SUMMARY:
Tositumomab and iodine I 131 tositumomab (Bexaar) therapeutic regimen targets monoclonal antibodies against the CD20 antigen expressed in non-Hodgkin lymphoma. This article reviews the mechanism of action and clinical indications for this regimen.
Tositumomab is a US Food and Drug Administration−approved murine immunoglobulin G2a λ monoclonal antibody directed against the CD20 antigen, which is a transmembrane phosphoprotein expressed on pre-B-lymphocytes and mature B-lymphocytes.1–3 The CD20 antigen is also expressed on >90% of B-cell non-Hodgkin lymphomas. The Bexaar therapeutic regimen (tositumomab and iodine I 131 tositumomab) is an antineoplastic radioimmunotherapeutic monoclonal antibody−based regimen composed of the monoclonal antibody, tositumomab, and the radio-labeled monoclonal antibody, iodine I 131 tositumomab.4–6 Once bound to the target cells, Bexxar delivers radiation, which enhances the killing effect of the antibody. Normal B-cells will recover in 6–9 months because the parent B-cells do not have the CD20 receptor.
Proposed Mechanism of Action
Multiple mechanisms of action have been proposed for tumor killing by the Bexaar regimen,4–8 which include the following: 1) apoptosis, 2) complement-dependent cytotoxicity, 3) antibody-dependent cellular cytotoxicity, and 4) ionizing radiation from the radioisotope. In addition, a potential vaccine-like effect leading to adaptive immunity against cells that survive initial treatment is also suggested.
Clinical Indications
The Bexaar therapeutic regimen is intended as a single course of treatment and is indicated for the treatment of patients with CD20 antigen-expressing relapsed or refractory, low-grade, follicular, or transformed non-Hodgkin lymphoma, including patients with rituximab-refractory non-Hodgkin lymphoma (Figs 1 and 2).4,7,9 Determination of the effectiveness of the therapeutic regimen is based on overall response rates in patients whose disease is refractory to chemotherapy alone or to chemotherapy and rituximab. The therapeutic regimen may also help improve long-term survival.9 This regimen is not indicated for the initial treatment of patients with CD20-positive non-Hodgkin lymphoma.4,7,9–11
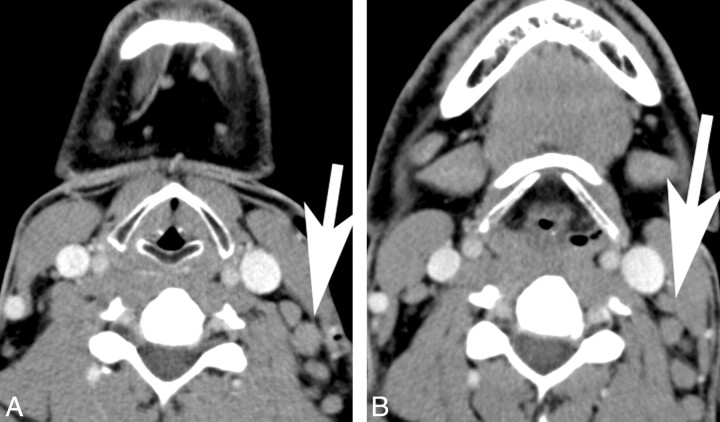
Fig 1.
Contrast-enhanced CT scans at the level of the cricoid cartilage (A) and hyoid bone (B) in a 56-year-old male patient with rituximab-refractory non-Hodgkins lymphoma demonstrate enlarged level III/IV and level II lymph nodes (arrows).
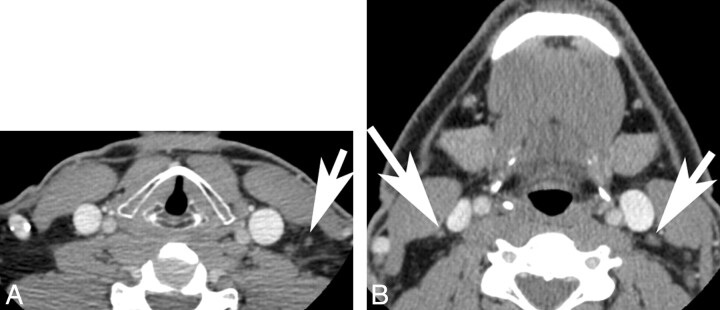
Fig 2.
