SUMMARY:
CLOVES syndrome is a complex disorder of congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and skeletal/scoliosis/spinal anomalies. We report the occurrence of spinal-paraspinal fast-flow lesions within or adjacent to the truncal overgrowth or a cutaneous birthmark in 6 patients with CLOVES syndrome.
Several overgrowth syndromes are associated with vascular anomalies; the best know are Klippel-Trenaunay syndrome and Parkes Weber syndrome. Vascular anomalies also have been reported in Proteus syndrome.1,2 Subsequent clinical studies have shown that many patients once labeled with Proteus syndrome have a newly characterized overgrowth condition known by the acronym CLOVES, as described by Sapp et al3 and Alomari.4 Multiple, often marked, anomalies make the management of patients with CLOVES syndrome particularly difficult. Furthermore, a subgroup in our CLOVES syndrome cohort was noted to have a debilitating spinal myelopathy due fast-flow spinal-paraspinal vascular lesions. Our aim was to report the clinical presentation and imaging features of this morbid association.
Case Reports
This study was approved by our Committee on Clinical Investigation. Six of the 26 patients with CLOVES syndrome were identified as having complex spinal-paraspinal fast-flow lesions. The available imaging studies included MR imaging in 6 patients, CT in 4, and spinal angiography in 5. Each patient is described below to emphasize the clinical course and imaging findings.
Patient 1
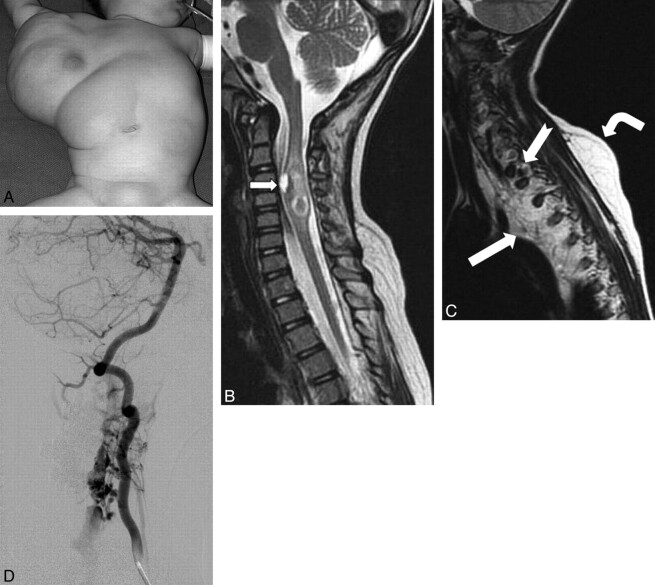
A 4-year-old girl presented to the emergency department of our hospital with a sudden onset of painless weakness of the left upper extremity and absent biceps reflexes. Her medical history was significant for a prenatal diagnosis of a complex lymphatic malformation of the right axilla and chest wall seen at 16 weeks' gestation. She was delivered via cesarean delivery. Truncal overgrowth was massive (Fig 1A) and continued to enlarge postnatally. A CT study demonstrated extensive fatty-lymphatic infiltration throughout the chest wall, axilla, and posterior mediastinum. At 3 months of age, she underwent partial resection of the truncal mass. At 9 months of age, she presented with a rapidly enlarging fatty mass of the abdominal wall, which was resected.
Fig 1.
A, Frontal photograph before the first surgical debulking. A large truncal mass extends into the right axilla, chest, and upper abdominal walls. Note a faint capillary malformation overlying the mass. B, Sagittal T2 MR image of the cervicothoracic spine demonstrates an expansile heterogeneous intramedullary lesion within the cervical spinal cord from the C4 to C6 levels, with signal-intensity characteristics consistent with blood products. A ventral cord lesion demonstrates a fluid-fluid level indicating an acute parenchymal hematoma (arrow). Perilesional T2 hyperintense rim is consistent with edema. C, Sagittal T2 MR image shows a diffuse involvement of the mediastinum and the paraspinal musculature with lymphatic malformation (white arrow) and fatty tissue, with marked deviation and compression of the spinal cord. There is an asymmetric fatty overgrowth of the spinal canal with the subcutaneous layer (bent arrow) insinuating into it via the neuroforamina, with large flow-void signals (notched arrow). D, Right vertebral arteriogram. The arterial supply to the epidural portion comes via the C3, C4, and C5 segmental branches of the right vertebral artery. The anterior and posterior spinal arteries are unremarkable. The lesion drains into markedly dilated epidural veins coursing inferiorly within the spinal canal and exits at a left upper thoracic neural foramen.
