Abstract
BACKGROUND AND PURPOSE:
An animal spinal tumor model is needed to better simulate the clinical situation and to allow percutaneous puncture, which may provide an experimental platform for the new nonvascular interventional therapies. We established a rabbit spinal tumor model through a CT-guided percutaneous puncture inoculation technique for nonvascular interventional therapy.
MATERIALS AND METHODS:
VX2 tumor cells were inoculated into the lumbar vertebrae of 32 rabbits through a CT-guided percutaneous puncture technique; then, the development of hind limb paraparesis was observed in the rabbits twice a day. MR imaging and CT were performed on days 14, 21, and 28 postinoculation and at the development of hind limb paraparesis. On days 21 and 28 postinoculation, 2 rabbits, whose imaging suggested successful modeling without hind limb paraparesis, were chosen on each day. The lumbar vertebrae were sampled from 1 rabbit for histopathologic examination, and the other rabbit underwent PET-CT examination before percutaneous vertebroplasty. Finally the lesion vertebrae were sampled for histopathologic examination.
RESULTS:
The success rate of modeling was 90.6% (29/32) in our study. On day 21 postinoculation, successful modeling was achieved in 21 rabbits, with 19 having no hind limb paraparesis. On day 28 postinoculation, another 7 achieved successful modeling, and only 1 developed hind limb paraparesis. Percutaneous vertebroplasty treatment was successful for the 2 rabbit models.
CONCLUSIONS:
Establishment of a rabbit spinal tumor model through a CT-guided percutaneous puncture technique and inoculation of VX2 tumor is easy and has a high success rate. The established model can be used to study nonvascular interventional therapies for spinal tumor, including percutaneous vertebroplasty.
Approximately 5%–10% of all patients with cancer have metastases to the spinal column.1,2 Vertebral metastasis has major clinical significance and can acutely impact patient quality of life. It is not only an indicator of bad prognosis but also produces severe pain, spinal instability, and neurologic compression following pathologic vertebral fracture. Despite multimodality treatment of spinal diseases, which includes a combination of surgery, radiation therapy, and chemotherapy, the median life expectancy for these patients is <1 year.3,4 Recently various interventional mini-invasive therapies, such as percutaneous vertebroplasty (PVP) and radiofrequency ablation through percutaneous puncture, have yielded encouraging preliminary clinical outcomes in the local treatment of spinal tumor.5–7 However, the treatment mechanism of these therapies remains unclear,8–10 and some have a higher rate of complications.11,12 In addition, more basic and preclinical research is needed to improve the curative efficacy of the newly developed treatment instruments and to reduce their complications. Due to lack of a live spinal tumor model for percutaneous puncture, previous research was mainly conducted with healthy animals, cadavers, or computer-simulated systems.13–15 A live animal spinal tumor model is urgently needed to better simulate the clinical situation and to allow percutaneous puncture, which may provide an experimental platform for the newly emerging mini-invasive interventional therapies. The present study, using CT-guided percutaneous puncture inoculation of VX2 tumor cells, created a rabbit spinal tumor model that allows mini-invasive therapies through a percutaneous puncture approach and observed the development and pathologic manifestation of hind limb paraparesis (HLP) in an animal model.
Materials and Methods
The protocol of this experiment was approved by the animal research committee of our institution and was conducted in accordance with the guidelines of the International Council on Animal Care. Healthy New Zealand white rabbits (n = 32), weighing 3–3.5 kg, were purchased from the Laboratory Animal Center of our university. Fasting was prescribed from the night before the inoculation in 32 rabbits, and 3% pentobarbital was used for general anesthesia via a rabbit ear vein at a dose of 30 mg/kg before inoculation, imaging examination, and PVP treatment.
Preparation of VX2 Tumor Mass
Rabbit VX2 carcinoma preparation was performed as previously described.16,17 Successful inoculation of the VX2 mass into the thigh muscles of the New Zealand rabbit could lead to a palpable tumor mass at the inoculation site 3 weeks later. At 30 minutes before inoculation, tumor was surgically obtained from the thigh of tumor-bearing rabbits; then, the fresh tumor tissues around the border of the tumor were harvested after removing the hemorrhage and necrotic tissues. The tumor tissues were cut into small blocks (approximately 1.0 mm3, 2 × 105 tumor cells), which were soaked in saline until use.
