SUMMARY:
Although a relatively rare neoplasm, primary carcinoid tumor has an unusual propensity to metastasize to the orbits. Within the orbit, metastatic EOM lesions have been described in scattered reports in the ophthalmology literature but have received little to no attention in the radiology literature. After a retrospective review, we identified CT and MR imaging studies of 7 patients with carcinoid tumor metastatic to the EOM. Our findings suggest that in patients with known carcinoid tumor, well-defined, round, or fusiform masses of the EOM should strongly suggest metastatic involvement. Our series suggests that bilateral lesions may occur and that any EOM can be involved. Knowledge of this pattern of metastatic disease may spare biopsies in some patients, and with current orbit-sparing therapy for patients with localized orbital disease, early and accurate diagnosis can significantly improve patient outcomes.
Carcinoid tumors are rare neuroendocrine neoplasms derived from enterochromaffin cells, which are found primarily in the gastrointestinal tract and bronchial tree.1,2 Liver metastases are the classic presentation of distant disease, which can lead to carcinoid syndrome (flushing, diarrhea, and wheezing) and right-sided valvular heart disease. However as treatment options improve and survival increases, new metastatic patterns have been increasing in frequency.3 Although rare, metastatic carcinoid to the extraocular muscles has been relatively well described in both retrospective case reports and clinical series in the ophthalmology literature.4–13 However, by our review of the radiology literature, there is a single dedicated case report detailing the imaging findings for this entity.14 Even within the ophthalmology literature, only a handful of reports focus exclusively on metastases involving the EOMs.4,8,15 We present the first series detailing the CT and MR imaging findings of carcinoid tumor metastases to the EOMs.
Materials and Methods
A waiver of informed consent was obtained from our institutional review board, and in a manner consistent with Health Insurance Portability and Accountability Act regulations, we retrospectively collected clinical data and CT and MR images available from our institution in patients with known carcinoid tumor. In addition, via a survey of head and neck radiologists from collaborating institutions, additional cases were collected. All imaging analyzed for this study was performed from January 2000 through October of 2010.
We found a total of 7 patients with known metastatic disease to the EOMs. The primary inclusion criterion was the presence of EOM lesions in patients with known metastatic carcinoid tumor, though the presence of non-EOM intraorbital disease was not considered an exclusion criterion. Patients were not included in this study if any alternate diagnosis such as an infectious or inflammatory condition of the EOM could not be could be excluded on the basis of the amalgam of clinical and imaging data available in the medical record or as directly provided to the radiologist by the referring physician. A clinical chart review was performed to collect patient age, sex, symptoms, site of original tumor, and means of diagnosis. Specific clinical symptoms at the time of the imaging were available for 5 of the 7 patients in the series.
We retrospectively reviewed the MR imaging (n = 5) or CT (n = 2) examinations for these patients. In all patients, contrast-enhanced orbital imaging was available for review. For all CT examinations, postcontrast imaging was performed after the administration of nonionic contrast material. Coronal reconstructions were available in all patients with CT examinations. All MR imaging sequences were performed with 1.5T or higher scanners, with, at the minimum, pre- and postgadolinium-enhanced T1-weighted images available for review.
All images were directly reviewed by a neuroradiology fellow, a senior radiology resident, and a head and neck radiologist with a Certificate of Added Qualification in Neuroradiology and >20 years of experience. We evaluated imaging findings including the following: 1) the distribution of EOMs involved (including the presence or absence of bilateral lesions), 2) the lesion morphology (fusiform, round, or irregular), 3) the margin of the lesion (well-circumscribed or infiltrative and involving adjacent structures), 4) involvement of the tendinous insertion of the muscle onto the globe, and 5) the attenuation or intensity of the lesion on postcontrast imaging relative to uninvolved EOM. We also reviewed for the presence or absence of other (non-EOM) orbital metastatic disease.
Results
Summary of Patient Data
The imaging and clinical findings are summarized in the Table. Figures 1–4 show representative cases. Six of 7 patients were women, and the mean age was 61.9 years. All lesions evaluated were well-defined, round or fusiform, without involvement of adjacent intraorbital structures, tendinous insertion of the globe, or osseous structures of the orbit. In the 5 patients with MR imaging, postgadolinium T1-weighted images demonstrated lesions that, relative to uninvolved EOM, were mildly hyperintense (n = 1), isointense (n = 1), and mildly to moderately hypointense (n = 3). In all patients for whom T2-weighted imaging was available for review (n = 4), the lesion intensity was mildly hyperintense to uninvolved muscle in all patients (though heterogeneously hyperintense in 1 patient, Fig 3A). In the 2 patients in whom postcontrast CT imaging was reviewed, the lesion was mildly hyperattenuated relative to uninvolved EOM. A total of 17 separate muscle lesions were noted in the 7 patients, with 5 patients demonstrating bilateral lesions. Of muscles involved, the most frequent occurrence was noted in the LR and the least frequent in the SR (number of lesions: LR = 7, IR = 3, MR = 3, SO = 2, IO = 1, and SR = 1). Although clinical presentations varied, proptosis and difficulty with gaze were reported most commonly.