Urgent MR imaging was performed and demonstrated an expansile hemorrhagic nonenhancing intramedullary lesion within the cervical spinal cord from the C4 to C6 levels (Fig 1B). There was a large infiltrative fatty and lymphatic lesion within the posterior mediastinum, right axilla, and chest wall (Fig 1C). Large fast-flow vessels were noted in the mediastinal-paravertebral portion of this mass.
She was initially given corticosteroids and improved neurologically. The interdisciplinary recommendation was no urgent intervention, given the high risk.
During the following year, the patient had 3 more hemorrhagic episodes in the same spinal region causing transient increased left-arm weakness, incontinence, and some clumsiness of the gait. Given the ongoing episodes, the spinal lesion was excised, with a histopathologic diagnosis of cavernous (venous) malformation. The patient had residual proximal left-arm weakness.
Follow-up MR imaging revealed the development of large flow voids in the paraspinal mass that insinuated intraspinally via midcervical neuroforamina. Angiography revealed a paraspinal-epidural AVM, with drainage into enlarged epidural veins, which compressed the cord (Fig 1D). She underwent staged embolization and surgical removal of the AVM nidus. The histopathologic examination revealed markedly dilated blood vessels surrounded by abundant hemosiderin and hyalinization of the wall.
Patient 2
A white teenage boy had an asymmetric torso and extremities, with left hemihypertrophy. Capillary stains and melanocytic nevi were noted along the left paraspinal and gluteal area (Fig 2A). Some of these patches concomitantly displayed the appearance of a port-wine stain (capillary malformation). There was an extensive linear epidermal nevus on the right shoulder and anterior chest wall, which followed the lines of Blaschko (Fig 2B). A spinal MR imaging study showed extensive flow voids within and around the lower third of the spinal cord around the T10-L1 vertebral bodies (Fig 2C). The dilated channels on the surface of the cord represented a dilated posterior pial arterial network. The adjacent dilated intradural radicular veins drained the lesion transdurally into the ipsilateral paraspinal region via the widened neuroforamina. Deformity and abnormal hyperintense signal-intensity changes in the L2 vertebral body resulted in a local lumbar kyphosis.
Fig 2.
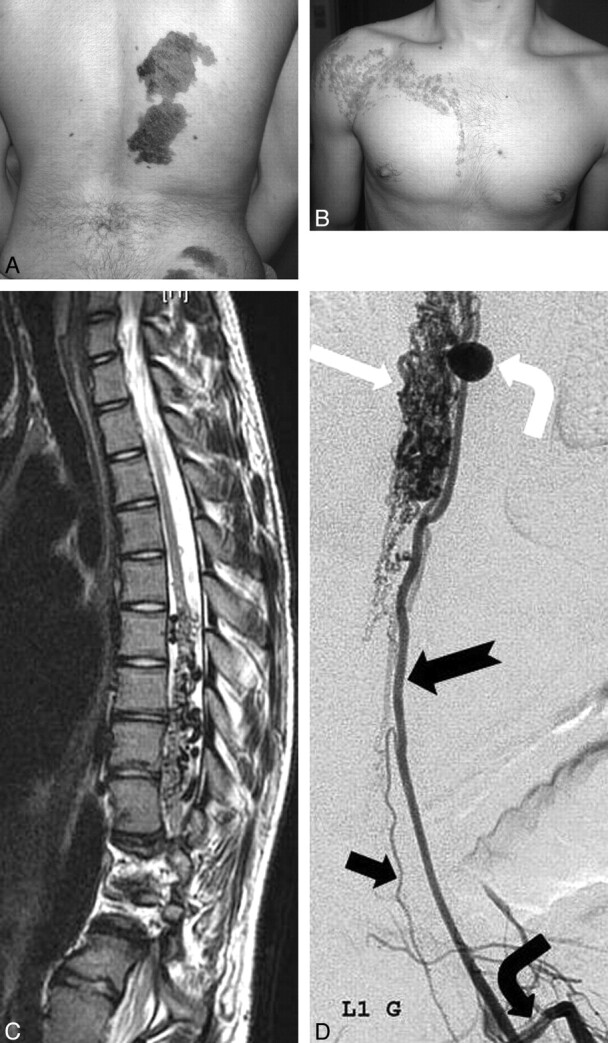
A, Multiple congenital dark nevi and capillary stains of the right paraspinal and gluteal areas. B, Linear epidermal nevus of the right shoulder and chest wall. Neither of these lesions crosses the midline. C, Spinal sagittal T2-weighted MR image demonstrates enlarged tortuous intradural flow voids (dorsally > ventrally) in the lower thoracic spine. The cord has an irregular contour due to the intradural dilated vessels, with focal hyperintense changes. Note also the extensive involvement of the adjacent vertebrae. D, Selective angiography of left first lumbar artery (bent black arrow) reveals an enlarged radiculomedullary artery (notched arrow) supplying an extensive dilated pial network (white arrow) on the surface of the cord via large pial branches. There is a large arterial aneurysm of the ascending pial branch (bent white arrow). Note the radiculomedullary supply to the anterior spinal artery (black arrow).