Percutaneous Puncture Inoculation Technique
The experimental rabbits were anesthetized and fixed in a prone position, and the skin on the left low back was prepared. CT localization set the junction of the head side of the L4 or L5 vertebral body and the left pedicle as the puncture point. A 17-ga coaxial introducer needle (Angiotech, Gainesville, Florida) was used to puncture the target vertebra under CT guidance, and the needle advancement was stopped when it reached the middle area of the punctured vertebral body (Figs 1 and 2); then, the inner core of the trocar was used to coaxially push the 2 tumor blocks into the vertebrae body through the sheathed needle, and finally a piece of Gelfoam (0.5 cm; Jinling Pharmaceutical Company, Nanjing, China) was used to seal the needle tract.
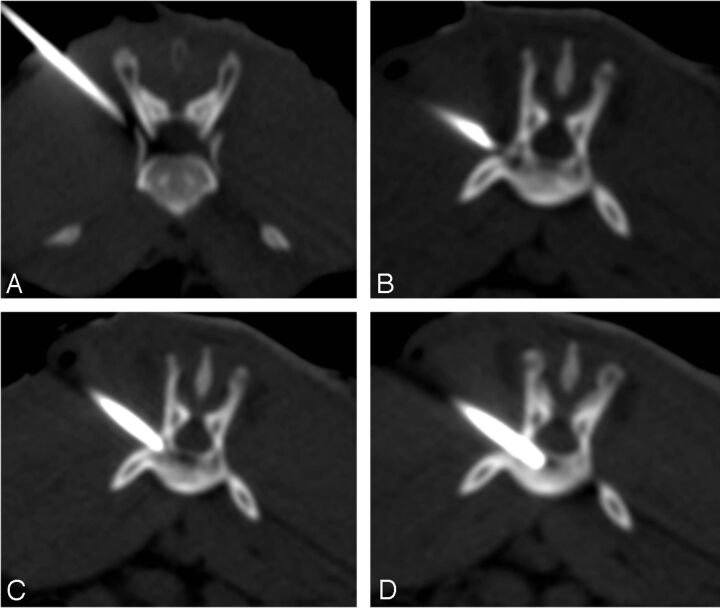
Fig 1.
CT-guided percutaneous puncture inoculation of a VX2 tumor mass. A, Incorrect positioning of puncture needle and the adjustment of needle position and direction. B, Correct positioning of puncture needle, just at the junction of the vertebral body and left vertebral pedicle. C, Puncture needle breaking the vertebral cortical bone and reconfirming that the needle positioning is not deep enough. D, Slow advancement of the puncture needle to the middle of vertebral body.
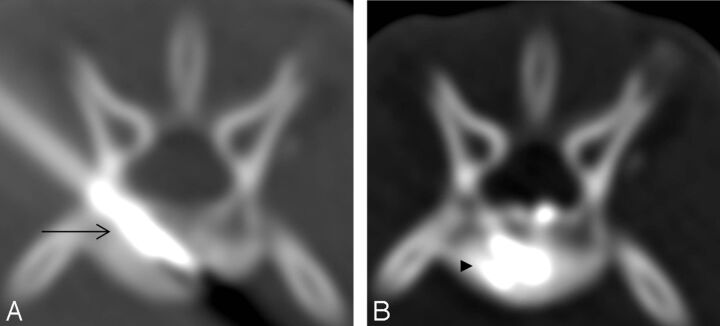
Fig 2.
CT scan reconstruction after successful puncture: coronal section (A), sagittal section (B), and axial section (C). The rabbit vertebra is narrow and long, with the central part being thin and small (arrowhead). The cross-section of the vertebral head is relatively thicker, and the puncture needle tip (arrow) is positioned at the relatively thicker side of vertebra head after successful puncture.
