from the coordinates of ROC curves. The analysis was performed using SPSS version 26 software package (IBM Corp, Armonk, NY). The value of P < .05 was considered statistically significant.
Results
A total of 168 patients with COVID-19 were admitted to the hospital between April 30 and May 29, 2020. At the time of data extraction, all subjects had been discharged or died, and every EMR was eligible for analysis. The number of males and females was equivalent (83 and 85, respectively). The age varied between 21 and 86 (mean, 58.3 ± 12.7) years. COVID-19 diagnosis was confirmed with polymerase chain reaction (PCR) on nasopharyngeal swabs in 140 patients (83%). The diagnosis was suspected in 28 (17%) subjects despite the negative result of PCR if a typical pattern of viral pneumonia was observed on chest CT scan (ground-glass opacity) as assessed by an experienced radiologist. This approach is consistent with the published data on the limited sensitivity of PCR on nasopharyngeal swabs (approximately 70%) and the high sensitivity of chest CT (over 90%).27 , 28 The local guidelines support a diagnosis of SARS-CoV-2 infection in PCR-negative patients who have a high clinical probability of disease and typical findings on chest CT.
According to the interim Russian national guidelines, the disease severity was classified as moderate (body temperature >38°C, respiratory rate >22 per min, dyspnea in physical activity, pneumonia by chest CT scan, SpO2 <95%, C-reactive protein >10 mg/mL) in 155 (92%) patients. Severe disease criteria (respiratory rate >30 per min, SpO2 ≤93%, PaO2/FiO2 ≤300 mm Hg, disease progression by chest CT scan, a decrease of consciousness or agitation, unstable hemodynamics, and serum lactate >2 mmol/L, quick sepsis related organ failure assessment >2) were found in 13 (8%) subjects. This classification was not contradictory with existing guidelines at the time of the study by the World Health Organization published in March 2020. Moderate disease is suggested as nonsevere pneumonia without the requirement for oxygen supplementation. Severe disease was suggested as respiratory infection with respiratory rate >30 per minute or severe respiratory distress or SpO2 ≤93% on room air.29
Time to admission from the onset of symptoms varied between 1 and 24 (mean, 7.2 ± 4.0) days consistent with previously published data on disease deterioration from days 5 to 8.30, 31, 32, 33 During this period, patients were treated at home with symptomatic therapy, chloroquine or hydroxychloroquine, and antibiotics. Antithrombotic treatment before hospitalization due to comorbidities was used by 37 (22.0%) patients. There were six individuals taking direct oral anticoagulants due to atrial fibrillation, and 31 subjects received antiplatelets due to cardiovascular diseases. All switched to LMWH according to the stated rules with cessation of previous therapy.
Prothrombin time at the admission ranged from 9.3 to 29.1 seconds (mean, 11.6 ± 1.9 seconds; normal range, 9.8-12.1 seconds). It was prolonged in nine (5%) patients. The activated partial thromboplastin time varied between 22.0 and 54.1 seconds (mean, 32.9 ± 5.0 seconds; normal range, 26.4-37.5 seconds), and it increased in 44 (26%) individuals. D-dimer varied from 0.1 to 6.7 mg/L (median: 0.6 mg/L, with an IQR of 0.4-1.0 mg/L; ULN is 0.55 mg/L). Any elevation of D-dimer was observed in 102 (61%) patients and elevation >3 times ULN in 15 (9%) individuals. We did not use the age-adjusted ULN for D-dimer similar to previous reports in patients with COVID-19.4 , 30, 31, 32 , 34, 35, 36, 37, 38
All patients received therapy with chloroquine or hydroxychloroquine, antibiotics, noninvasive and invasive oxygen therapy, symptomatic treatment, and prophylactic anticoagulation. All patients in the ICU received additional treatment with tocilizumab. No other specific treatment for COVID-19 was used. The LMWH was used in the standard prophylactic dose in four (2.4%) patients, in the intermediate dose in 129 (76.8%) patients, and in therapeutic doses in 35 (20.8%) patients. Of those on therapeutic anticoagulation, 29 patients had hypoxemia disproportional to known respiratory pathology (n = 15) or right ventricular dysfunction (n = 14) and were suspected with PA thrombosis (15 admitted to the ICU, 4 required mechanical ventilation). Six had a critical elevation of D-dimer. The duration of inpatient treatment varied from 1 to 29 days (mean, 13.9 ± 4.0 days).
The risk factor distribution by the Caprini score is shown in Table I. In the original scale, the score ranged from 2 to 12 (mean, 5.4 ± 1.8) as calculated at admission and between 2 and 15 (mean, 5.9 ± 2.5) as calculated at discharge or death. Among all modifications, the highest score was observed with Caprini[COVID-19] version followed by Caprini[Dd>ULN], Caprini[Dd>3ULN], and the original version. All scores significantly increased when they were measured at the end of hospitalization (Table II ).
Table II.
The value of the Caprini score according to the version and time of evaluation
| Version of the Caprini score | Time of evaluation |
P1 | |
|---|---|---|---|
| At admission | At discharge or death | ||
| Caprini[orig] | 5.4 ± 1.8 | 5.9 ± 2.5 | <.001 |
| Caprini[Dd>ULN] | 7.3 ± 2.6 | 7.7 ± 3.3 | <.001 |
| Caprini[Dd>3ULN] | 5.7 ± 2.1 | 6.1 ± 2.9 | <.001 |
| Caprini[COVID-19] | 9.6 ± 2.3 | 10.0 ± 3.0 | <.001 |
| P2 | <.001 | <.001 | – |
Caprini[COVID-19], Considering two points for asymptomatic infection; three points for symptomatic infection; five points for symptomatic infection with positive D-dimer; Caprini[Dd>3ULN], considering three points if D-dimer increased >3 times over ULN; Caprini[Dd>ULN], considering three points if D-dimer increased over the ULN; Caprini[orig], the original score; P1, paired t-test; P2, analysis of variance; ULN, upper limit of normal.
