Whiplash injuries may damage the anterior longitudinal ligament, which is not critical because as long as the injury is isolated the spine remains stable. These authors studied 91 patients acutely and 12 months after whiplash injury with special attention to damaged transverse and alar ligaments. High proton density signal was seen in both injured and control patients with similar prevalence. Moreover, in those with a history of whiplash injury, these areas of high signal were seen in identical numbers acutely and on follow-up studies. Thus, these high signal areas, commonly assumed to represent injury to the ligaments, cannot be solely explained by trauma.
Abstract
BACKGROUND AND PURPOSE:
The cause and clinical relevance of upper neck ligament high signal intensity on MR imaging in WAD are controversial. The purpose of this study was to explore changes in the signal intensity of the alar and transverse ligaments during the first year after a whiplash injury.
MATERIALS AND METHODS:
Dedicated high-resolution upper neck proton attenuation–weighted MR imaging was performed on 91 patients from an inception WAD1–2 cohort, both in the acute phase and 12 months after whiplash injury, and on 52 controls (noninjured patients with chronic neck pain). Two blinded radiologists independently graded alar and transverse ligament high signal intensity 0–3, compared initial and follow-up images to assess alterations in grading, and solved any disagreement in consensus. The Fisher exact test was used to compare proportions.
RESULTS:
Alar and transverse ligament grading was unchanged from the initial to the follow-up images. The only exceptions were 1 alar ligament changing from 0 to 1 and 1 ligament from 1 to 0. The prevalence of grades 2–3 high signal intensity in WAD was thus identical in the acute phase and after 12 months, and it did not differ from the prevalence in noninjured neck pain controls (alar ligaments 33.0% versus 46.2%, P = .151; transverse ligament 24.2% versus 23.1%, P = 1.000).
CONCLUSIONS:
Alar and transverse ligament high signal intensity in patients with WAD1–2 observed within the first year after injury cannot be explained by the trauma. Dedicated upper neck MR imaging cannot be recommended as a routine examination in these patients.
The alar and transverse ligaments are important stabilizers at the craniovertebral junction—the alar ligaments prevent excessive rotation and lateral flexion and the transverse ligament prevents anterior dislocation of atlas on axis during flexion.1–3 These ligaments can show high signal intensity on proton attenuation–weighted high-resolution MR imaging.4–9 The high signal intensity has an unknown etiology, a debated relation to trauma, and uncertain clinical relevance. It has been reported in patients with chronic WAD6,9,10 but also in noninjured controls.4,5,7,8
In the only study of alar and transverse ligament high signal intensity in acute WAD, such high signal intensity was not related to crash factors, was not more frequent when compared with noninjured controls without neck pain, and did not influence clinical outcome after 12 months.8,11 Trauma-related high signal intensity can appear in ligaments some time after an acute injury due to repair processes of scarring and fibrosis or fat replacement.12 In one study, patients with WAD imaged 2–6 years after trauma were more likely than controls to have alar and transverse ligament high signal intensity.6 Changes in the signal intensity of these ligaments have never been examined prospectively. Such changes over time could be related to a trauma but also to altered ligament function due to neck pain per se, regardless of trauma. Any time-related development of a high signal intensity would shed light on its pathogenesis and morphology.
In this study, patients with acute WAD grade 1 or 2 (ie, acute neck complaints after trauma but no fractures, dislocations, or neurologic signs)13 underwent high-resolution upper neck MR imaging both in the acute phase and at 12 months' follow-up. The aim was to explore changes in the signal intensity of the alar and transverse ligaments during the first year after a whiplash injury causing acute neck pain. We also compared the prevalence of ligament high signal intensity between the WAD group and a control group of noninjured patients with chronic neck pain.
Materials and Methods
The appropriate Research Ethics Committee approved this prospective controlled study. Written informed consent was obtained from all study participants. To detect a difference in prevalence of high signal intensity from 35% in the WAD group to 13% in the control group as statistically significant in a 2:1 design (significance level, 5%; power, 80%), 90 patients with WAD and 45 controls would be needed.
