SUMMARY:
Although hydrocephalus associated with NF-1 is not rare, up to now the MR imaging findings in these patients and the role of ETV in the treatment of hydrocephalus associated with NF-1 have not been investigated thoroughly. We present the MR imaging findings of hydrocephalus associated with NF-1 in 7 of 54 patients with NF-1. Although the types of obstruction were various, including aqueductal web, superior velum medullary synechia, periaqueductal/tectal hamartomas, cerebellar and pontine tegmentum hamartomas, brain stem glioma, or a combination, the presence of hamartomas was a consistent finding in patients with NF-1 with hydrocephalus. In 5 cases, 8 ETV procedures were performed and followed for up to 53 months. All children treated with ETV were shunt-free at their most recent examinations. ETV may be the primary procedure for the treatment of hydrocephalus associated with NF-1, regardless of the cause and the level of the obstruction.
NF-1 is a tumor disorder that is caused by the malfunction of a gene on chromosome 17 that is responsible for control of cell division.1,2 The cranial manifestations are rich and usually include glial tumors of the central nervous system, primarily optic pathway gliomas and astrocytomas.3 Other CNS manifestations of NF-1 are the so-called “hamartomas” or focal areas of high signal intensity on T2-weighted images.1 Reports of the coexistence of hydrocephalus and NF-1 have already been published.4–16 However, the publications were mostly case reports and only a few study cohorts. Furthermore, ETV as a treatment of choice in patients with NF-1 and hydrocephalus has rarely been reported.17 The aim of this study was to evaluate the MR imaging characteristics of hydrocephalus in NF-1 and to assess the effectiveness of ETV in the treatment of hydrocephalus.
Materials and Methods
Patients
Fifty-four consecutive patients with NF-1 who underwent cranial MR imaging at our hospital between January 2005 and May 2010 were enrolled in the study. The criteria of the National Institutes of Health were used for the diagnosis of NF-1 in all patients.18 We analyzed patients' clinical records and initial and follow-up MR imaging findings. The internal review board of our hospital approved the study. The coexistence of hydrocephalus and NF-1 was observed in 7 patients. Five of 7 were treated with ETV and followed for ≤54 months. Altogether 8 ETV procedures were performed in the 5 patients. The sixth patient was being treated with chemotherapy due to an optic pathway glioma, and surgical intervention was postponed. In the seventh patient, a shunt had been inserted at another institution and brain stem surgery was performed in our hospital.
MR Imaging Technique
All patients were examined with a 3T scanner by using an 8-channel head coil (Trio; Siemens, Erlangen, Germany). Patients were examined under sedation and had completed a conventional study including axial TSE T1, axial fluid-attenuated inversion recovery, and axial/sagittal/coronal TSE T2. If the conventional MR imaging sequences demonstrated hydrocephalus, 3D-CISS followed by sagittal and oblique axial cine PC was performed at the same session. If necessary, axial TSE T1 and sagittal 3D turbo fast low-angle shot T1 after gadolinium chelates administration were performed as well. Our imaging paradigm and sequence parameters for hydrocephalus are described in detail elsewhere.19,20
MR Imaging Analysis
For each patient, conventional sequences, sagittal 3D CISS, and sagittal and axial-oblique cine PC imaging were used to assess the standard criteria for hydrocephalus diagnosis. If triventricular hydrocephalus was observed, particular attention was paid to the cerebral aqueduct, brain stem, cerebellum, and fourth ventricle. The pathologic findings in the cerebral aqueduct and neighboring structures in patients with NF-1 with obstructive triventricular hydrocephalus were classified as follows:
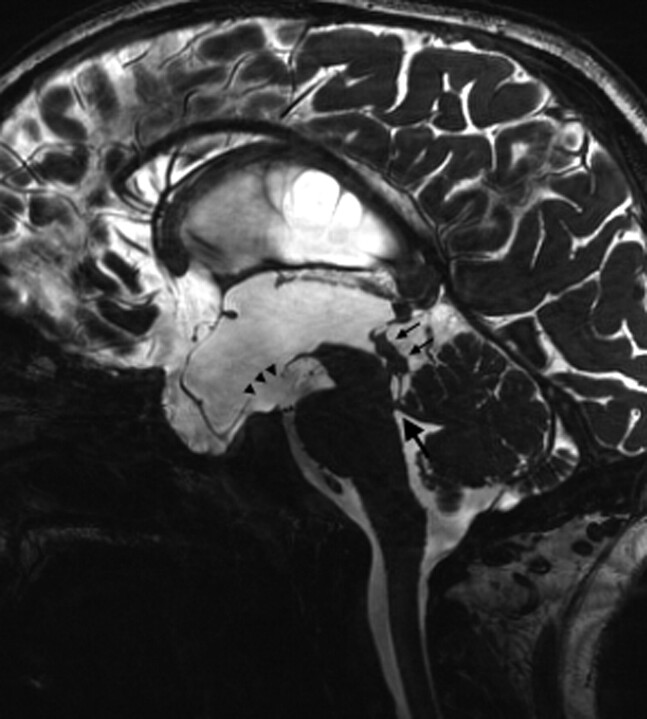
Fig 1.