Observation of the Postinoculation Procedure
Animals were examined twice daily for signs of HLP after transplantation. On days 14, 21, and 28 postinoculation, MR imaging and CT were performed. On days 21 and 28 postinoculation, 2 rabbits, whose imaging examination suggested tumor growth but with no signs of paralysis, were chosen each day. One animal was used to harvest lesion vertebrae for pathologic examination, the other underwent an 18F-FDG PET-CT examination before using polymethylmethacrylate bone cement (Cranioplastic; Codman, Raynham, Massachusetts) to conduct PVP treatment, and finally the lesion vertebrae samples were harvested for histopathology. For the rest of the experimental rabbits, both MR imaging and CT were performed immediately after development of HLP; then, lesion vertebrae samples were harvested for pathologic examination. Animals with no HLP at 3 months postinoculation and no tumor growth on imaging examination were sacrificed, and the spines were processed for histopathology.
Imaging Examination and PVP Technique
An MX 8000 4-channel CT scanner (Marconi Medical Systems, Cleveland, Ohio) was used. The scanning parameters were the following: tube voltage, 120 kV(peak); tube current, 200 mA; section thickness, 2 mm; reconstruction interval, 2 mm; and rotation time, 1 second.
MR imaging was performed with a 1.5T imaging unit (Eclipse; Philips Healthcare, Best, the Netherlands) and a spine coil. Sagittal T1-weighted (TR/TE, 500/12 ms) and sagittal and axial T2-weighted (TR/TE, 4500/112 ms) images were obtained, and sagittal T1-weighted images were obtained after intravenous administration of 0.1-mmol/kg gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany).
Fasting was prescribed for 4 hours before PET-CT, and 18F-FDG was injected via a rabbit ear vein (27.8 MBq/Kg); 45 minutes later the rabbits were fixed in a prone position for PET-CT scanning (Discovery LS; GE Healthcare, Milwaukee, Wisconsin). The parameters for CT were as follows: An initial scout view was obtained with 10 mAs and 120 kVp, followed by spiral CT at a table speed of 17.5 mm/s and a pitch of 1.75 with 120 mAs, 140 kV. PET images were obtained with a weight-based protocol and 4–6 minutes of acquisition time per bed position. All PET images were reconstructed by using an iterative algorithm, with CT-based attenuation correction applied. Metabolic images from PET and anatomic images from CT were fused in a postprocessing workstation (Xeleris 1.1; GE Healthcare).
PVP was performed under CT guidance. An 18-ga vascular access needle punctured the spinal tumor to deliver approximately 0.5-mL polymethylmethacrylate bone cement (Corinplast 3; Corin, Gloucester, UK) for PVP therapy.
Results
Successful Modeling Rate
All 32 rabbits underwent successful puncture with no acute paralysis, and successful modeling was achieved in 29, with a success rate of 90.6% (29/32), which was confirmed by histopathologic results. Among the 3 failed cases, 1 had no tumor growth inside the vertebra, but inside paravertebral soft tissues; and 2 had no spinal tumor growth after inoculation or paralysis 3 months later and reimaging examination still found no tumor growth. The 2 rabbits were sacrificed and pathologic examination of the lumbar vertebrae undergoing tumor inoculation found no tumor cells, indicating inoculation failure.
Hind Limb Paralysis Time
On day 14 postinoculation, only 1 rabbit had spinal tumor growth as shown by MR imaging, and HLP occurred on day 19 postinoculation. On day 21 postinoculation, 21 rabbits had spinal tumor growth as shown by both MR imaging and CT and 2 had HLP. On day 28 postinoculation, spinal tumor growth was observed in another 7 rabbits as shown by MR imaging and CT and 1 had HLP. Among the 29 rabbits that achieved successful modeling, 25 developed HLP on average 26.4 ± 4.2 days after inoculation (range, 19–36 days). On days 19, 28, 33, 34, 35, and 36 postinoculation, 1 paralysis occurred each day. On days 21, 23, and 24, two cases of paralysis occurred each day; on days 25 and 26, four cases of paralysis occurred each day, and on day 27, five cases of paralysis occurred; so the peak time for successful modeling animals to show signs of HLP was on days 25–27 postinoculation. For the 4 rabbits achieving successful modeling but with no HLP, pathologic samples were still harvested on days 21 and 28, or vertebra samples were obtained for pathologic examination after PET-CT and PVP treatment.