A and B, Subsequent to Bexaar therapeutic regimen, the follow-up contrast-enhanced CT images show significant reduction in the size of these lymph nodes (arrows).
To our knowledge, the safety of multiple courses of the Bexaar therapeutic regimen or a combination of this regimen with other forms of irradiation or chemotherapy has not been evaluated.
Administration and Effects
The Bexaar therapeutic regimen is administered in 2 discrete steps: the dosimetric and therapeutic steps.9,12,13 The dosimetric step involves only an infusion of tositumomab. The therapeutic step is performed 7–14 days later, involving administration of an infusion of tositumomab followed by an additional smaller dose of tositumomab that is attached to the radioactive isotope iodine 131. This step delivers approximately 75 cGy of radiation to the body.
Adverse Effects
The most serious adverse reactions observed have been severe and prolonged cytopenias and the sequelae of cytopenias, which included infections (sepsis) and hemorrhage in patients with thrombocytopenia, allergic reactions (bronchospasm and angioedema), secondary leukemia, and myelodysplasia.9,12,13 Less common but severe adverse reactions are pneumonia, pleural effusion, and dehydration.
Economic Issues
The Bexaar therapeutic regimen is expensive and may cost approximately $25,000 per patient. The regimen is covered under Medicare.
Footnotes
Disclosures: Suresh K. Mukherji, Consultant: Philips Healthcare.
References
- 1. Rutar FJ, Augustine SC, Kaminski MS, et al. Feasibility and safety of outpatient Bexxar therapy (tositumomab and iodine I 131 tositumomab) for non-Hodgkin's lymphoma based on radiation doses to family members. Clin Lymphoma 2001;2:164–72 [DOI] [PubMed] [Google Scholar]
- 2. Kaminski MS, Zelenetz AD, Press OW, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol 2001;19:3918–28 [DOI] [PubMed] [Google Scholar]
- 3. Kaminski MS, Estes J, Zasadny KR, et al. Radioimmunotherapy with iodine (131) I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood 2000;96:1259–66 [PubMed] [Google Scholar]
- 4. Davies AJ. A review of tositumomab and I (131) tositumomab radioimmunotherapy for the treatment of follicular lymphoma. Expert Opin Biol Ther 2005;5:577–88 [DOI] [PubMed] [Google Scholar]
- 5. Kaminski MS, Tuck M, Estes J, et al. 131 I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med 2005;352:441–49 [DOI] [PubMed] [Google Scholar]
- 6. Davies AJ. Tositumomab and iodine [131I] tositumomab in the management of follicular lymphoma: an oncologist's view. Q J Nucl Med Mol Imaging 2004;48:305–16 [PubMed] [Google Scholar]
- 7. Kaminski MS, Radford JA, Gregory SA, et al. Re-treatment with I-131 tositumomab in patients with non-Hodgkin's lymphoma who had previously responded to I-131 tositumomab. J Clin Oncol 2005;23:7985–93 [DOI] [PubMed] [Google Scholar]
- 8. Davies AJ, Rohatiner AZ, Howell S, et al. Tositumomab and iodine I 131 tositumomab for recurrent indolent and transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol 2004;22:1469–79 [DOI] [PubMed] [Google Scholar]
- 9. Kaminski M. Bexxar, iodine I 131 tositumomab, effective in long-term follow-up of non-Hodgkin's lymphoma. Cancer Biol Ther 2007;6:996–97 [PubMed] [Google Scholar]
- 10. Smith S, Sweetenham JW. Iodine 131 tositumomab in the treatment of non-Hodgkin's lymphoma. Future Oncol 2007;3:255–62 [DOI] [PubMed] [Google Scholar]
- 11. Dosik AD, Coleman M, Kostakoglu L, et al. Subsequent therapy can be administered after tositumomab and iodine I-131 tositumomab for non-Hodgkin lymphoma. Cancer 2006;106:616–22 [DOI] [PubMed] [Google Scholar]
- 12. William BM, Bierman PJ. I-131 tositumomab. Expert Opin Biol Ther 2010;10:1271–78 [DOI] [PubMed] [Google Scholar]
- 13. Cheung MC, Maceachern JA, Haynes AE, et al. I-tositumomab in lymphoma. Curr Oncol 2009;16:32–47 [DOI] [PMC free article] [PubMed] [Google Scholar]