Symptomatic VTE was detected in 11 of 168 (6.5%; 95% CI, 3.7%-11.3%) patients including catheter-related vein thrombosis of upper limbs in three (1.8%; 95% CI, 0.6%-5.1%) cases; isolated lower limb DVT in two (1.2%; 95% CI, 0.3%-4.3%) cases; combination of lower limb DVT and PE in one (0.6%; 95% CI, 0.1%-3.3%) case, and isolated PE in five (3.0%; 95% CI, 1.3%-6.8%) cases. Of the three lower limb DVT cases, two affected the distal veins and one affected the proximal veins. Thrombosis of calf muscle veins was seen only in PE. Of the six PEs, five were fatal and confirmed by autopsy; the last one was confirmed by computed tomography pulmonary angiogram. All lesions affected segmental and subsegmental branches of the PA. All VTE events developed despite intermediate (n = 5) or therapeutic (n = 6) doses of LMWH with the incidence of 4.7% and 14.3%, respectively; no VTE event was observed in those who received prophylactic LMWH (P = .107, Supplementary Table II, online only). VTE was more frequently observed in critically ill patients admitted to the ICU (28.6%) compared with those on the ward (2.1%; P < .001). The original Caprini score at admission was significantly higher in patients who required treatment in the ICU (7.25 ± 1.90 vs 5.06 ± 1.49; P < .001) and predicted the admission to the ICU (odds ratio, 2.07; 95% CI, 1.55%-2.75%; P < .001).
Of the total, 28 (16.7%; 95% CI, 11.8%-23.1%) patients required admission to the ICU, 8 (4.8%; 95% CI, 2.5%-9.2%) patients required mechanical ventilation, and 8 (4.8%; 95% CI, 2.5%-9.2%) patients died in the hospital. Of eight individuals on mechanical ventilation, seven (88%) died. Pulmonary embolism was suggested as the underlying cause of death in two patients and as a contributing cause in three patients. Thus, five of eight deaths were associated with PE. The other immediate causes of death were acute respiratory insufficiency (n = 4) and acute cardiac-respiratory insufficiency (n = 2). Bleeding was detected in only two (1.2%; 95% CI, 0.3%-4.3%) subjects represented by subcutaneous hematoma requiring surgical drainage and uterine bleeding that was classified as CRNM bleeding. Both occurred while receiving a therapeutic and intermediate dose of LMWH, respectively.
In total, 151 of 168 (90%) patients were followed by a telephone interview at 6 months. Of those 17 who were not followed, 8 died in the hospital and 9 did not respond to the several phone calls that were performed weekly over 1 month. Extended antithrombotic treatment was prescribed to 49 (29%) patients including 13 (8%) with anticoagulants and 36 (21%) with antiplatelet agents. The treatment duration varied between 2 and 24 weeks (median: 12 weeks, with an IQR of 4-24 weeks) after discharge. Anticoagulation was usually stopped within 6 months after discharge, and only three patients with atrial fibrillation prolonged the therapy. In contrast, approximately half of patients continued treatment with antiplatelets due to comorbidities, and 19 subjects ceased it within 2 to 12 weeks. Thus, 20 of 37 individuals who had been using antithrombotic therapy before admission and survived resumed their treatment after discharge, and the last seven patients died in the hospital. The outpatient facilities were responsible for the extended pharmacological prophylaxis after discharge, and reliable indications could not be evaluated.
A new symptomatic VTE event was reported by only one patient who did not receive antithrombotic treatment and was diagnosed with distal DVT 1 week after discharge. The diagnosis was confirmed by DUS, and therapeutic anticoagulation was initiated. This 63-year-old woman had moderate disease and a score of 7 on the original Caprini model. She had increased D-dimer <3 UNL and received intermediate doses of LMWH in the hospital. Thus at 6 months, VTE events were observed in 12 of 168 patients with a cumulative incidence of 7.1% (95% CI, 4.1%-12.0%). The only episode of CRNM bleeding due to hemorrhoids was reported by one gentleman who received acetylsalicylic acid with a cumulative incidence of 1.8% (95% CI, 0.6%-5.1%). No new death was registered, and the overall mortality rate did not change at 6 months (4.8%; 95% CI, 2.5%-9.2%).