Patients with Whiplash
From May 2007 to March 2009, 114 patients with acute WAD1–2 were included in an inception cohort and completed adequate high-resolution MR imaging of their upper neck ligaments within 0–13 days (median, 5 days) after injury.8 They were consecutively recruited from a primary ward and a hospital clinic and were all Norwegian-speaking drivers or passengers, aged 18–80 years, sustaining a car crash during the last 7 days, reporting onset of neck pain within 48 hours after the crash. They had no neurologic signs and no clinical or radiologic signs of neck fracture or dislocation. N.V. ascertained the WAD grading by interviewing the patients and reviewing reports from clinicians and radiologists. The exclusion criteria were prior neck injury or whiplash trauma; prior neck pain of >30 days in total; reported treatment for neck problems during the past 10 years; prior severe head injury; previous cervical spine surgery, rheumatic disease, cancer, or any other serious somatic or psychiatric conditions; and pregnancy/MR incompatibility. How ligament high signal intensity on MR images in the acute phase of injury related to initial clinical characteristics and to clinical outcome at 12 months has been reported previously.8,11
All 114 patients were invited to undergo follow-up MR imaging 12 months after injury; 20 patients declined a second MR examination and 3 patients did not respond to phone or mail reminders. This left 91 patients constituting the present study sample.
Noninjured Patients with Neck Pain: Symptomatic Controls
Noninjured patients with neck pain were consecutively recruited from an outpatient spine clinic as symptomatic controls. The inclusion criteria, fulfilled by 188 patients attending from June 2007 to October 2008, were Norwegian-speaking patients, aged 18–80 years, with a main complaint of >3 months' neck pain. The exclusion criteria were any neck or whiplash trauma (n = 109), severe head injury (n = 5), cervical spine surgery (n = 4), rheumatic disease (n = 1), cancer or any other serious somatic or psychiatric condition (n = 0), clinical sign of myelopathy or known cervical nerve root syndrome (n = 3), pregnancy/MR incompatibility (n = 1), and unwillingness to participate (n = 12). Of the remaining 53 patients with neck pain, 1 patient aborted MR imaging due to claustrophobic discomfort, leaving 52 patients in the study.
Clinical Data
All included patients with WAD had completed a questionnaire 0–13 days (median, 4 days) after their car crash regarding clinical data in the acute phase of injury and accident-related factors.8 They also filled in a follow-up questionnaire 51–56 weeks (median, 52 weeks) after the crash. It included a modified version of the NDI,14,15 calculated only when at least 8 of 10 items were answered and then given as a percentage of the highest achievable score.15 NDI was dichotomized into NDI ≤ 8% (recovered) or NDI > 8% (not recovered).14,16 Neck pain during the preceding week was registered at follow-up on an NRS-11.17,18 All 91 patients returned valid data for both NDI and neck pain NRS-11. The 12-month follow-up in the study did not include a clinical examination.
Clinicians at the outpatient spine clinic examined and included noninjured symptomatic controls according to the study criteria. Of the 52 controls, 49 underwent a physical examination by a physiotherapist that included goniometric cervical AROM measurements19 (48 the same day they were included and 1 delayed for 9 days). Fifty-one controls filled in a questionnaire including NDI, NRS-11 of preceding week neck pain, and questions regarding pain duration (50 the same day they were included and 1 delayed for 11 days).
MR Protocol
All MR examinations were performed with subjects' head and neck in a neutral position in a standard 1-channel circular polarized receive-only head coil, by using the same 1.5T scanner (Symphony Mastroclass; Siemens Medical System, Erlangen, Germany) and the same established MR protocol.9,20 This protocol included proton attenuation–weighted fast spin-echo sequences in 3 orthogonal planes, axial, coronal, and sagittal; TR/TE, 2150–2660/15 ms; section thickness, 1.5 mm; intersection gap, 0.0 or 0.3 mm (sagittal); FOV, 175 × 200 mm or 200 × 200 mm (coronal); voxel size, 0.6–0.7 × 0.4 × 1.5 mm3; and echo-train length, 13.