Midsagittal 3D-CISS image of a 12-year-old boy with NF-1 reveals periaqueductal/tectal hamartomas (thin arrows), superior velum medullary synechia (thick arrow), and the stoma at the floor of the third ventricle (arrowheads) at 3-month follow-up after ETV.
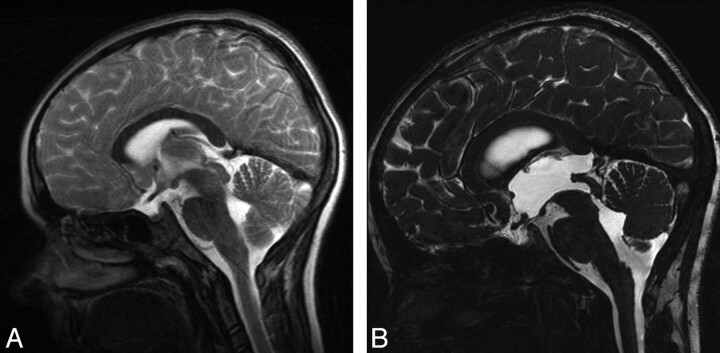
Fig 2.
A, Midsagittal TSE T2 image of a 9-year-old boy with NF-1 shows minimal T2 hyperintensity and slight enlargement in the tectum without causing hydrocephalus. B, Twenty months later, midsagittal 3D-CISS image demonstrates an inferior aqueductal web in addition to hamartomas in the tectum causing severe triventricular hydrocephalus.
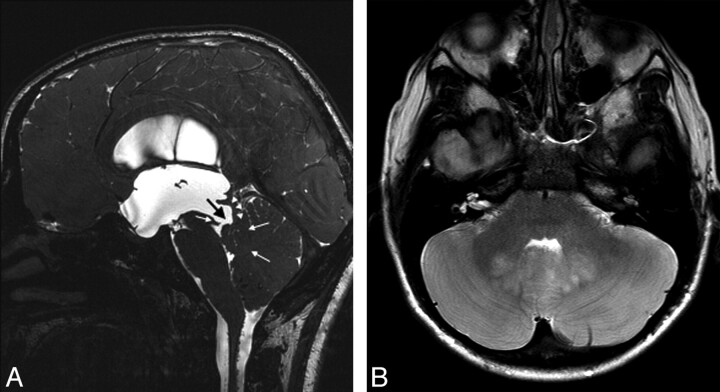
Fig 3.
A, Midsagittal 3D-CISS image of a 10-year-old boy with NF-1 demonstrates severe triventricular hydrocephalus. The cerebral aqueduct is wide open (black arrow). The tectum (white arrowheads) is thin and displaced posteriorly. However, the fourth ventricle inlet is obliterated due to hamartomas filling the ventricle (white arrows). B, Axial TSE T2 image obtained through the midbrain reveals extensive cerebellar hamartomas mostly in the cerebellar vermis.
ETV technique
All ETV procedures were performed by a senior neurosurgeon (M.M.Ö). The patient was placed in a supine position, and the head was elevated to 20°-30°. After a burr-hole was created just in front of the coronal suture on the midpupillary line, the dura was opened and a rigid sheath was introduced into the frontal horn of the lateral ventricle. The endoscope was passed into the frontal horn and then through the foramen of Monro. After the floor of the third ventricle was punctured by using a blunt probe at the tuber cinereum midway between the infundibular recess and the intermamillary point, the fenestrated site was enlarged by a Fogarty catheter; and finally, the prepontine space was explored for cisternal membranes. If there was an obstructing cisternal membrane, it was also fenestrated as described above. When the procedure was complete, the endoscope was withdrawn slowly, exploring the ventricles. A piece of Gelfoam (Phadia, Uppsala, Sweden) was placed in the burr-hole, and the scalp was closed in standard fashion.