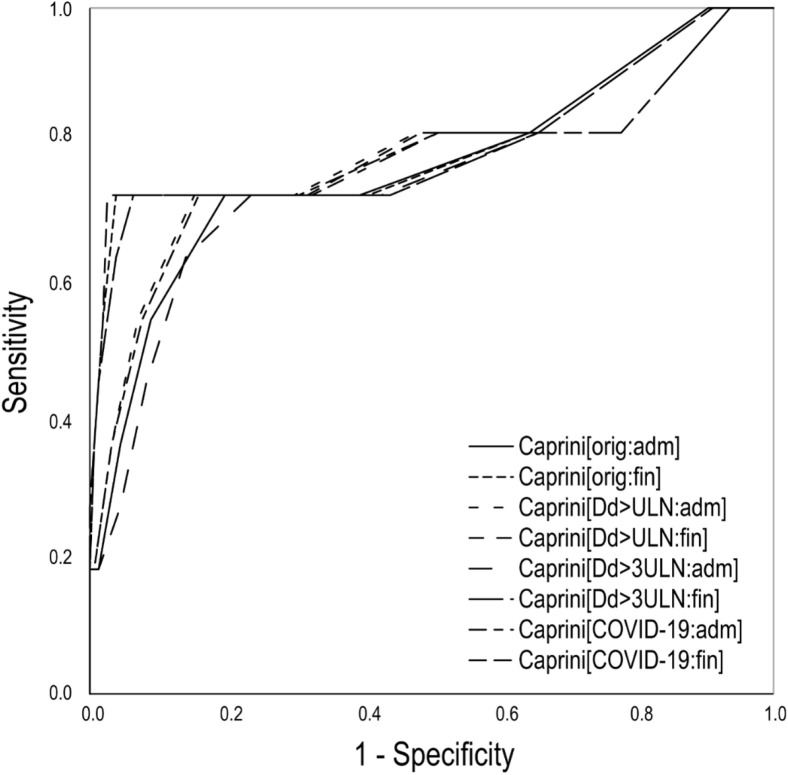
A statistically significant association with symptomatic VTE detected during inpatient treatment was observed for all versions of the Caprini score calculated at admission or discharge (P < .001, Figs 1 and 2 ). The high predictability was confirmed by the single-factor logistic regression analysis (Supplementary Table III, online only) and the analysis of ROC curves (Table III ; Supplementary Fig 1, online only). Of the scores calculated on admission, the highest predictability was for the AUC and was found for the version considering any elevation of D-dimer (Caprini[Dd>ULN:adm]). A score of ≥10 with a sensitivity of 73% and specificity of 85% predicted the occurrence of symptomatic VTE. However, the standard version (Caprini[orig:adm]) demonstrated high predictability with a sensitivity of 73% and a specificity of 80% with a score of ≥7 predicting VTE. Among the final scores, the best predictability was observed for the original version of the scale (Caprini[orig:fin]) with a sensitivity of 73% and a specificity of 96% with a score of ≥11 predicting VTE. The second highest predictability was observed for the version considering any elevation of D-dimer (Caprini[Dd>ULN:fin]) with a sensitivity of 73% and a specificity of 97% with a score of ≥14 predicting VTE. Generally, improved predictability and higher specificity were observed for the final Caprini score. In the subgroup of ICU patients, the same tendency with slightly improved predictability was registered (Supplementary Tables IV and V, online only).
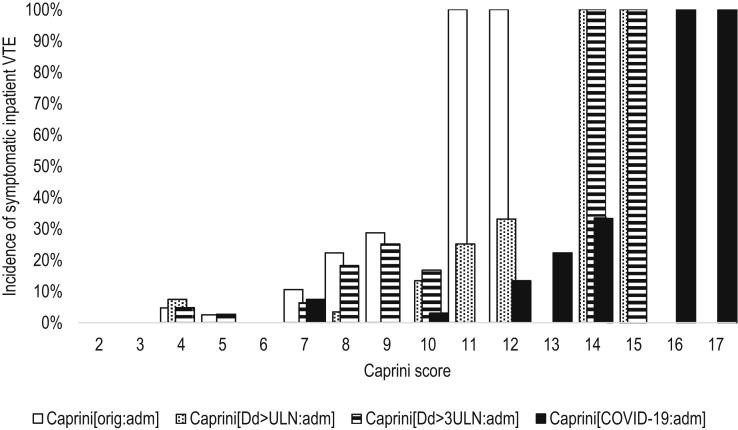
Fig 1.
The incidence of symptomatic inpatient venous thromboembolism (VTE) according to the different modifications of the Caprini score assessed at the time of admission. Caprini[COVID-19:adm]: Considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at admission (V = 0.525; P < .001); Caprini[Dd>3ULN:adm]: considering three points if D-dimer increased >3 times over ULN, score assessed at admission (V = 0.493; P < .001); Caprini[Dd>ULN:adm]: considering three points if D-dimer increased over ULN, score assessed at admission (V = 0.531; P < .001); Caprini[orig:adm]: the original score assessed at admission (V = 0.505; P < .001); ULN: upper limit of normal; V: Cramer's V.
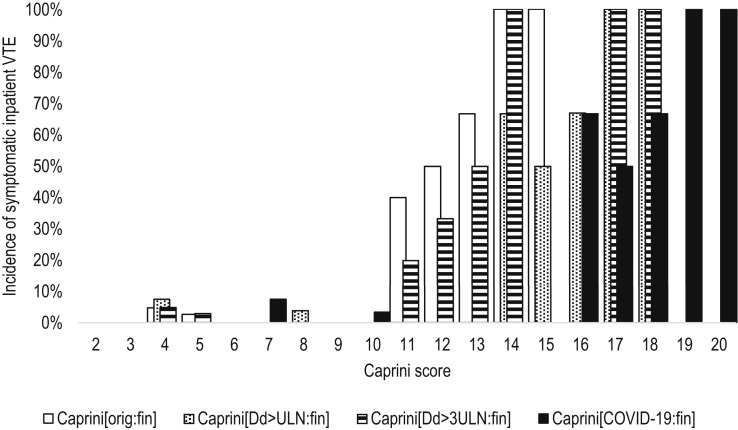
Fig 2.
The incidence of symptomatic inpatient venous thromboembolism (VTE) according to the different modifications of the Caprini score assessed at the time of discharge or death. Caprini[COVID-19:fin]: Considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at discharge or death (V = 0.706; P < .001); Caprini[Dd>3ULN:fin]: considering three points if D-dimer increased >3 times over ULN, score assessed at discharge or death (V = 0.645; P < .001); Caprini[Dd>ULN:fin]: considering three points if D-dimer increased over ULN, score assessed at discharge or death (V = 0.707; P < .001); Caprini[orig:fin]: the original score assessed at discharge or death (V = 0.664; P < .001); ULN: upper limit of normal; V: Cramer's V.
Table III.