The 91 patients with WAD1–2 underwent initial MR imaging 0–13 days (median, 5 days) after the crash and follow-up MR imaging 51–56 weeks (median, 52 weeks) after the crash. The 52 noninjured symptomatic controls were imaged within 0–25 days (median, 4 days) after inclusion.
Image Interpretation
The alar and transverse ligaments were graded 0–3 on the proton attenuation–weighted sequences based on the ratio between any high signal intensity part and the total cross-sectional area of the ligament as judged visually.6,9,12 High signal intensity in one-third or less of the total cross-sectional area was graded 1, high signal intensity in one-third to two-thirds of the total cross-sectional area was graded 2, and high signal intensity in two-thirds or more of the total cross-sectional area was graded 3. No high signal intensity was graded 0. The right and left sides were graded separately, by using the image with the largest cross-sectional area of high signal intensity. Alar ligaments were graded on sagittal sections and transverse ligaments on sagittal or coronal sections. Any high signal intensity had to be seen in at least 2 imaging planes to be graded 1–3. Homogeneous gray ligaments were graded 2.
Two radiologists (6 and 26 years experience) who were blinded to group allocation and clinical data independently graded the initial MR images of the WAD1–2 cohort and the MR images of the symptomatic controls. The images were de-identified and presented in a random order interspersed between images of noninjured asymptomatic individuals (not reported here). Both radiologists thereafter solved all disagreements by consensus reading of images. Their consensus grading was used in the analyses, where grades 0 and 1 were combined and grades 2 and 3 were combined. Regarding presence of grades 2–3 alar and transverse ligament high signal intensity per subject, the 2 radiologists disagreed in 25 (17%) and 27 (19%) subjects and achieved κ 0.59 and 0.46 for interobserver agreement, respectively.
The same 2 radiologists, blinded to all clinical data except group allocation, interpreted the follow-up MR images of the WAD1–2 cohort with both the initial images and the consensus grading of the initial images available. Each independently compared ligament high signal intensity between the initial and the follow-up images and assessed whether the grading was altered at follow-up (no versus yes, and to which grade). For 2 patients (2/91, 2.2%), they disagreed on such alterations and performed a consensus grading, which was used in the analyses.
Statistical Analyses
The Fisher exact test was used to compare proportions between groups. To compare means, the Mann-Whitney U-test was used as normality could not be assumed. SPSS 16.0 for Windows (SPSS, Chicago, Illinois) was used to analyze data. P ≤ .05 indicated statistical significance.
Results
Characteristics of Patients with WAD
Table 1 shows characteristics of the 91 patients with WAD. Their median age was 29.2 years; 53 (58.1%) were women; and 33.0% and 24.2% had grades 2–3 alar and transverse ligament high signal intensity, respectively, in the acute phase of injury. Four patients reported a new minor whiplash trauma during follow-up. The 23 patients with WAD not completing follow-up imaging did not differ significantly from the 91 included WAD patients in age, sex, initial pain, or ligament high signal intensity in the acute phase. Twenty of these 23 dropouts completed the follow-up questionnaire 12 months after injury, and they were more likely to have recovered than the 91 patients with WAD completing follow-up MR imaging (NDI ≤ 8%; 80.0% versus 50.5%; P = .024).
Table 1:
Clinical characteristics of the 91 patients with follow-up MR imaging 12 months after whiplash injury
| n | % | Median (Range) | |
|---|---|---|---|
| Clinical characteristics at the acute phase of injury | |||
| Women | 53 | 58.1 | |
| Age (yr) | 29.2 (18.1–69.2) | ||
| Initial neck pain intensity, NRS-11 score (0–10) | 4.0 (1.0–9.0) | ||
| Crash-related factors | |||
| Rear-end collision | 60 | 65.9 | |
| Head turned at impact (n = 77) | 26 | 33.8 | |
| Head injury at crash | 9 | 9.9 | |
| Seat belt used at impact | 88 | 96.7 | |
| Head restraint present at impact (n = 87) | 75 | 86.2 | |
| Patient car speed at impact (km/h; n = 91) | 0.0 (0.0–75.0) | ||
| Relative car speeda at impact (km/h; n = 69) | 45.0 (10.0–150.0) | ||
| Clinical characteristics at follow-up | |||
| Last week's neck pain intensity, NRS-11 score | 2.0 (0.0–8.0) | ||
| Neck disability, NDI score (%) | 8.0 (0.0–64.0) |
Difference between vehicle speeds if rear-end collision; otherwise, sum of vehicle speeds.