Results
A total of 7 patients (mean age, 9.7 years; range, 4–12 years) were diagnosed as having triventricular obstructive hydrocephalus with NF-1. In all patients, there were either periaqueductal/tectal hamartomas or hamartomas in the cerebellum and pontine tegmentum filling the fourth ventricle. The types of obstruction were various, including periaqueductal/tectal plate hamartomas (5 patients), hamartomas in the cerebellum and pontine tegmentum filling the fourth ventricle (2 patients), inferior aqueductal web (1 patient), superior velum medullary synechia (1 patient), and brain stem glioma (2 patients). In 4 patients, there were multiple lesions causing obstruction. In 2 patients, in addition to hamartomas, there were an inferior aqueductal web and a superior velum medullary synechia. In 1 patient, mesencephalic pilocytic astrocytoma and periaqueductal hamartomas were both responsible for hydrocephalus. In another patient, a brain stem glioma accompanied hamartomas in the cerebellum and pontine tegmentum, filling the fourth ventricle. The radiologic diagnosis suggested a brain stem glioma due to tiny contrast enhancements in the anterior and anterolateral part of the pons. However, biopsy taken from the wall of the fourth ventricle during the ETV procedure demonstrated no sign of tumor. Furthermore, brain stem MR imaging findings have not changed in ≤53 months since the initial diagnosis.
In 1 patient, cranial MR imaging performed 20 months before the onset of hydrocephalus symptoms demonstrated a normal cerebral aqueduct and ventricles with periaqueductal hamartomas, but without obstructive hydrocephalus (Fig 2A). Follow-up MR imaging at the beginning of clinical symptoms demonstrated an inferior aqueductal web, a tectal hamartoma, and severe triventricular hydrocephalus (Figs 2B).
ETV was performed as a primary procedure for the treatment of hydrocephalus in 5 patients. In 3 patients, follow-up MR imaging demonstrated an open functional stoma at 32, 42, and 53 months. In 1 patient, 27-month follow-up MR imaging demonstrated cisternal membranous obstruction in the interpeduncular cistern, which was not observed on the previous MR imaging, and repeat ETV confirmed the diagnosis. After the second ETV, 15-month follow-up MR imaging demonstrated an open and functional stoma without clinical symptoms. In another patient, 23-month follow-up MR imaging revealed closure of the stoma, and a second ETV was performed. Twenty months after the second ETV, MR imaging demonstrated repeat stoma obliteration. After the third ETV, the patient was well at 17-month follow-up. All children who were treated with ETV were shunt-free at their most recent follow-up examinations.
In 1 patient, hydrocephalus was mild, despite a huge optic pathway glioma. The patient was still being treated with chemotherapy, and ETV was planned after reduction in tumor size in case ventricular enlargement progressed. In this case, a mesencephalic pilocytic astrocytoma with extensive periaqueductal/tectal hamartomas, which compressed the cerebral aqueduct, was diagnosed and treated with a shunt in another hospital. The tumor was removed by a transcallosal approach by using intraoperative MR imaging guidance. Twenty-four-hour MR imaging demonstrated gross total tumor resection. Although the mass effect on the cerebral aqueduct caused by the mesencephalic mass had disappeared, obstruction of the cerebral aqueduct still persisted due to extensive periaqueductal/tectal hamartomas.
Discussion
Hamartomas or focal areas of high signal intensity can be seen in ≤93% of patients with NF-1.1,3,21,22 They tend to show T2 hyperintensity but no mass effect, edema, or contrast enhancement. The brain stem, cerebellar peduncles, and basal ganglia are the most preferred locations. The exact nature of hamartomas is not clear. Although the morphologic criteria are often diagnostic in making a differentiation between hamartomas and brain stem glioma, some cases can present diagnostic difficulties and MR spectroscopy can provide additional information.1,22 However, the range of NF-1 lesions, from hamartomas to low-grade glial tumors, is not clearly understood and has not been investigated adequately, to our knowledge. Also, the clinical significance and the biologic behavior of hamartomas are yet to be clarified; at least we know that hamartomas or focal areas of signal intensity adjacent to the cerebral aqueduct or the fourth ventricle inlet cause hydrocephalus in the course of NF-1.