Imaging Manifestation of the Spinal Tumor Model
CT of the lumbar vertebrae showed irregular osteolytic bone destruction inside, with high-attenuation osteogenesis also observed in some spinal tumors, and vertebral posterior border bone destruction observed at the late stage (Fig 3). MR imaging revealed that L4 or L5 vertebrae showed low signal on T1WI and high signal on T2WI, with uneven signal attenuation. Enhanced scanning showed heterogeneous enhancement. For cases with a larger tumor, the border with the spinal cord was unclear, and the latter was compressed locally (Fig 4). CT images of the PET-CT results were the same as those described above, but the fused images revealed increased uptake of radionuclide in the tumor inoculation area (Fig 5), with the standardized uptake value increased notably. For the 2 rabbits receiving PVP treatment, the puncture of the spinal tumor model was smooth, and the sedimentation of bone cement inside the vertebral body was satisfactory (Fig 6).
Fig 3.
CT of a successful model of a spinal tumor. A and B, Irregular bone destruction inside the lumbar vertebrae and cortical bone destruction at the vertebra posterior border. C and D, Another experimental animal with vertebral bone destruction. The vertebral cortical bone broke at the vertebra border, with a small amount of high-attenuation osteogenesis inside.
Fig 4.
MR images of the vertebra of a paralyzed rabbit with abnormal signal (arrow) from the L4 vertebra. A, Low signal on T1WI. B, High signal on T2WI. C, Enhanced scan shows significantly enhanced lesions and spinal cord compression.
Fig 5.
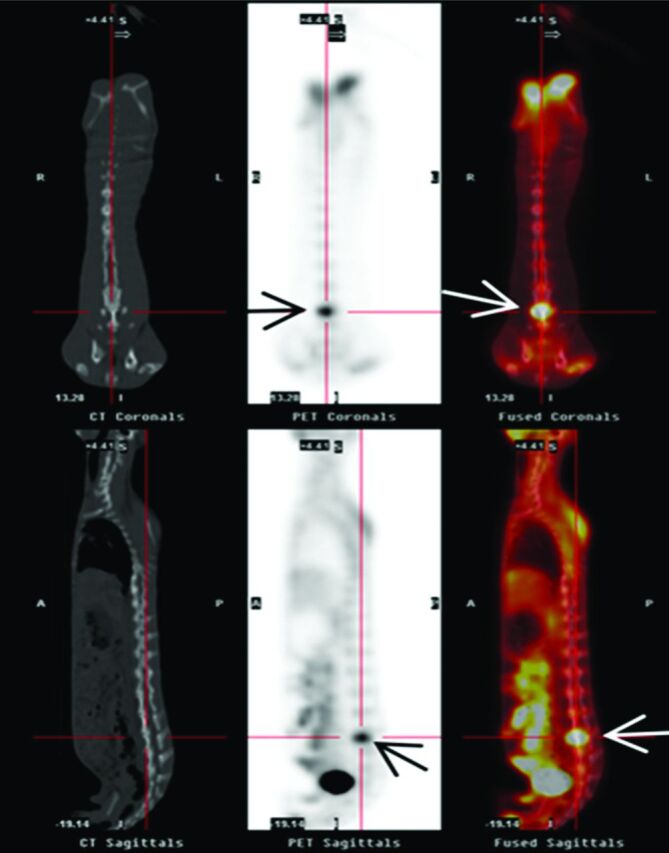
PET-CT images of a spinal tumor in a successful rabbit model with increased uptake of radionuclide at L5 vertebra (arrow). The left column shows a reconstructed image at CT coronal and sagittal positions, the middle column shows PET image, and the right column shows PET-CT fused image.
Fig 6.
CT-guided PVP treatment of a rabbit spinal tumor. A, High-attenuation metallic puncture needle (arrow) into the vertebral body during PVP. B, Satisfactory sedimentation of high-attenuation bone cement (arrowhead) in the vertebral body after PVP.