Ligament High Signal Intensity 12 Months after Whiplash Injury
All patients with WAD had interpretable follow-up MR images that could be directly compared with the initial images. The grade of high signal intensity was altered from 0 to 1 in one alar ligament (Fig 1) and from 1 to 0 in another alar ligament. All other alar and transverse ligaments showed exactly the same grade of high signal intensity at follow-up as initially on both the left and the right side (Figs 2 and 3). The prevalence of grades 2–3 high signal intensity in patients with WAD was thus the same 12 months after injury as in the acute phase: 33.0% (95% CI, 23.1%–42.8%) for the alar ligaments and 24.2% (95% CI, 15.2%–33.1%) for the transverse ligament (Table 2).
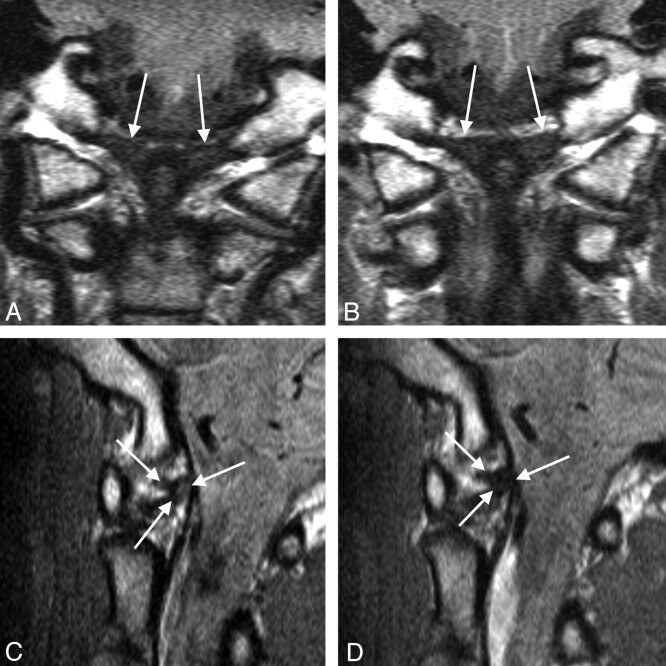
Fig 1.
Alar ligaments (arrows) on coronal (A, B) and sagittal (C, D) MR sections in a WAD patient. Left alar ligament altered from grade 1 on the initial examination (A, C) to grade 0 at follow-up (B, D).
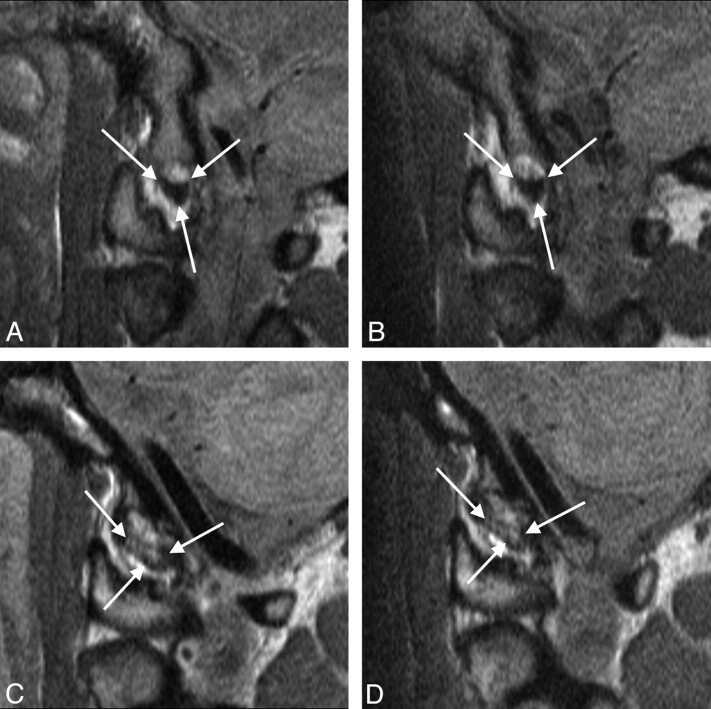
Fig 2.