Predictability of different versions of the Caprini score for symptomatic venous thromboembolism (VTE) in patients with COVID-19 by the analysis of receiver operating characteristic (ROC) curve
| Version of the Caprini score | AUC ± SD | P | Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Symptomatic VTE during inpatient treatment | |||||
| Caprini[orig:adm] | 0.769 ± 0.090 | .003 | 7 | 73 | 80 |
| Caprini[Dd>ULN:adm] | 0.776 ± 0.096 | .002 | 10 | 73 | 85 |
| Caprini[Dd>3ULN:adm] | 0.757 ± 0.090 | .004 | 7 | 73 | 76 |
| Caprini[COVID-19:adm] | 0.772 ± 0.097 | .003 | 12 | 73 | 84 |
| Caprini[orig:fin] | 0.803 ± 0.095 | .001 | 11 | 73 | 96 |
| Caprini[Dd>ULN:fin] | 0.801 ± 0.100 | .001 | 14 | 73 | 97 |
| Caprini[Dd>3ULN:fin] | 0.797 ± 0.096 | .001 | 11 | 73 | 94 |
| Caprini[COVID-19:fin] | 0.799 ± 0.100 | .001 | 16 | 73 | 97 |
| Symptomatic VTE at 6 months | |||||
| Caprini[orig:fin] | 0.805 ± 0.087 | <.001 | 7 | 75 | 78 |
| Caprini[Dd>ULN:fin] | 0.804 ± 0.092 | <.001 | 10 | 75 | 81 |
| Caprini[Dd>3ULN:fin] | 0.796 ± 0.880 | .001 | 7 | 75 | 75 |
| Caprini[COVID-19:fin] | 0.803 ± 0.092 | <.001 | 12 | 75 | 81 |
AUC, Area under the curve; Caprini[COVID-19:adm], considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at admission; Caprini[COVID-19:fin], considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at discharge or death; Caprini[Dd>3ULN:adm], considering three points if D-dimer increased >3 times over ULN, score assessed at admission; Caprini[Dd>3ULN:fin], considering three points if D-dimer increased >3 times over ULN, score assessed at discharge or death; Caprini[Dd>ULN:adm], considering three points if D-dimer increased over ULN, score assessed at admission; Caprini[Dd>ULN:fin], considering three points if D-dimer increased over ULN, score assessed at discharge or death; Caprini[orig:adm], the original score assessed at admission; Caprini[orig:fin], the original score assessed at discharge or death; SD, standard deviation; ULN, upper limit of normal.
Supplementary Fig 1 (online only).
Predictability of different versions of the Caprini score for symptomatic venous thromboembolism (VTE) detected during inpatient treatment by the receiver operating characteristic (ROC) curves. Caprini[COVID-19:adm], Considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at admission; Caprini[COVID-19:fin], considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at discharge or death; Caprini[Dd>3ULN:adm], considering three points if D-dimer increased >3 times over ULN, score assessed at admission; Caprini[Dd>3ULN:fin], considering three points if D-dimer increased >3 times over ULN, score assessed at discharge or death; Caprini[Dd>ULN:adm], considering three points if D-dimer increased over ULN, score assessed at admission; Caprini[Dd>ULN:fin], considering three points if D-dimer increased over ULN, score assessed at discharge or death; Caprini[orig:adm], the original score assessed at admission; Caprini[orig:fin], the original score assessed at discharge or death; ULN, upper limit of normal.
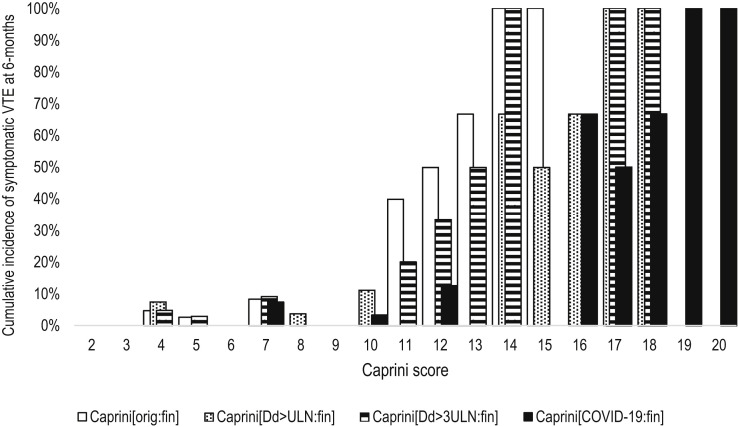
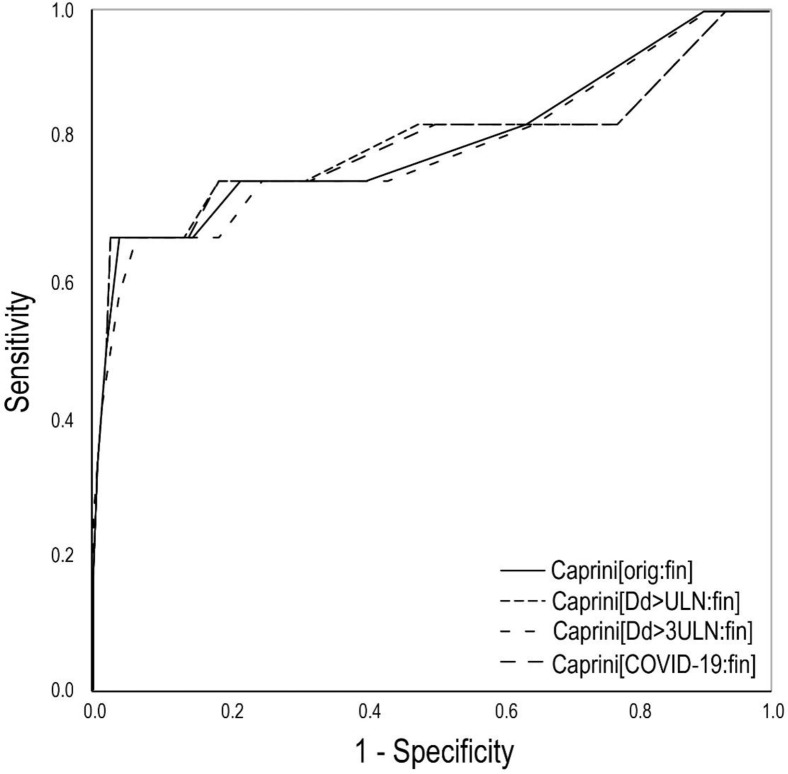
Only one new symptomatic VTE event was detected at 6 months of follow-up; this subject did not significantly affect the predictability of all versions of the final Caprini score (Fig 3 and Table III; Supplementary Table III and Supplementary Fig 2, online only). However, the areas under the ROC curves slightly increased for most versions parallel with decreased cutoff scores and specificity. The best characteristics were obtained for the version Caprini[orig:fin]. At a score of ≥7, it had a sensitivity of 75% and a specificity of 78% predicting symptomatic VTE at 6 months.