Pathology of the Spinal Tumor Model
Pathologic samples of the lesion vertebrae showed tumor growth inside the vertebral body. For the rabbits without paralysis, cortical bone on the posterior border was intact; for the rabbits with paralysis, tumor mass intruded into and compressed the spinal cord (Fig 7). Hematoxylin-eosin staining showed extensive osteolytic activity induced by tumor cells inside the vertebrae, complicated by mild osteogenic activity. The tumor cell was large, with well-demarcated borders, cellular atypia, and a visible pathologic mitotic count. Tumor cells had a nest arrangement, with regional invasive growth destroying the cortical bone (Fig 7).
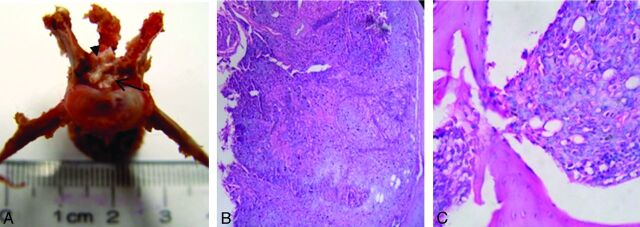
Fig 7.
General pathology and hematoxylin-eosin staining of a successful animal model. A, Bone destruction at the posterior border of the vertebra, with the spinal cord (arrowhead) being compressed by the back side of the tumor (arrow). B, Magnification ×40. C, Hematoxylin-eosin staining. Tumor cells have a nest arrangement under the microscope, and the cells are bulky, with clear boundaries, a high level of cell atypia, an increased nuclear cytoplasm ratio, visible pathologic mitotic count, and a staggered arrangement of tumor cells and bone. Magnification ×400.
Discussion
The previous rat- or mice-based spinal tumor models were mainly used for studying tumor metastasis mechanisms, radiation therapy, and drug screening.18,19 However, the rat vertebra is small, making it difficult to accommodate a thick needle in various nonvascular interventional treatments (such as PVP, radiofrequency ablation) by using a percutaneous puncture technique. Recent rabbit models of spinal tumors created by different surgical methods requiring more experimental techniques and more advanced equipment also sustained even greater trauma, which is not helpful for conducting the ensuing experiments.16,17 More important, those tumor models were through direct-inoculation surgery. The inoculation approach of the model of Amundson et al16 was through the posterior pedicle, breaching the vertebral lamina directly into the spinal canal, then into the posterior vertebral edge, so that the tumors were mainly located on the posterior vertebral edge. Meanwhile, the same approach is also needed when the model is used for research on nonvascular interventional therapy; this will make puncturing very difficult because it entails breaching the spinal canal, which may easily cause nerve damage and is disadvantageous for observing the efficacy of ensuing treatments. So Amundson et al's model is mainly used for studying surgical procedures.20 Our model is prepared through a lateral pedicle approach, particularly for percutaneous puncture of the vertebrae, through which the ensuing experiments could also be performed for nonvascular interventional therapy, which makes our model more suitable for research on this therapy.
In 2010, Sciubba et al21 reported a successful rabbit vertebral tumor model constructed through a percutaneous puncture technique, which was used for studying ultrasonic ablation treatment of spinal tumor. This model had a grossly apparent spinal and paraspinal tissue mass. We noticed that Sciubba et al mainly focused on how to treat the model; they did not give sufficient description on the details of establishment procedure, including the specific sites for puncture, images of the model at different time points, and the development of paralysis. We also noticed that some radiofrequency electrodes used in Sciubba et al's research for tumor treatment actually punctured the paravertebral soft-tissue tumor, instead of vertebral tumor. Differences exist between paravertebral tumor and vertebral tumor. According to the literature, factors like bone cement polymerization temperature during PVP and temperature changes inside the vertebral body and spinal canal during radiofrequency ablation treatment may be directly associated with the treatment efficacy and complications.5,12
The vertebral body has its special anatomic structure. CSF adjacent to the back of the vertebral body can take away some heat; at the same time, differences exist in specific heat and thermal conductivity between bone tissue and soft tissue, which could all directly affect the actual temperature inside the spinal tumor and spinal canal during treatment.13,22 The above pathophysiologic traits of vertebral tumor are not easily found in paravertebral tumor. Therefore, establishment of a real vertebral tumor model for the percutaneous puncture technique will contribute greatly to the emerging nonvascular interventional therapies for vertebral tumors.