Sagittal MR images showing unaltered grading of the alar ligament from the initial (A, C) to the follow-up (B, D) MR examination in 1 patient with WAD with grade 0 (A, B) and another patient with WAD with grade 3 (C, D) high signal intensity.
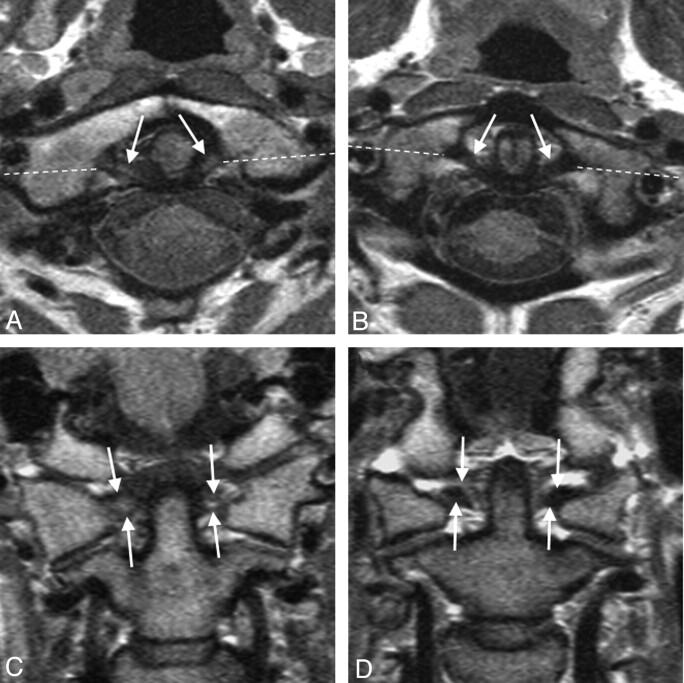
Fig 3.
Transverse ligament (arrows) on axial (A, B) and coronal (C, D) MR sections taken at follow-up in one patient with WAD with grade 3 high signal intensity (A, C) and another patient with grade 0 high signal intensity (B, D). Broken lines mark the coronal plane. Both patients' transverse ligament grading was unchanged compared with the initial MR images taken at injury.
Table 2:
MR imaging grades 2–3 ligament high signal intensity in patients with WAD1-2 12 months after injury and in noninjured controls with chronic neck pain
| Group | Alar Ligament Grades 2–3 High Signala |
Transverse Ligament Grades 2–3 High Signala |
||||
|---|---|---|---|---|---|---|
| n | % | Pb | n | % | Pb | |
| WAD, NDI > 8% at follow-up (n = 45) | 18 | 40.0 | .681 | 12 | 26.7 | .814 |
| WAD, NDI ≤ 8% at follow-up (n = 46) | 12 | 26.1 | .058 | 10 | 21.7 | 1.000 |
| All WAD (n = 91) | 30 | 33.0 | .151 | 22 | 24.2 | 1.000 |
| Controls, all NDI > 8% (n = 52) | 24 | 46.2 | 12 | 23.1 | ||
Highest assigned grade if different between right and left side.
P value based on Fisher exact test compared with the controls.
Ligament High Signal Intensity in Symptomatic Controls
Among the 52 noninjured patients with neck pain, the prevalence of grades 2–3 high signal intensity was 46.2% (95% CI, 32.1%–60.2%) for the alar ligaments and 23.1% (95% CI, 11.2%–34.9%) for the transverse ligament (Table 2). In this control group, 37 (71.2%) were women, age was 22.0–59.9 years (median, 41.9 years), NRS-11 score of last week's neck pain was 2–10 (median, 6), NDI was 14–72 (median, 36), total cervical AROM was 150–393° (median, 295°), and time since first neck pain episode varied between 4.5 months and 36 years (median, 42.0 months). None of these clinical factors were related to the presence of grades 2–3 ligament high signal intensity (P > .134). Alar high signal intensity tended to be more likely in male controls (P = .073).