Spinal angiography showed a metameric shunt supplied predominantly by radiculopial branches from the lower thoracic intercostal arteries (Fig 2D). Additional supply was recruited from enlarged pial branches of a radiculomedullary artery from a left upper lumbar artery. The drainage was into an extensive dilated perimedullary pial network on the surface of the cord and markedly ectatic extradural veins.
Patient 3
A 17-year-old white boy was referred with the diagnosis of Klippel-Trenaunay syndrome. He was born with extensive vascular malformations of the legs and spine (Fig 3A) and had a history of seizures and upper gastrointestinal bleeding. A few months earlier, he had developed sharp back pain, urinary incontinence, fatigue, and dyspnea. Pulmonary embolism was excluded by CT angiography. On physical examination, there was overgrowth of both lower extremities and the dorsal trunk, with right paraspinal overgrowth covered by a large faint capillary stain, a facial epidermal nevus, and multiple-toe macrodactyly. Moderate scoliosis and a sharp-degree thoracolumbar kyphosis were also evident. Ambulation was limited as a result of advanced right-hip developmental dysplasia, and he used a wheelchair. Spinal MR imaging showed an extensive lumbosacral fatty overgrowth in the paraspinal region and asymmetric paraspinal muscles (Fig 3B). There were flow-void signals within the mass with extension into the spinal canal and erosion of at least 1 lamina. A large epidural venous channel was seen inside the lumbosacral spinal canal, almost equal in size to the thecal sac. The cord was tethered, with the conus terminating at the L4 level. There was compression of the conus medullaris and thecal sac along most of the lumbar and sacral regions and a Chiari 1 malformation. The paraspinal muscles were extensively infiltrated with a large conglomerate of vessels in a fatty substance.
Fig 3.
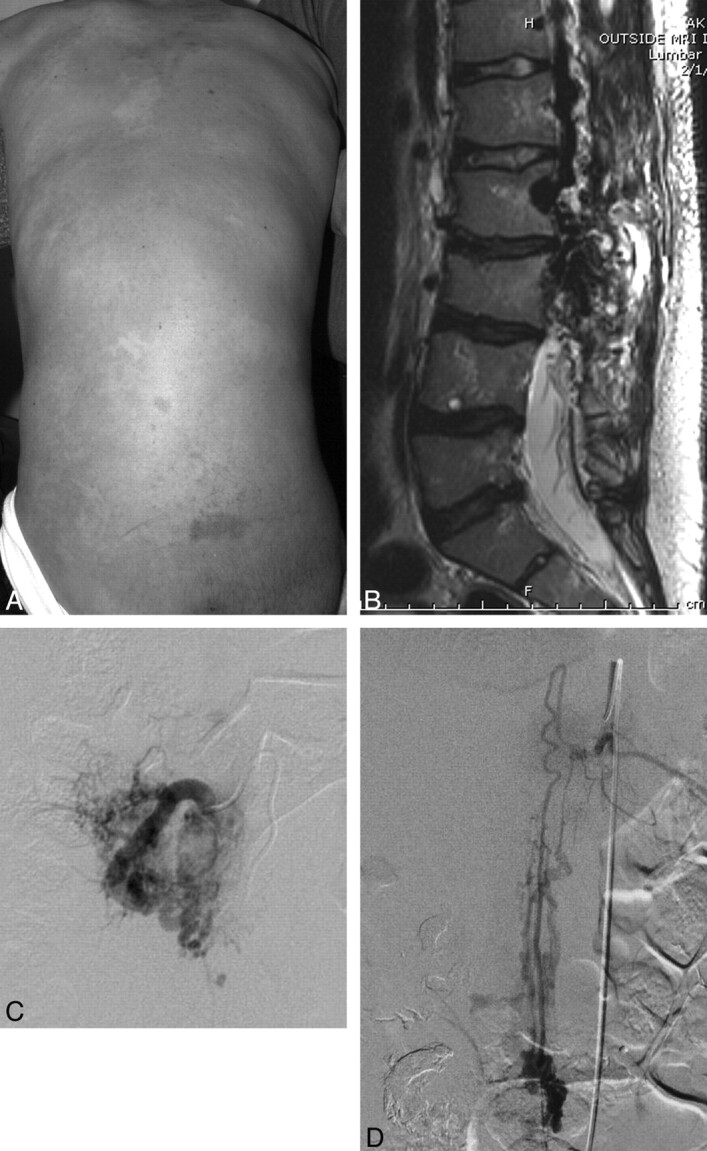
A, Faint diffuse capillary stain covering asymmetrically thickened soft tissue of the back and scoliosis. B, Sagittal T2-weighted MR image demonstrates a large area of abnormal heterogeneous tissue and signal-intensity void involving the paraspinal muscles, thecal sac, and adjacent lumbar vertebrae. Some fatty thickening and flow voids are also seen in the retroperitoneum. There is some loss of anterior height of the lumbar vertebrae with irregular contour, dehydration of the intervertebral disks, and kyphosis. C, Selective angiogram of the right L3 segmental artery demonstrates arterial dilation with extensive supply to the paravertebral AVM, centered within the paraspinal musculature. D, Selective study of the artery of Adamkiewicz (left T11) demonstrates a pial arteriovenous fistula at the L2 level.