Fig 3.
The cumulative incidence of symptomatic venous thromboembolism (VTE) at 6 months according to the different modifications of the Caprini score assessed at the time of discharge or death. Caprini[orig:fin]: The original score assessed at discharge or death (V = 0.634; P < .001); Caprini[Dd>ULN:fin]: considering three points if D-dimer increased over ULN, score assessed at discharge or death (V = 0.677; P < .001); Caprini[Dd>3ULN:fin]: considering three points if D-dimer increased >3 times over ULN, score assessed at discharge or death (V = 0.616; P < .001); Caprini[COVID-19:fin]: considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at discharge or death (V = 0.678; P < .001); ULN: upper limit of normal; V: Cramer's V.
Supplementary Fig 2 (online only).
Predictability of different versions of the Caprini score for symptomatic venous thromboembolism (VTE) detected 6 months after discharge by the receiver operating characteristic (ROC) curves. Caprini[COVID-19:fin], Considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at discharge or death; Caprini[Dd>3ULN:fin], considering three points if D-dimer increased >3 times over ULN, score assessed at discharge or death; Caprini[Dd>ULN:fin], considering three points if D-dimer increased over ULN, score assessed at discharge or death; Caprini[orig:fin], the original score assessed at discharge or death; ULN, upper limit of normal.
Discussion
The high prevalence of VTE in hospitalized patients with COVID-19 requires validation for specific predictive tools to identify subjects at the highest risk who may benefit from escalated pharmacological prophylaxis. The American Venous Forum has already recommended using the Caprini score for VTE risk assessment at admission and discharge.13 They referenced the original model and suggested a score of ≥8 to escalate the pharmacological prophylaxis in the hospital and extend it after discharge. In the current study, we compared the COVID-19 modifications including theoretical changes based on the D-dimer level with the original scale. The results showed that an original score of ≥7 calculated at admission as well as a score of ≥11 calculated at discharge is associated with the increased risk of inpatient VTE despite the administration of LMWH (predominantly intermediate doses). Such patients are the highest-risk group that requires special attention. The question arises if therapeutic doses of heparin may be beneficial in these patients.
The importance of elevated D-dimer levels in COVID-19 was introduced recently. It appears to be associated with unfavorable outcomes, increased mortality, and VTE.22 , 23 The thresholds for VTE verification were found at 1.0 to 2.3 mg/L.4 , 5 , 38 Prophylactic doses of LMWH have increased survival in patients with the D-dimer level >6 times ULN.39 The American Venous Forum proposed therapeutic anticoagulation for subjects with the D-dimer level >3 times ULN.13 However, in our study, the suggested level of D-dimer supplementing the original scale did not improve the predictability of the original Caprini score. No significant difference in D-dimer level was found between patients with and without VTE: median of 0.96 (IQR, 0.62-1.75) mg/L compared with 0.64 (0.39-0.95) mg/L, respectively (P = .094). At the same time, any D-dimer level over the ULN improved the predictability and specificity of the original score when calculated at admission but not at discharge. In general, all evaluated models demonstrated good predictability without significant differences. However, the fact of higher AUC and specificity of the final scores highlights the need for repeated assessment and consideration of new risk factors during hospitalization.
Any change in the original Caprini score affects the predictive threshold for the highest-risk group, which could vary by four to five points. Thus, the prognosis for VTE and detection of patients requiring escalated prophylaxis and/or additional imaging tests for VTE should be based on a specific threshold of every modification (Table III).
Interestingly, the incidence of VTE and mortality rate in our study appeared to be lower than previously reported for the general ward.2 , 4 , 40 , 41 This fact may be related to the predominance of moderate disease and the use of intermediate to therapeutic doses of LMWH in 97.6% of all patients. There is limited evidence that escalated anticoagulation can improve survival in patients with COVID-19.42 , 43 Also, anti-inflammatory properties and potential antiviral effects of heparins may be relevant.44, 45, 46 However, there was no difference in the crude incidence of VTE between subgroups not balanced for the severity of infection, comorbidities, or other cofounders who received prophylactic, intermediate, or therapeutic anticoagulation in the current study. The risk-benefit ratio of therapeutic anticoagulation should be confirmed in robust randomized clinical trials.
Despite the challenging clinical benefits of Caprini score assessment and routine pharmacological prophylaxis in stable medically ill patients, we decided to combine individuals admitted to the ward and ICU.19 The reason is that the VTE incidence in patients with COVID-19 on the ward is much higher than in other medical patients and reaches the values for critically ill patients.2 , 47 , 48 Moreover, admission to the ICU is associated with additional VTE risk factors considered by the Caprini score (confined to the bed >72 hours, central venous access, sepsis) and requires repeated recalculations. The predictability of all versions of the Caprini score in a subgroup of ICU patients demonstrated a slight improvement compared with the overall population (Supplementary Tables IV and V, online only). However, the original score assessed at admission did not consider ICU-related risk factors or laboratory findings and accurately predicted VTE. Although most VTE events occurred in the ICU, most of these patients were initially admitted to the ward and had a higher original score at baseline.