In this study, the rabbit VX2 spinal tumor model was established through percutaneous puncture inoculation under CT guidance. All the rabbits in our study successfully underwent percutaneous puncture for inoculating VX2 tumor mass; posttransplantation imaging and pathologic examination confirmed the high success rate of tumor inoculation. Meanwhile, we noticed that PET-CT has been increasingly used to evaluate the treatment efficacy in malignant bone tumors. In the present article, the PET-CT results of 2 animal models both clearly displayed the spinal tumor, suggesting that PET-CT could also evaluate the efficacy of all types of newly emerging interventional therapies for the tumor model.23
PVP treatment based on the rabbit model also achieved preliminary success, indicating its potential in studying mini-invasive interventional treatments through percutaneous puncture. In addition, in another study, we are using our model to study PVP treatment for spinal tumor and to develop a new type of PVP bone cement. For example, detecting the polymerization temperature of bone cement inside the spinal tumor model can better reflect the temperature change during polymerization of bone cement in the clinical setting. To sum up, our model can be used for further exploration of the mechanism of various nonvascular interventional therapies for spinal tumor; the efficacy and safety of some newly developed interventional equipment and materials could also be tested with this model. Eventually, we hope our model can serve as a better platform for studying various emerging mini-invasive interventional treatments for vertebral tumors.
To make it easier to puncture the target vertebra, one should target the larger lumbar vertebrae of the rabbit for modeling. However, because the sixth lumbar vertebra is usually obscured by the ilium, which may affect the puncture approach, the L4 or L5 vertebra is a more suitable target. The rabbit vertebra is narrow and long, with a thin middle part, making it difficult to accommodate the puncture needle.24 However, the size of the vertebral head side is larger, with a triangular cross-section and a maximum oblique diameter of approximately 1.0 cm, where the pedicle is located; thus, the area suitable for tumor inoculation should be the narrow area 0.5 cm from the endplate of the vertebral head side (Fig 2). Conventional clinical practice is to puncture the lumbar vertebra through a pedicle approach; however, the rabbit pedicle is thin and is difficult to puncture. Puncture through the lateral pedicle route is relatively easy and can avoid damage to the spinal canal structures. The rabbit was in a prone position when undergoing puncture, and the junction of the vertebral body and the left pedicle was chosen as the optimal approach for the convenience of the right-handed laboratory personnel. All 32 rabbits underwent such an approach for puncture without postoperative acute HLP, indicating that the transpedicular lateral approach is feasible and safe.
CT-guided puncture technology is the key to successful modeling. The advancement of the puncture needle should be stopped promptly when the needle tip reaches the cortical bone of the lumbar vertebra; then CT should be used to guide the puncture needle angle until the needle tip is positioned just at the left lateral margin of the vertebra head side and to penetrate the needle into vertebral body along the adjusted direction (Fig 1). The penetration depth of the puncture needle was approximately 0.5 cm. Caution should be exercised during the process to prevent the needle from penetrating too deep and damaging the cortical bone of the contralateral vertebra, avoiding the tumor mass being pushed into contralateral paraspinal soft tissue. To ensure that the tumor mass is pushed into the vertebral body, one should push the inner core of the puncture trocar 3–4 times inside the needle sheath. Finally, Gelfoam should be pushed along the sheath to embolize the needle tract to avoid paraspinal tumor mass implantation caused by the shedding of the tumor mass.
For various surgical techniques used to establish a rabbit model of vertebral tumors, the average time for the experiment animal to develop signs of HLP was 18–30 days.16,17 The average time in the present study was 26.4 ± 4.2 days, consistent with other experiments, and paralysis occurred mostly on days 25–27 postinoculation. Amundson et al16 reported that imaging could not find vertebral tumors in the experiment animals within 14 days postinoculation. In this study, MR imaging revealed that on day 14 postinoculation, there was only 1 case of vertebral tumor growth, accounting for 3.5% (1/29) of the total successful models, suggesting that rabbit VX2 spinal tumor growth was hard to detect by imaging examination within 2 weeks postinoculation. For the total 29 successful modeling animals in this study, 72.4% (21/29) of the vertebral lesions could be confirmed by MR imaging and CT examination 21 days postinoculation. Meanwhile, the peak time for successful modeling animals to show signs of HLP was on days 25–27 postinoculation. Therefore, it is recommended that when using this model, the treatment should begin on day 21 postinoculation, when successful modeling can be found and confirmed by imaging examinations in most cases, while HLP has not yet developed.