Sonography of the urinary bladder showed significant postvoid residual urine, which required home catheterization. The pediatric neurosurgical consult advised a nonoperative approach. Spinal angiography showed a large region of arteriovenous paraspinal and spinal shunt surgeries occupying the right side of the spinal canal in the midlumbar region (Fig 3C). Arterial supply was primarily via multiple branches of L1-L4 segmental arteries and the artery of Adamkiewicz (left T11, Fig 3D). Venous drainage was via epidural and intrathecal veins ascending to the lower thoracic region.
Patient 4
A 3-year-old boy from Brazil was referred to our Vascular Anomalies Center with the diagnosis of the truncal form of Klippel-Trenaunay syndrome. The child was born with a large soft-tissue overgrowth of the back. There were capillary stains on the chest wall, forehead, and occipital areas. The left foot was large at birth and had grown proportionally since. The sole of the left foot had a furrowed appearance, and the first interdigital space was abnormally wide (sandal-gap toe). MR imaging showed an expansive infiltrative lesion with heterogeneous signal intensity located in the left paravertebral musculature with extension into the left retroperitoneal, pelvic, and gluteal areas. Fatty tissue and large vessels extended into the epidural space displacing the spinal cord. There was a small syrinx. Scoliosis and renal asymmetry were also noted.
Several resections of the truncal mass were performed. The histopathologic examination revealed complex benign tissue with lymphatic malformation and infiltrative hamartomatous tissue associated with arteriovenous fistulas. With time, there has been significant regrowth of the lesion.
Patient 5
A 4-year-old white girl was referred with a diagnosis of AVM-hemangioma-angiolipoma involving the left side of her trunk. She was born with capillary stains on both paraspinal regions. Retroperitoneal, pelvic, and gluteal extension of the paraspinal lesion was noted, and scoliosis was evident. The patient had a gait disturbance, with dysfunction of the right leg and a limb-length discrepancy. Occasionally, she felt some back pain with physical activity. She underwent resections of the lesion after preoperative embolization. CT of the trunk showed a large left paraspinal overgrowth composed largely of fat extending into the adjacent retroperitoneal and spinal canal. There was expansion of the spinal canal and some displacement of the cord in the lower thoracic area. Spinal angiography showed arteriovenous shunt surgery involving the overgrown paraspinal area as well as the vertebral body, with intradural and extradural drainage.