Regardless of the lack of extended pharmacological prophylaxis prescribed in only one-third of patients, there was no further increase in the VTE rate after discharge as expected. Our observation confirms the previously published data reporting that postdischarge VTE risk in COVID-19 does not differ from other acute medical illness and does not require routine pharmacological prophylaxis.49 , 50 Moreover, the predictability of the final Caprini score did not change significantly when it was assessed in relation to symptomatic VTE detected at 6 months.
The limitations of the study include its retrospective character, small sample size, absence of the total instrumental screening for VTE, the predominance of moderate disease, the inconsistency of pharmacological prophylaxis with various doses of LMWH and duration, and an empirical suggestion for the novel COVID-19 scores that were not supported by reliable statistical calculations. The follow-up data were collected via telephone interviews to reduce the contacts during pandemics. Despite these disadvantages, the results may be useful to improve VTE risk stratification in patients with COVID-19.
Conclusions
The study identified a significant correlation between the Caprini score and the risk of VTE in patients with COVID-19. A score of ≥7 predicts symptomatic VTE despite pharmacological prophylaxis. The original Caprini score is essential and identifies patients with COVID-19 who are at risk. Further studies are needed to determine if there are any clinical benefits using the modified COVID-19 Caprini score.
Author contributions
Conception and design: SZ, VB, KL
Analysis and interpretation: KL, JC
Data collection: ST, IS, VB
Writing the article: KL, JC
Critical revision of the article: ST, IS, SZ, VB, KL, JC
Final approval of the article: ST, IS, SZ, VB, KL, JC
Statistical analysis: KL
Obtained funding: Not applicable
Overall responsibility: KL
Footnotes
Author conflict of interest: none.
Additional material for this article may be found online at www.jvsvenous.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvsvenous.org.
Appendix (online only).
Supplementary Table I (online only).
The standard questionnaire for a telephone interview performed at 6 months after discharge (translation from Russian)
| Question | Comment |
|---|---|
| After you had been discharged from the hospital with COVID-19, have you been repeatedly admitted to the hospital? | If yes, please provide the medical documentation on repeated hospitalization by e-mail |
| After you had been discharged from the hospital with COVID-19, have you been diagnosed with deep vein thrombosis, superficial vein thrombosis, pulmonary embolism, any other kind of thrombosis? | If yes, please provide the medical documentation on this event by e-mail |
| After you had been discharged from the hospital with COVID-19, have you continued, restarted, or been newly prescribed with any blood thinners? | If yes, please provide the medical prescription of such drugs by e-mail. |
| Do you use it now, or have you used it after discharge from the hospital with COVID-19 such drugs as heparin, low-molecular-weight heparin, enoxaparin, nadroparin, dalteparin, bemiparin, parnaparin, rivaroxaban, apixaban, dabigatran, warfarin, acetylsalicylic acid, clopidogrel, ticagrelor, prasugrel, dipyridamole, and sulodexide? | If yes, please provide the medical prescription of such drugs, doses, and duration by e-mail If you are not sure, please provide the list of all medications you have been taking after discharge |
| After you had been discharged from the hospital with COVID-19, have you been suffering from shortness of breath, chest pain, cough, blood in sputum, loss of consciousness, leg pain, leg swelling and redness, tender masses on the leg? | If yes, you need to contact your physician immediately. Please provide his conclusion by e-mail |
| After you had been discharged from the hospital with COVID-19, have you been suffered from any kind of bleeding? | If yes, have it lead to special medical attention? Have you undergone specific medical interventions for diagnosis or treatment? Have you been admitted to the hospital? If so, please provide medical documentation related to this bleeding event by e-mail |
Supplementary Table II (online only).
The number of COVID-19 patients with symptomatic venous thromboembolism (VTE) according to the anticoagulation regimen and admission to intensive care unit (ICU)
| Ward | Transferred to ICU | Admitted to ICU | Totally | |
|---|---|---|---|---|
| Prophylactic LMWH | 0/4 (0) | 0/0 | 0/0 | 0/4 (0) |
| Intermediate LMWH | 3/116 (2.6) | 3/12 (25) | 0/1 (0) | 6/129 (4.7) |
| Therapeutic LMWH | 0/20 (0) | 3/10 (30) | 2/5 (40) | 5/35 (14.3) |
| Totally | 3/140 (2.1) | 6/22 (27.3) | 2/6 (33.3) | 11/168 (6.5) |
The figures represent the number of patients with confirmed symptomatic VTE divided by the number of patients within a specific subgroup (percentage). Totally 140 patients were admitted to the ward and never transferred to the ICU; 22 patients were admitted to the ward and further transferred to the ICU due to deterioration; 6 patients were admitted initially to the ward with severe disease. Intermediate LMWH: Subcutaneous enoxaparin 80 mg once daily; Prophylactic low-molecular-weight heparin (LMWH): subcutaneous enoxaparin 40 mg once daily; Therapeutic LMWH: subcutaneous enoxaparin 1 mg/kg twice daily.
Supplementary Table III (online only).