Despite of the above effort, we admit that heterogeneity in the amount and location of placement of the tumor cells may exist in this study. The left side of the rabbit L4 or L5 vertebral body and the lateral pedicle approach were chosen for puncture in all the animals, and the vertebral head side with a larger triangular cross-section was chosen for tumor inoculation to keep the utmost consistency in the inoculation location. Most of the animals reached the experimental end point of HLP within a relatively tight range. There were 3 cases of failed animal modeling in this experiment, with 1 case having paraspinal soft-tissue tumor growth, which might be caused by tumor cell leakage, while the reason for the other 2 failed cases was not clear and should be addressed in further experiments. Also, this study aimed to investigate the feasibility of establishing a rabbit model of vertebral tumors through percutaneous puncture and the associated imaging manifestations, so the mechanism of paralysis was not discussed, which may also need to be addressed in future studies.
Conclusions
Our method in the present study is easy to perform and less invasive with a low animal mortality rate and a high successful modeling rate. Our model, which was established in the rabbit by using an interventional method, may provide a better platform for studying various mini-invasive interventional therapies for vertebral tumor via percutaneous puncture and other treatments, including radiation therapy and surgery.
ABBREVIATIONS:
- HLP
hind limb paraparesis
- PVP
percutaneous vertebroplasty
Footnotes
Disclosures: Long Chen, Yi-Wei Wu, Kun-Yuan Ge, Yong-Chuang Chang, Chao Yang, Cai-Fang Ni—RELATED: Grant: a grant from the National Natural Science Foundation of China (grant No. 81101136),* a grant from Jiangsu Provincial Special Program of Medical Science, China (BL2012004);* and a grant from the International Exchange Health Program of Jiangsu Province, China (grant No. 2012020).* Jian Xiao—RELATED: Grant: a grant from the Shanghai Municipal Natural Science Foundation, China (grant No. 11ZR1448300).* *Money paid to the institution.
This work was supported by a grant from the National Natural Science Foundation of China (grant No. 81101136); a grant from the Shanghai Municipal Natural Science Foundation, China (grant No. 11ZR1448300); a grant from Jiangsu Provincial Special Program of Medical Science, China (BL2012004); and a grant from the International Exchange Health Program of Jiangsu Province, China (grant No. 2012020).
References
- 1. Barron KD, Hirano A, Araki S, et al. Experiences with metastatic neoplasms involving the spinal cord. Neurology 1959;9:91–106 [DOI] [PubMed] [Google Scholar]
- 2. Geldof AA, Rao BR. Prostatic tumor (R3327) skeletal metastasis. Prostate 1990;16:279–90 [DOI] [PubMed] [Google Scholar]
- 3. Fourney DR, Abi-Said D, Rhines LD, et al. Simultaneous anterior-posterior approach to the thoracic and lumbar spine for the radical resection of tumors followed by reconstruction and stabilization. J Neurosurg 2001;94(2 suppl):232–44 [DOI] [PubMed] [Google Scholar]
- 4. Cooper PR, Errico TJ, Martin R, et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery 1993;32:1–8 [DOI] [PubMed] [Google Scholar]
- 5. Nakatsuka A, Yamakado K, Takaki H, et al. Percutaneous radiofrequency ablation of painful spinal tumors adjacent to the spinal cord with real-time monitoring of spinal canal temperature: a prospective study. Cardiovasc Intervent Radiol 2009;32:70–75 [DOI] [PubMed] [Google Scholar]
- 6. Lee B, Franklin I, Lewis JS, et al. The efficacy of percutaneous vertebroplasty for vertebral metastases associated with solid malignancies. Eur J Cancer 2009;45:1597–602 [DOI] [PubMed] [Google Scholar]