Patient 6
A 2-year-old girl was born with massive bilateral truncal overgrowth and extensive involvement of the chest wall, flank, axillae, and anterior abdominal wall with overlying capillary stains. There was asymmetric overgrowth of the lower extremities and gluteal areas with enlargement of the feet, macrodactyly, and syndactyly. The lower extremities progressively became paraparetic and spastic; contractures caused an inability to walk. There was notable cognitive delay. Skeletal anomalies included scoliosis and bilateral hip dysplasia. MR imaging showed extensive truncal overgrowth comprising predominantly fatty and lymphatic tissue, extending into the mediastinal, abdominal, and pelvic spaces. The left kidney was small. Spinal angiography at our hospital showed extensive hypervascularity and arteriovenous shunt surgery in the paraspinal soft tissues between the T2 and T5 levels, with extension into the epidural space. The arterial feeders arose from numerous small branches of the segmental arteries with epidural drainage into the azygous vein.
After preoperative embolization, shunt surgery through the intraspinal portion was almost completely eliminated. Thereafter, cord compression by the intraspinal vascular tissue and lack of spinal cord function below the affected level necessitated exploration and T1-T5 laminectomy. Hypervascular tissue found in the intradural-extramedullary space communicated with the pial vessels. The debilitating course of the disease resulted in paraparesis, chronic pain, seizures, learning disabilities, malnourishment, chronic constipation, and urine incontinence.
Discussion
CLOVES syndrome is a recently described sporadic overgrowth disorder with features of truncal fatty overgrowth, vascular malformations, epidermal nevus, and skeletal anomalies (including scoliosis and variable acral anomalies).1,2 Patients with CLOVES syndrome are typically born with lipomatous masses of the thoracic and abdominal wall (commonly in the posterolateral chest wall and flank) with variable contiguous extension to the anterior abdominal wall, groin, retroperitoneum, mediastinum, and gluteal area. Slow-flow vascular malformations (including lymphatic malformations and phlebectasia) are common.4 Other manifestations of the syndrome include musculoskeletal (leg-length discrepancy, chondromalacia patellae, dislocated knees, scoliosis, wide hands and feet, furrowed soles, sandal-gap toe, macrodactyly, talipes, windswept hand, hemihypertrophy), neurologic (neural tube defect, tethered cord), cutaneous (capillary malformation, epidermal nevus, multiple small nevi), and other anomalies (such as renal agenesis/hypoplasia).
Spinal cord arteriovenous shunts are a rare and heterogeneous group of vascular anomalies. Although the natural history of these lesions is variable, the overall prognosis is considered unfavorable.5 An exceedingly rare form of arteriovenous shunt, located outside the spinal canal, can potentially result in spinal cord injury (ie, a paraspinal or paravertebral AVM with epidural venous drainage).6 Draining via the epidural venous plexus into spinal cord veins can potentially result in venous hypertension and myelopathy.7
In a review of the cohort of patients with CLOVES syndrome at Children's Hospital Boston, Alomari4 described the presence of spinal-paraspinal fast-flow lesions in 5 of 18 patients. The spinal column can be affected in several ways in patients with CLOVES. Direct insinuation of the mass through the neuroforamina into the epidural space can compress the cord, thecal sac, or nerve roots. Parasitization of blood supply from segmental feeders (both pial and, more commonly, nonpial branches) has also been noted to follow the distribution of the fatty-lymphatic overgrowth. Engorged dilated dural veins within the spinal canal can also cause mass effect. In the rare paravertebral AVMs, such as seen in CLOVES syndrome, venous hypertension in the paravertebral plexus may affect the spinal cord through reflux into the pial veins via an epidural route, causing compromise of the cord function.8 Other spinal comorbidities in CLOVES syndrome include scoliosis, vertebral anomalies, neural tube defects, and tethered cord.
The treatment of spinal AVMs is a neurosurgical challenge.9 Resection of the fatty truncal mass in CLOVES syndrome, if feasible, is associated with recurrence.4 Endovascular techniques can be helpful in controlling the flow in AV shunts and alleviating symptoms in some of these lesions, as well as being a useful adjunct to resection.6
Some of the cardinal features of CLOVES syndrome may appear to overlap other overgrowth disorders, such as Klippel-Trenaunay syndrome, Cobb syndrome, Proteus syndrome, and Parkes Weber syndrome. Bannayan-Riley-Ruvalcaba syndrome and Lhermitte-Duclos disease (2 rare syndromes resulting from mutation in the PTEN gene) also have been reported to have paraspinal arteriovenous shunts.10,11 Many of the case reports presumed to be examples of Cobb syndrome are more likely examples of CLOVES syndrome.8,12–17 The index case by Stanley Cobb in 1915 described the association of a cutaneous nevus and a spinal AVM in the same metamere.18 Since then, the Cobb syndrome diagnosis has been indiscriminately applied to various pathologies in which a spinal AVM and cutaneous lesions coexist.