The prevalence of grades 2–3 ligament high signal intensity did not differ between the 52 symptomatic controls and the 91 patients with WAD (alar, P = .151; transverse, P = 1.000) or between these controls and patients with WAD who recovered (NDI ≤ 8%) or not (NDI > 8%) at follow-up (alar, P = .058–0.681; transverse, P = .814–1.000; Table 2). Alar high signal intensity tended to be more frequent in symptomatic controls than in recovered patients with WAD (P = .058; Table 2).
Discussion
This study shows that the signal intensity of the alar and transverse ligaments on dedicated MR imaging does not change during the first year after a whiplash injury. The prevalence of grades 2–3 ligament high signal intensity in patients with WAD was identical in the acute phase and after 12 months, and it was similar to the prevalence in noninjured patients with chronic neck pain.
The unchanged signal intensity 12 months after injury strongly indicates that the high signal intensity was not caused by the traumatic event. Any high signal intensity due to edema or bleeding from acute mechanical injury would be expected to decrease as the acute responses resolve.21–23 The unaltered signal intensity also does not support the theory that acute ligament injury invisible on MR images in the acute phase can cause the development of high signal intensity seen only at a later stage of injury, eg, after a repair process or due to accelerated degeneration.11 We cannot completely rule out that such high signal intensity may occur later than 12 months after injury. However, in a large group of patients with neck pain with a history of neck trauma, neither alar nor transverse ligament high signal intensity was related to time since trauma (median, 5 years; range, 39 days–59 years).9
The similar prevalence of ligament high signal intensity in our WAD cohort at 12 month-follow-up and in noninjured patients with chronic neck pain also shows that the high signal intensity was hardly due to the trauma. The prevalence of alar high signal intensity in noninjured patients with chronic neck pain (46.2%) was comparable to that of a previous report (33.3%).7 The high signal intensity could theoretically be due to altered ligament function caused by neck pain. Pain-induced immobility causes morphologic changes in muscles, tendons, and ligaments.24–26 In a group of patients with chronic WAD2, alar ligament high signal intensity was related to reduced cervical AROM and high NDI.10,27 In contrast, we found no relation to cervical AROM, NDI, or neck pain in our symptomatic controls. These noninjured patients with chronic neck pain tended to have more alar high signal intensity than patients who recovered from WAD. However, alar and transverse ligament high signal intensity is reported to be frequent also in healthy noninjured persons without neck pain.4,5,7,8 The high signal intensity is unlikely to be caused by pain-induced neck immobility.
Reported prevalence of ligament high signal intensity on upper neck high-resolution MR images has varied between cohorts, both in patients with chronic WAD and healthy controls. The significantly higher prevalence in patients with chronic WAD2 compared with noninjured controls found by Kråkenes et al6 has not been confirmed.5,7 The 45 nonrecovered patients with WAD in the present study (NDI > 8% at follow-up in Table 2) had similar prevalence of grades 2–3 high signal intensity as 157 noninjured asymptomatic controls in a previous study8 (alar ligaments 40% versus 31%; transverse ligament 27% versus 30%).
Twenty-three eligible patients (20%) did not complete follow-up MR examination. This represents an obvious limitation of the study. The dropouts were probably less motivated for repeated imaging as they had more often fully recovered. However, they had similar clinical and imaging characteristics in the acute phase as the 91 patients completing a second MR examination. The dropout of recovered patients would be expected to increase the prevalence of any trauma-related signal intensity changes and thus strengthened our findings of no such changes. The higher median age of the controls versus the patients with WAD (41.9 versus 29.2 years; P < .001) hardly influenced the frequency of ligament high signal intensity. This frequency did not vary with age, neither in the control group nor in the original WAD cohort.8
A major strength of this study is the prospective and consecutive inclusion of both patients with WAD and noninjured neck pain controls. Our cohort is highly representative of patients with WAD1–2 with no previous neck problems who seek medical care shortly after a car crash. The same magnet and protocol were used for all MR examinations providing comparable images. Direct comparison of the follow-up images with the previous images reflects clinical practice and is preferred for assessing changes in imaging findings over time.28–30 Had the follow-up images been interpreted blinded to the initial images, unwanted variation in the assessment of any changes could have been introduced. The MR imaging results were not transferred to the patients or their health care providers, avoiding possible effects on response rates and clinical outcome.