Hydrocephalus in patients with NF-1 is not a very rare coexistence, and an incidence from 1% to 5% has been published as in the literature.11–14 In the past, the main cause for hydrocephalus associated with NF-1 was reported as posterior fossa tumors.13 This finding can be attributed to the early diagnosis of tumors before hydrocephalus could even occur in patients with NF-1. The first report of aqueductal stenosis in NF-1 was made by Pennybacker in 1940.23 However, the reports gradually increased in the era of modern imaging, but most were case reports. Almost always periaqueductal/tectal hamartomas were demonstrated in these cases, and the term “primary aqueductal stenosis” was used. Rarely, brain stem glioma or tectal glioma was demonstrated in these patients.24,25 In only 1 case has an aqueductal web been shown.26 However, superior velum medullary synechia or hamartomas in the cerebellum and pontine tegmentum filling the fourth ventricle have never been described to date in the literature in patients with hydrocephalus and NF-1. Almost always the shunt insertion was performed as a primary procedure for the treatment of hydrocephalus associated with NF-1.11–16 ETV was performed in only 1 patient with hydrocephalus and NF-1.17
Our study demonstrates that the coexistence of hydrocephalus and NF-1 may not be as rare as previously stated in the literature. Although the types of obstruction are various, including aqueductal web, superior velum medullary synechia, periaqueductal/tectal hamartomas, cerebellar and pontine tegmentum hamartomas, brain stem glioma, or a combination, the presence of hamartomas either in the periaqueductal area or the fourth ventricle inlet is a consistent finding. Therefore, in the presence of brain stem or cerebellar hamartomas adjacent to the cerebral aqueduct or the forth ventricle inlet but in the absence of hydrocephalus, patients should be followed up at regular intervals for ≤12 years due to the proliferative nature of hamartomas in NF-1.21
Although ETV has been used effectively in the treatment of hydrocephalus due to various etiologies, the application of it in patients with NF-1 with hydrocephalus has been very rarely published, to our knowledge. Our study demonstrates that ETV can be used as a primary procedure for the treatment of hydrocephalus associated with NF-1 regardless of the type and the level of the obstruction, with the aim of avoiding possible shunt complications. If a stoma closure or membranous cisternal obstruction is detected after ETV in the follow-up MR imaging examinations, second or even third attempts could be performed effectively instead of shunt insertion.
Conclusions
Hydrocephalus associated with NF-1 is not rare. Although the types of obstruction are various, including aqueductal web, superior velum medullary synechia, periaqueductal/tectal hamartomas, cerebellar and pontine tegmentum hamartomas, brain stem glioma, or a combination, the presence of hamartomas at either the periaqueductal area or the fourth ventricle inlet is a consistent finding in patients with NF-1 with hydrocephalus. The findings demonstrated herein indicate the potential of ETV in the treatment of hydrocephalus associated with NF-1.
Abbreviations
- cine PC
cine phase-contrast
- CNS
central nervous system
- ETV
endoscopic third ventriculostomy
- 3D-CISS
3D constructive interference in steady state
- NF-1
neurofibromatosis type 1
- TSE
turbo spin-echo
References
- 1. Castillo M, Green C, Kwock L, et al. Proton MR spectroscopy in patients with neurofibromatosis type 1: evaluation of hamartomas and clinical correlation. AJNR Am J Neuroradiol 1995;16:141–47 [PMC free article] [PubMed] [Google Scholar]
- 2. Pont MS, Elster AD. Lesions of skin and brain: modern imaging of the neurocutaneous syndromes. AJNR Am J Neuroradiol 1992;158:1193–203 [DOI] [PubMed] [Google Scholar]
- 3. Zamboni SL, Loenneker T, Boltshauser E, et al. Contribution of diffusion tensor MR imaging in detecting cerebral microstructural changes in adults with neurofibromatosis type 1. AJNR Am J Neuroradiol 2007;28:773–76 [PMC free article] [PubMed] [Google Scholar]
- 4. Afifi AK, Jacoby CG, Bell WE, et al. Aqueductal stenosis and neurofibromatosis: a rare association. J Child Neurol 1988;3:125–30 [DOI] [PubMed] [Google Scholar]
- 5. Balestrazzi P, de Gressi S, Donadio A, et al. Periaqueductal gliosis causing hydrocephalus in a patient with neurofibromatosis type 1. Neurofibromatosis 1989;2:322–25 [PubMed] [Google Scholar]