Weon et al12 reported a case of a young adult woman who presented with a spinal AVM in the lumbar region associated with a solid fatty mass. The authors referred to the mass as a “lipomyelomeningocele,” which had an intradural extension and associated spinal arteriovenous shunt supplied by the radiculopial branches of the lumbar arteries and drained by tortuous dilated perimedullary veins. Spinal MR imaging revealed a lumbosacral fatty mass with extension into the spinal canal, spina bifida, and tethered cord. Zala and Mumenthaler13 described a case of presumed Cobb syndrome in which the patient demonstrated hemihypertrophy, verrucous angioma, and café au lait spots. Clinton et al14 reported a boy with a verrucous vascular malformation of the right chest and upper back associated with a symptomatic “cavernous” vascular malformation in the corresponding thoracic spinal cord. Shim et al15 associated Cobb syndrome with lymphangioma circumscriptum. Brant et al16 reported an interesting case of a neonate girl born with a large subcutaneous mass covered by a cutaneous hemangioma of the lumbosacral region. Ratzenhofer et al17 described spinal arteriovenous shunts and spinal angiomas, linear epidermal nevus, leg length discrepancy, and other pedal and osseous changes in a 12-year-old boy. Berenstein et al8 also referred to a large pulsatile mass of the right paraspinal mass associated with a purely extraspinal AVM. Analysis of these cases involving truncal fatty masses (lipomeningocele, paraspinal mass), lymphatic lesions (lymphangioma circumscriptum, verrucous vascular lesions), other cutaneous lesions (linear epidermal nevus, hemangioma), and musculoskeletal anomalies (hemihypertrophy, foot anomalies) suggests that they are all likely to be examples of CLOVES syndrome.
The etiology of overgrowth syndromes associated with vascular anomalies, including CLOVES, is largely unknown. These rare sporadic and asymmetric phenotypes could result from genetic mutations, which, in autosomal form, would be lethal but which survive through mosaicism, as proposed by Happle.19 The coexistence of fatty overgrowth and fast-flow shunts in CLOVES syndrome is further evidence of a strong relation between the adipocytes and the endothelial cells. The pioneering work of Rupnick et al20 and Folkman21 showed that adipose tissue growth is angiogenesis-dependent and angiogenesis inhibitors cause decreased endothelial cell proliferation and increased apoptosis in the adipose tissue of treated animals. The angioarchitecture of these complex shunts displays a metameric disposition with arterial feeders derived from consecutive segmental arteries.
This retrospective analysis relied on available data regarding a new rare disorder. Not all the subjects in the CLOVES cohort were evaluated for the presence of paraspinal shunts. Specifically, relevant diagnostic studies, such as spinal angiography and MR imaging, were not performed or available in all patients. The presence of other spinal abnormalities in CLOVES (such as paraspinal masses, tethered cord, nerve and cord compression, kyphoscoliosis, skeletal anomalies, and so forth) could also account for some of the patients' signs and symptoms, precluding further imaging work-up. These limitations might result in an underestimation of the true incidence of the vascular paraspinal lesions in these patients.
All patients in our cohort with spinal-paraspinal involvement by fast-flow lesions had clearly abnormal vascular structures on imaging; there were no examples of subtle lesions with small arterial feeders. As such, we would expect that cross-sectional imaging would be adequate as a screening tool for patients with CLOVES and that in the absence of intraspinal flow voids on MR imaging, spinal angiography for the sole purpose of establishing the diagnosis appears to be unnecessary.
Conclusions
Spinal-paraspinal fast-flow infiltrative lesions are a relatively common cause of myelopathy in patients with CLOVES syndrome. Heightened awareness of this association may allow earlier diagnosis.