Conclusions
In this first study on follow-up MR imaging of upper neck ligaments, the signal intensity of the alar and transverse ligaments did not alter during the first year after a whiplash injury. A high signal intensity was similarly prevalent after whiplash injury and in noninjured patients with chronic neck pain. It cannot be explained by the acute trauma or altered ligament function due to neck pain. More likely it represents normal variants. Upper neck MR imaging is not recommended for routine use in the examination and follow-up of patients after a whiplash injury.
Acknowledgments
Geir E. Eide supervised the statistical analyses.
Abbreviations
- AROM
active range of motion
- CI
confidence interval
- NDI
Neck Disability Index
- NRS-11
11-point numeric rating scale
- WAD
whiplash-associated disorders
Footnotes
This study was funded by the Grieg Foundation and Norwegian ExtraFoundation for Health and Rehabilitation.
References
- 1. Dvorak J, Panjabi MM. Functional anatomy of the alar ligaments. Spine (Phila Pa 1976) 1987;12:183–89 [DOI] [PubMed] [Google Scholar]
- 2. Dvorak J, Schneider E, Saldinger P, et al. Biomechanics of the craniocervical region: the alar and transverse ligaments. J Orthop Res 1988;6:452–61 [DOI] [PubMed] [Google Scholar]
- 3. Heller JG, Amrani J, Hutton WC. Transverse ligament failure: a biomechanical study. J Spinal Disord 1993;6:162–65 [PubMed] [Google Scholar]
- 4. Lummel N, Zeif C, Kloetzer A, et al. Variability of morphology and signal intensity of alar ligaments in healthy volunteers using MR imaging. AJNR Am J Neuroradiol 2011;32:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dullerud R, Gjertsen O, Server A. Magnetic resonance imaging of ligaments and membranes in the craniocervical junction in whiplash-associated injury and in healthy control subjects. Acta Radiol 2010;51:207–12 [DOI] [PubMed] [Google Scholar]
- 6. Krakenes J, Kaale BR. Magnetic resonance imaging assessment of craniovertebral ligaments and membranes after whiplash trauma. Spine (Phila Pa 1976) 2006;31:2820–26 [DOI] [PubMed] [Google Scholar]
- 7. Myran R, Kvistad KA, Nygaard OP, et al. Magnetic resonance imaging assessment of the alar ligaments in whiplash injuries: a case-control study. Spine (Phila Pa 1976) 2008;33:2012–16 [DOI] [PubMed] [Google Scholar]
- 8. Vetti N, Kråkenes J, Damsgaard E, et al. MRI of the alar and transverse ligaments in acute whiplash-associated disorders 1–2—a cross-sectional controlled study. Spine 2011;36:E434–40 [DOI] [PubMed] [Google Scholar]
- 9. Vetti N, Kråkenes J, Damsgaard E, et al. MRI of the alar and transverse ligaments in whiplash-associated disorders (WAD) grades 1–2: high-signal changes by age, gender, event and time since trauma. Neuroradiology 2009;51:227–35 [DOI] [PubMed] [Google Scholar]
- 10. Kaale BR, Krakenes J, Albrektsen G, et al. Whiplash-associated disorders impairment rating: neck disability index score according to severity of MRI findings of ligaments and membranes in the upper cervical spine. J Neurotrauma 2005;22:466–75 [DOI] [PubMed] [Google Scholar]
- 11. Vetti N, Krakenes J, Eide GE, et al. Are MRI high-signal changes of alar and transverse ligaments in acute whiplash injury related to outcome? BMC Musculoskelet Disord 2010;11:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krakenes J, Kaale BR, Moen G, et al. MRI assessment of the alar ligaments in the late stage of whiplash injury–a study of structural abnormalities and observer agreement. Neuroradiology 2002;44:617–24 [DOI] [PubMed] [Google Scholar]