- 7. Itagaki MW, Talenfeld AD, Kwan SW, et al. Percutaneous vertebroplasty and kyphoplasty for pathologic vertebral fractures in the Medicare population: safer and less expensive than open surgery. J Vasc Interv Radiol 2012;23:1423–29 [DOI] [PubMed] [Google Scholar]
- 8. Anselmetti GC, Manca A, Kanika K, et al. Temperature measurement during polymerization of bone cement in percutaneous vertebroplasty: an in vivo study in humans. Cardiovasc Intervent Radiol 2009;32:491–98 [DOI] [PubMed] [Google Scholar]
- 9. Paul L, Santonja C, Izquierdo E. Complete necrosis of a spinal giant cell tumor after vertebroplasty. J Vasc Interv Radiol 2006;17:727–31 [DOI] [PubMed] [Google Scholar]
- 10. Chen L, Ni RF, Liu SY, et al. Percutaneous vertebroplasty as a treatment for painful osteoblastic metastatic spinal lesions. J Vasc Interv Radiol 2011;22:525–28 [DOI] [PubMed] [Google Scholar]
- 11. Nakatsuka A, Yamakado K, Maeda M, et al. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol 2004;15:707–12 [DOI] [PubMed] [Google Scholar]
- 12. Munk PL, Murphy KJ, Gangi A, et al. Fire and ice: percutaneous ablative therapies and cement injection in management of metastatic disease of the spine. Semin Musculoskelet Radiol 2011;15:125–34 [DOI] [PubMed] [Google Scholar]
- 13. Wegener B, Zolyniak N, Gulecyuz MF, et al. Heat distribution of polymerisation temperature of bone cement on the spinal canal during vertebroplasty. Int Orthop 2012;36:1025–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tschirhart CE, Finkelstein JA, Whyne CM. Optimization of tumor volume reduction and cement augmentation in percutaneous vertebroplasty for prophylactic treatment of spinal metastases. J Spinal Disord Tech 2006;19:584–90 [DOI] [PubMed] [Google Scholar]
- 15. Lu J, Deng J, Zhao H, et al. Safety and feasibility of percutaneous vertebroplasty with radioactive (153)Sm PMMA in an animal model. Eur J Radiol 2011;78:296–301 [DOI] [PubMed] [Google Scholar]
- 16. Amundson E, Pradilla G, Brastianos P, et al. A novel intravertebral tumor model in rabbits. Neurosurgery 2005;57:341–46; discussion 341–46 [DOI] [PubMed] [Google Scholar]
- 17. Takahashi M, Ogawa J, Kinoshita Y, et al. Experimental study of paraplegia caused by spinal tumors: an animal model of spinal tumors created by transplantation of VX2 carcinoma. Spine J 2004;4:675–80 [DOI] [PubMed] [Google Scholar]
- 18. Ushio Y, Posner R, Kim JH, et al. Treatment of experimental spinal cord compression caused by extradural neoplasms. J Neurosurg 1977;47:380–90 [DOI] [PubMed] [Google Scholar]
- 19. Arguello F, Baggs RB, Duerst RE, et al. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer 1990;65:98–106 [DOI] [PubMed] [Google Scholar]
- 20. Yamada K, Terai H, Matsumoto T, et al. Effect of spinal fixation in rabbits with metastatic tumor using a novel spinal fusion model. J Spinal Disord Tech 2012. July 19. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21. Sciubba DM, Burdette EC, Cheng JJ, et al. Percutaneous computed tomography fluoroscopy-guided conformal ultrasonic ablation of vertebral tumors in a rabbit tumor model. Laboratory investigation. J Neurosurg Spine 2010;13:773–79 [DOI] [PubMed] [Google Scholar]
- 22. Aebli N, Goss BG, Thorpe P, et al. In vivo temperature profile of intervertebral discs and vertebral endplates during vertebroplasty: an experimental study in sheep. Spine (Phila Pa 1976) 2006;31:1674–78; discussion 1679 [DOI] [PubMed] [Google Scholar]
- 23. Peller PJ. Role of positron emission tomography/computed tomography in bone malignancies. Radiol Clin North Am 2013;51:845–64 [DOI] [PubMed] [Google Scholar]
- 24. Knipe MF. Principles of neurological imaging of exotic animal species. Vet Clin North Am Exot Anim Pract 2007;10:893–907, vii [DOI] [PubMed] [Google Scholar]