Abbreviations
- AVM
arteriovenous malformation
- CLOVES syndrome
congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and skeletal/scoliosis/spinal anomalies
References
- 1. Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet 1999;84:389–95 [DOI] [PubMed] [Google Scholar]
- 2. Turner JT, Cohen MM, Jr,, Biesecker LG. Reassessment of the Proteus syndrome literature: application of diagnostic criteria to published cases. Am J Med Genet A 2004;130A:111–22 [DOI] [PubMed] [Google Scholar]
- 3. Sapp JC, Turner JT, van de Kamp JM, et al. Newly delineated syndrome of congenital lipomatous overgrowth, vascular malformations, and epidermal nevi (CLOVE syndrome) in seven patients. Am J Med Genet A 2007;143A:2944–58 [DOI] [PubMed] [Google Scholar]
- 4. Alomari AI. Characterization of a distinct syndrome that associates complex truncal overgrowth, vascular, and acral anomalies: a descriptive study of 18 cases of CLOVES syndrome. Clin Dysmorphol 2009;18:1–7 [DOI] [PubMed] [Google Scholar]
- 5. Aminoff MJ, Logue V. The prognosis of patients with spinal vascular malformations. Brain 1974;97:211–18. [DOI] [PubMed] [Google Scholar]
- 6. Hui F, Trosselo MP, Meisel HJ, et al. Paraspinal arteriovenous shunts in children. Neuroradiology 1994;36:69–73 [DOI] [PubMed] [Google Scholar]
- 7. Goyal M, Willinsky R, Montanera W, et al. Paravertebral arteriovenous malformations with epidural drainage: clinical spectrum, imaging features, and results of treatment. AJNR Am J Neuroradiol 1999;20:749–55 [PMC free article] [PubMed] [Google Scholar]
- 8. Berenstein A, Lasjaunias P. Spine and spinal cord vascular lesions. In: Surgical Neuroangiography: Endovascular Treatment of Spine and Spinal Cord Lesions. Berlin, Germany: Springer-Verlag; 2004:752–53 [Google Scholar]
- 9. Djindjian R. Embolization of angiomas of the spinal cord. Surg Neurol 1975;4:411–20 [PubMed] [Google Scholar]
- 10. Akiyama Y, Ikeda J, Ibayashi Y, et al. Lhermitte-Duclos disease with cervical paraspinal arteriovenous fistula. Neurol Med Chir (Tokyo) 2006;46:446–49 [DOI] [PubMed] [Google Scholar]
- 11. Tan WH, Baris HN, Burrows PE, et al. The spectrum of vascular anomalies in patients with PTEN mutations: implications for diagnosis and management. J Med Genet 2007;44:594–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weon YC, Chung JI, Roh HG, et al. Combined spinal intramedullary arteriovenous malformation and lipomyelomeningocele. Neuroradiology 2005;47:774–79 [DOI] [PubMed] [Google Scholar]
- 13. Zala L, Mumenthaler M. Cobb syndrome: association with verrucous angioma, ipsilateral hypertrophy of the extremities and café-au-lait spots [in German]. Dermatologica 1981;163:417–25 [PubMed] [Google Scholar]
- 14. Clinton TS, Cooke LM, Graham BS. Cobb syndrome associated with a verrucous (angiokeratomalike) vascular malformation. Cutis 2003;71:283–87 [PubMed] [Google Scholar]
- 15. Shim JH, Lee DW, Cho BK. A case of Cobb syndrome associated with lymphangioma circumscriptum. Dermatology 1996;193:45–47 [DOI] [PubMed] [Google Scholar]
- 16. Brant AJ, James HE, Tung H. Cutaneomeningospinal angiomatosis (Cobb syndrome) with tethered cord. Pediatr Neurosurg 1999;30:93–95 [DOI] [PubMed] [Google Scholar]
- 17. Ratzenhofer E, Hohlbrugger H, Gebhart W, et al. Linear epidermal nevus with multiple malformations (author's transl) [in German]. Klin Padiatr 1981;193:117–19 [DOI] [PubMed] [Google Scholar]
- 18. Maramattom BV, Cohen-Gadol AA, Wijdicks EF, et al. Segmental cutaneous hemangioma and spinal arteriovenous malformation (Cobb syndrome): case report and historical perspective. J Neurosurg Spine 2005;3:249–52 [DOI] [PubMed] [Google Scholar]
- 19. Happle R. Lethal genes surviving by mosaicism: a possible explanation for sporadic birth defects involving the skin. J Am Acad Dermatol 1987;16:899–906 [DOI] [PubMed] [Google Scholar]
- 20. Rupnick MA, Panigrahy D, Zhang CY, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A 2002;99:10730–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Folkman J. Angiogenesis: initiation and control. Ann N Y Acad Sci 1982;401:212–27 [DOI] [PubMed] [Google Scholar]