- 13. Spitzer WO, Skovron ML, Salmi LR, et al. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: redefining “whiplash” and its management. Spine (Phila Pa 1976) 1995;20:1S–73S [PubMed] [Google Scholar]
- 14. Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther 1991;14:409–15 [PubMed] [Google Scholar]
- 15. Ackelman BH, Lindgren U. Validity and reliability of a modified version of the neck disability index. J Rehabil Med 2002;34:284–87 [DOI] [PubMed] [Google Scholar]
- 16. Sterling M, Kenardy J, Jull G, et al. The development of psychological changes following whiplash injury. Pain 2003;106:481–89 [DOI] [PubMed] [Google Scholar]
- 17. Fejer R, Jordan A, Hartvigsen J. Categorising the severity of neck pain: establishment of cut-points for use in clinical and epidemiological research. Pain 2005;119:176–82 [DOI] [PubMed] [Google Scholar]
- 18. Jensen MP, Karoly P, O'Riordan EF, et al. The subjective experience of acute pain. An assessment of the utility of 10 indices. Clin J Pain 1989;5:153–59 [DOI] [PubMed] [Google Scholar]
- 19. Balogun J, Abereoje O, Olaogun M, et al. Inter- and intratester reliability of measuring neck motions with tape measure and Myrin gravity-reference goniometer. J Orthop Sports Phys Ther 1989;10:248–53 [Google Scholar]
- 20. Krakenes J, Kaale BR, Rorvik J, et al. MRI assessment of normal ligamentous structures in the craniovertebral junction. Neuroradiology 2001;43:1089–97 [DOI] [PubMed] [Google Scholar]
- 21. Zanetti M, De Simoni C, Wetz HH, et al. Magnetic resonance imaging of injuries to the ankle joint: can it predict clinical outcome? Skeletal Radiol 1997;26:82–88 [DOI] [PubMed] [Google Scholar]
- 22. Kreitner KF, Ferber A, Grebe P, et al. Injuries of the lateral collateral ligaments of the ankle: assessment with MR imaging. Eur Radiol 1999;9:519–24 [DOI] [PubMed] [Google Scholar]
- 23. Fujimoto E, Sumen Y, Ochi M, et al. Spontaneous healing of acute anterior cruciate ligament (ACL) injuries–conservative treatment using an extension block soft brace without anterior stabilization. Arch Orthop Trauma Surg 2002;122:212–16 [DOI] [PubMed] [Google Scholar]
- 24. Trudel G, Koike Y, Ramachandran N, et al. Mechanical alterations of rabbit Achilles' tendon after immobilization correlate with bone mineral density but not with magnetic resonance or ultrasound imaging. Arch Phys Med Rehabil 2007;88:1720–26 [DOI] [PubMed] [Google Scholar]
- 25. Woo SL, Gomez MA, Sites TJ, et al. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg Am 1987;69:1200–11 [PubMed] [Google Scholar]
- 26. Kasper CE, McNulty AL, Otto AJ, et al. Alterations in skeletal muscle related to impaired physical mobility: an empirical model. Res Nurs Health 1993;16:265–73 [DOI] [PubMed] [Google Scholar]
- 27. Kaale BR, Krakenes J, Albrektsen G, et al. Active range of motion as an indicator for ligament and membrane lesions in the upper cervical spine after a whiplash trauma. J Neurotrauma 2007;24:713–21 [DOI] [PubMed] [Google Scholar]
- 28. Oei EH, Koster IM, Hensen JH, et al. MRI follow-up of conservatively treated meniscal knee lesions in general practice. Eur Radiol 2010;20:1242–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu HT, Morrison WB, Schweitzer ME. Edematous Schmorl's nodes on thoracolumbar MR imaging: characteristic patterns and changes over time. Skeletal Radiol 2006;35:212–19 [DOI] [PubMed] [Google Scholar]
- 30. Mitra D, Cassar-Pullicino VN, McCall IW. Longitudinal study of high intensity zones on MR of lumbar intervertebral discs. Clin Radiol 2004;59:1002–08 [DOI] [PubMed] [Google Scholar]