Predictability of different versions of the Caprini score for symptomatic venous thromboembolism (VTE) in patients with COVID-19 by the single-factor logistic regression
| Version of the Caprini score | OR | 95% CI | P |
|---|---|---|---|
| Symptomatic VTE during inpatient treatment | |||
| Caprini[orig:adm] | 1.87 | 1.34-2.61 | <.001 |
| Caprini[Dd>ULN:adm] | 1.63 | 1.23-2.17 | .001 |
| Caprini[Dd>3ULN:adm] | 1.59 | 1.24-2.05 | <.001 |
| Caprini[COVID-19:adm] | 1.73 | 1.27-2.34 | <.001 |
| Caprini[orig:fin] | 1.73 | 1.36-2.20 | <.001 |
| Caprini[Dd>ULN:fin] | 1.58 | 1.28-1.95 | <.001 |
| Caprini[Dd>3ULN:fin] | 1.61 | 1.30-2.00 | <.001 |
| Caprini[COVID-19:fin] | 1.63 | 1.31-2.03 | <.001 |
| Symptomatic VTE at 6 months | |||
| Caprini[orig:fin] | 1.68 | 1.34-2.10 | <.001 |
| Caprini[Dd>ULN:fin] | 1.55 | 1.27-1.89 | <.001 |
| Caprini[Dd>3ULN:fin] | 1.56 | 1.28-1.91 | <.001 |
| Caprini[COVID-19:fin] | 1.60 | 1.30-1.96 | <.001 |
Caprini[COVID-19:adm]: Considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at admission; Caprini[COVID-19:fin]: considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at discharge or death; Caprini[Dd>3ULN:adm]: considering three points if D-dimer increased >3 times over ULN, score assessed at admission; Caprini[Dd>3ULN:fin]: considering three points if D-dimer increased >3 times over ULN, score assessed at discharge or death; Caprini[Dd>ULN:adm]: considering three points if D-dimer increased over ULN, score assessed at admission; Caprini[Dd>ULN:fin]: considering three points if D-dimer increased over ULN, score assessed at discharge or death; Caprini[orig:adm]: the original score assessed at admission; Caprini[orig:fin]: the original score assessed at discharge or death; CI: confidence interval; OR: odds ratio; ULN: upper limit of normal.
Supplementary Table IV (online only).
Predictability of different versions of the Caprini score for symptomatic venous thromboembolism (VTE) in patients with COVID-19 admitted or transferred to the intensive care unit (ICU) (n = 28) by the single-factor logistic regression
| Version of the Caprini score | OR | 95% CI | P |
|---|---|---|---|
| Symptomatic VTE during inpatient treatment | |||
| Caprini[orig:adm] | 2.30 | 1.16-4.53 | .017 |
| Caprini[Dd>ULN:adm] | 2.50 | 1.20-5.24 | .015 |
| Caprini[Dd>3ULN:adm] | 1.98 | 1.06-3.70 | .033 |
| Caprini[COVID-19:adm] | 2.52 | 1.19-5.32 | .015 |
| Caprini[orig:fin] | 3.30 | 1.20-9.06 | .021 |
| Caprini[Dd>ULN:fin] | 3.10 | 1.23-7.80 | .016 |
| Caprini[Dd>3ULN:fin] | 2.93 | 1.05-8.15 | .040 |
| Caprini[COVID-19:fin] | 3.18 | 1.26-8.05 | .015 |
Caprini[COVID-19:adm]: Considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at admission; Caprini[COVID-19:fin]: considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at discharge or death; Caprini[Dd>3ULN:adm]: considering three points if D-dimer increased >3 times over ULN, score assessed at admission; Caprini[Dd>3ULN:fin]: considering three points if D-dimer increased >3 times over ULN, score assessed at discharge or death; Caprini[Dd>ULN:adm]: considering three points if D-dimer increased over ULN, score assessed at admission; Caprini[Dd>ULN:fin]: considering three points if D-dimer increased over ULN, score assessed at discharge or death; Caprini[orig:adm]: the original score assessed at admission; Caprini[orig:fin]: the original score assessed at discharge or death; CI: confidence interval; OR: odds ratio; ULN: upper limit of normal.
Supplementary Table V (online only).
Predictability of different versions of the Caprini score for symptomatic venous thromboembolism (VTE) in patients with COVID-19 admitted or transferred to the intensive care unit (ICU) (n = 28) by the analysis of receiver operating characteristic (ROC) curve
| Version of the Caprini score | AUC ± SD | P | Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Symptomatic VTE during inpatient treatment | |||||
| Caprini[orig:adm] | 0.844 ± 0.074 | .005 | 7 | 75 | 80 |
| Caprini[Dd>ULN:adm] | 0.881 ± 0.064 | .002 | 10 | 75 | 85 |
| Caprini[Dd>3ULN:adm] | 0.816 ± 0.082 | .010 | 7 | 88 | 65 |
| Caprini[COVID-19:adm] | 0.875 ± 0.066 | .002 | 12 | 75 | 85 |
| Caprini[orig:fin] | 0.913 ± 0.053 | .001 | 11 | 75 | 85 |
| Caprini[Dd>ULN:fin] | 0.925 ± 0.048 | .001 | 14 | 75 | 85 |
| Caprini[Dd>3ULN:fin] | 0.903 ± 0.057 | .001 | 11 | 88 | 75 |
| Caprini[COVID-19:fin] | 0.925 ± 0.048 | .001 | 16 | 75 | 85 |
AUC, Area under the curve; Caprini[COVID-19:adm], considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at admission; Caprini[COVID-19:fin], considering two points for asymptomatic infection, three points for symptomatic infection, five points for symptomatic infection with positive D-dimer, score assessed at discharge or death; Caprini[Dd>3ULN:adm], considering three points if D-dimer increased >3 times over ULN, score assessed at admission; Caprini[Dd>3ULN:fin], considering three points if D-dimer increased >3 times over ULN, score assessed at discharge or death; Caprini[Dd>ULN:adm], considering three points if D-dimer increased over ULN, score assessed at admission; Caprini[Dd>ULN:fin], considering three points if D-dimer increased over ULN, score assessed at discharge or death; Caprini[orig:adm], the original score assessed at admission; Caprini[orig:fin], the original score assessed at discharge or death; SD, standard deviation; ULN, upper limit of normal.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiménez D., García-Sanchez A., Rali P., Muriel A., Bikdeli B., Ruiz-Artacho P. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159:1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nahum J., Morichau-Beauchant T., Daviaud F., Echegut P., Fichet J., Maillet J.-M. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19) JAMA Network Open. 2020;3:e2010478. doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142:114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 5.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020;296:E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center,clinicopathologic case series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carsana L., Sonzogni A., Nasr A., Rossi R., Pellegrinelli A., Zerbi P. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. medRxiv. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198-209 doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wichmann D., Sperhake J.P., Lutgehetmann M., Steurer S., Edler C., Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75 doi: 10.1016/j.jacc.2020.04.031. 2950-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The COVID-19 Sub-Committee of the American Venous Forum Considerations in prophylaxis and treatment of VTE in COVID-19 patients; 2020. https://www.veinforum.org/wp-content/uploads/2020/04/COVID-19-White-Paper-04-17-2020-FINAL-1.pdf Available at:
- 14.Oudkerk M., Buller H.R., Kuijpers D., van Es N., Oudkerk S.F., McLoud T.C. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297:E216–E222. doi: 10.1148/radiol.2020201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health COVID-19 treatment guidelines panel. Coronavirus disease 2019 (COVID-19) treatment guidelines; 2020. https://www.covid19treatmentguidelines.nih.gov/ Available at: [PubMed]
- 16.Xu J.-F., Wang L., Zhao L., Li F., Liu J., Zhang L. Risk assessment of venous thromboembolism and bleeding in COVID-19 patients. Respir Res. 2020 doi: 10.1111/crj.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T., Chen R., Liu C., Liang W., Guan W., Tang R. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng D.X., Xu J.L., Mao Q.X., Liu R., Zhang W.Y., Qian H.Y. Association of Padua prediction score with in-hospital prognosis in COVID-19 patients. QJM. 2020;113:789–793. doi: 10.1093/qjmed/hcaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant P.J., Greene M.T., Chopra V., Bernstein S.J., Hofer T.P., Flanders S.A. Assessing the Caprini score for risk assessment of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129:528–535. doi: 10.1016/j.amjmed.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pannucci C.J., Swistun L., MacDonald J.K., Henke P.K., Brooke B.S. Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094–1103. doi: 10.1097/SLA.0000000000002126. [DOI] [PubMed] [Google Scholar]
- 21.Hanh B.M., Cuong L.Q., Son N.T., Duc D.T., Hung T.T., Hung D.D. Determination of risk factors for venous thromboembolism by an adapted Caprini scoring system in surgical patients. J Pers Med. 2019;9:36. doi: 10.3390/jpm9030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gungor B., Atici A., Baycan O.F., Alici G., Ozturk F., Tugrul S. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: a systematic review and meta-analysis. Am J Emerg Med. 2021;39:173–179. doi: 10.1016/j.ajem.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nopp S., Moik F., Jilma B., Pabinger I., Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;4:1178–1191. doi: 10.1002/rth2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobastov K., Barinov V., Schastlivtsev I., Laberko L., Rodoman G., Boyarintsev V. Validation of the Caprini risk assessment model for venous thromboembolism in high-risk surgical patients in the background of standard prophylaxis. J Vasc Surg Venous Lymphat Disord. 2016;4:153–160. doi: 10.1016/j.jvsv.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Kaatz S., Ahmad D., Spyropoulos A.C., Schulman S., Subcommittee on Control of Anticoagulation Definition of clinically relevant nonmajor bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in nonsurgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–2126. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 26.Schulman S., Angeras U., Bergqvist D., Eriksson B., Lassen M.R., Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8:202–204. doi: 10.1111/j.1538-7836.2009.03678.x. [DOI] [PubMed] [Google Scholar]
- 27.Böger B., Fachi M.M., Vilhena R.O., Cobre A.F., Tonin F.S., Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am J Infect Control. 2021;49:21–29. doi: 10.1016/j.ajic.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383:e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization . World Health Organization; 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. [Google Scholar]
- 30.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han H., Yang L., Liu R., Liu F., Wu K.L., Li J. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 35.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Lu X., Chen H., Chen T., Su N., Huang F. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paranjpe I., Fuster V., Lala A., Russak A., Glicksberg B.S., Levin M.A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nadkarni G.N., Lala A., Bagiella E., Chang H.L., Moreno P.R., Pujadas E. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Downing L.J., Strieter R.M., Kadell A.M., Wilke C.A., Greenfield L.J., Wakefield T.W. Low-dose low-molecular-weight heparin is anti-inflammatory during venous thrombosis. J Vasc Surg. 1998;28:848–854. doi: 10.1016/s0741-5214(98)70060-6. [DOI] [PubMed] [Google Scholar]
- 45.Vicenzi E., Canducci F., Pinna D., Mancini N., Carletti S., Lazzarin A. Coronaviridae and SARS-associated coronavirus strain HSR1. Emerg Infect Dis. 2004;10:413–418. doi: 10.3201/eid1003.030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindahl U., Li J.-P. Heparin—an old drug with multiple potential targets in Covid-19 therapy. J Thromb Haemost. 2020;18:2422–2424. doi: 10.1111/jth.14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minet C., Potton L., Bonadona A., Hamidfar-Roy R., Somohano C.A., Lugosi M. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care. 2015;19:287. doi: 10.1186/s13054-015-1003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gafter-Gvili A., Drozdinsky G., Zusman O., Kushnir S., Leibovici L. Venous thromboembolism prophylaxis in acute medically ill patients: a retrospective cohort study. Am J Med. 2020;133:1444–1452.e3. doi: 10.1016/j.amjmed.2020.04.026. [DOI] [PubMed] [Google Scholar]
- 49.Roberts L.N., Whyte M.B., Georgiou L., Giron G., Czuprynska J., Rea C. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136:1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patell R., Bogue T., Koshy A., Bindal P., Merrill M., Aird W.C. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]