- 6. Chattopadhyay A, Kher AS, Thamke RM, et al. Neurofibromatosis presenting with aqueductal stenosis. Indian J Pediatr 1994;61:586–87 [DOI] [PubMed] [Google Scholar]
- 7. Gelabert Gonzalez M, Bollar Zabala A, Prieto Gonzalez A, et al. Neurofibromatosis and stenosis of the aqueduct of Sylvius: a magnetic resonance assessment [in Spanish]. Rev Med Univ Navarra 1990;34:17–19 [PubMed] [Google Scholar]
- 8. Horwich A, Riccardi VM, Francke U. Brief clinical report: aqueductal stenosis leading to hydrocephalus—an unusual manifestation of neurofibromatosis. Am J Med Genet 1983;14:577–81 [DOI] [PubMed] [Google Scholar]
- 9. Kawano H, Hayashi M, Kabuto M, et al. Von Recklinghausen neurofibromatosis complicated with aqueductal stenosis: report of two adult cases [in Japanese]. Rinsho Shinkeigaku 1985;25:336–41 [PubMed] [Google Scholar]
- 10. Krajewska G, Perniola T. Central von Recklinghausen disease: report of a clinical case with multifocal symptoms and hydrocephalus due to stenosis of the aqueduct (author's transl) [in Italian]. Riv Patol Nerv Ment 1979;99:308–16 [PubMed] [Google Scholar]
- 11. Pascual-Castroviejo I, Pascual-Pascual SI, Velazquez-Fragua R, et al. Aqueductal stenosis in the neurofibromatosis type 1: presentation of 19 infantile patients [in Spanish]. Rev Neurol 2007;45:18–21 [PubMed] [Google Scholar]
- 12. Pou-Serradell A, Ugarte-Elola AC. Hydrocephalus in neurofibromatosis: contribution of magnetic resonance imaging to its diagnosis, control and treatment. Neurofibromatosis 1989;2:218–26 [PubMed] [Google Scholar]
- 13. Radhakrishnan K, Kak VK, Sridharan R, et al. Adult aqueductal stenosis with Recklinghausen's neurofibromatosis. Surg Neurol 1981;16:262–65 [DOI] [PubMed] [Google Scholar]
- 14. Riviello JJ, Jr, Marks HG, Lee MS, et al. Aqueductal stenosis in neurofibromatosis. Neurofibromatosis 1988;1:312–17 [PubMed] [Google Scholar]
- 15. Senveli E, Altinors N, Kars Z, et al. Association of von Recklinghausen's neurofibromatosis and aqueduct stenosis. Neurosurgery 1989;24:99–101 [DOI] [PubMed] [Google Scholar]
- 16. Spadaro A, Ambrosio D, Moraci A, et al. Nontumoral aqueductal stenosis in children affected by von Recklinghausen's disease. Surg Neurol 1986;26:487–95 [DOI] [PubMed] [Google Scholar]
- 17. Umemura K, Hayashi N, Hamada H, et al. Endoscopic third ventriculostomy in a case of obstructive hydrocephalus with neurofibromatosis (NF-1) [in Japanese]. No To Shinkei 1998;50:427–31 [PubMed] [Google Scholar]
- 18. Williams VC, Lucas J, Babcock MA, et al. Neurofibromatosis type 1 revisited. Pediatrics 2009;123:124–33 [DOI] [PubMed] [Google Scholar]
- 19. Dincer A, Kohan S, Ozek MM. Is all “communicating” hydrocephalus really communicating? Prospective study on the value of 3D-constructive interference in steady state sequence at 3T. AJNR Am J Neuroradiol 2009;30:1898–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dinçer A, Yıldız E, Kohan S, et al. Analysis of endoscopic third ventriculostomy patency by MRI: value of different pulse sequences, the sequence parameters and the imaging planes for investigation of flow void. Childs Nerv Syst 2011;27:127–35 [DOI] [PubMed] [Google Scholar]
- 21. Griffiths PD, Blaser S, Mukonoweshuro W, et al. Neurofibromatosis bright objects in children with neurofibromatosis type 1: a proliferative potential? Pediatrics 1999;104:e49. [DOI] [PubMed] [Google Scholar]
- 22. Gonen O, Wang ZJ, Viswanathan AK, et al. Three-dimensional multivoxel proton MR spectroscopy of the brain in children with neurofibromatosis type 1. AJNR Am J Neuroradiol 1999;20:1333–41 [PMC free article] [PubMed] [Google Scholar]
- 23. Pennybacker J. Stenosis of the aqueduct of Sylvius: (Section of Neurology). Proc R Soc Med 1940;33:507–12 [PMC free article] [PubMed] [Google Scholar]
- 24. Hosoda K, Kanazawa Y, Tanaka J, et al. Neurofibromatosis presenting with aqueductal stenosis due to a tumor of the aqueduct: case report. Neurosurgery 1986;19:1035–37 [DOI] [PubMed] [Google Scholar]
- 25. Pollack IF, Shultz B, Mulvihill JJ. The management of brainstem gliomas in patients with neurofibromatosis 1. Neurology 1996;46:1652–60 [DOI] [PubMed] [Google Scholar]
- 26. Van Es S, North KN, McHugh K, et al. MRI findings in children with neurofibromatosis type 1: a prospective study. Pediatr Radiol 1996;26:478–87 [DOI] [PubMed] [Google Scholar]