Demographic, clinical, and imaging findings for the patient cohort
| Patient No. | Age (yr) | Sex | Clinical Presentation | CT or MRI Available | EOMs Involved | Lesion Morphology | Lesion Margins | Involvement of Insertion of EOM on Globe | Density/Intensity on Postcontrast Relative to Normal EOM |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | Proptosis and CN VI palsy | MRI | Rt. MR | Fusiform | Well-defined | No | Mildly hyperintense |
| Lt. LR | |||||||||
| 2 | 42 | F | Gaze difficulty and tearing | CT | Lt. LR | Fusiform | Well-defined | No | Moderately hyperdense |
| 3 | 71 | F | N/A | MRI | Rt. SO | Round | Well-defined | No | Mildly hypointense |
| 4 | 71 | F | N/A | MRI | Rt. LR, MR, SO, Lt. LR and IR | Round and fusiform | Well-defined | No | Mildly hypointense |
| 5 | 62 | F | Proptosis and pain | MRI | Rt. LR | Round | Well-defined | No | Moderately hypointense |
| Lt. IR | |||||||||
| 6 | 53 | F | Proptosis and gaze difficulty | CT | Rt. LR, SR, MR | Round and fusiform | Well-defined | No | Mildly hyperdense |
| Lt. LR | |||||||||
| 7 | 70 | M | Blurry vision | MRI | Rt. IR | Fusiform | Well-defined | No | Isointense |
| Lt. IO |
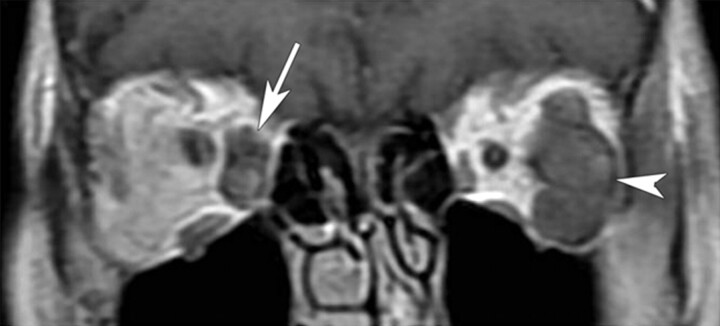
Fig 1.
Coronal postcontrast T1-weighted image of the orbits in patient 1 demonstrates a heterogeneously enhancing ovoid lesion involving the right medial rectus (arrow). A similarly enhancing but larger lobulated lesion involves the left lateral rectus (arrowhead).
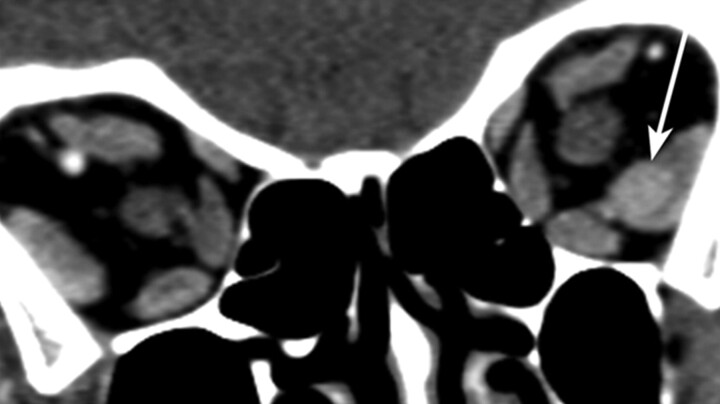
Fig 2.
Coronal reformat contrast-enhanced CT image of the orbits in patient 2 demonstrates a homogeneously enhancing well-circumscribed rounded mass within the left lateral rectus (arrow).
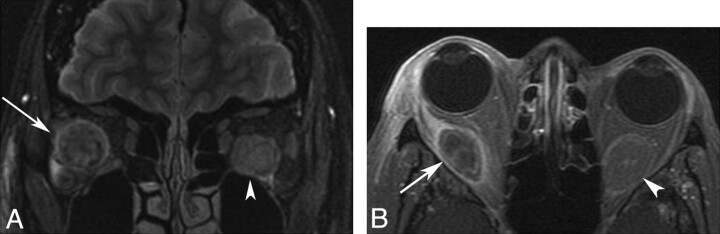
Fig 3.
A, Coronal fat-saturated T2-weighted image of the orbits in patient 5 demonstrates a heterogeneously hyperintense well-circumscribed masses involving the right lateral (arrow) and left inferior recti (arrowhead). B, Axial fat-saturated T1-weighted postgadolinium image in this same patient demonstrates heterogeneous hypoenhancement of the fusiform masses of the right lateral (arrow) and left inferior recti (arrowhead).
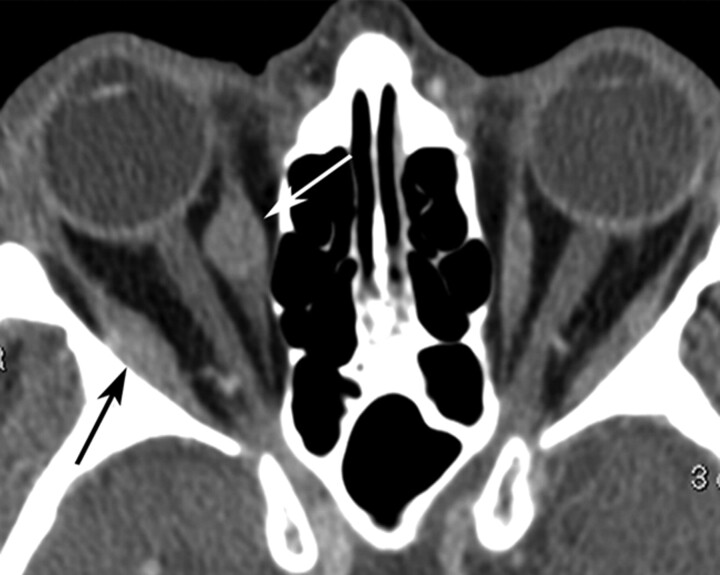
Fig 4.
Axial contrast-enhanced CT image of the orbits in patient 6 shows ovoid well-defined lesions of the right medial (black arrow) and lateral (white arrow) recti.
Pathology samples (available in 3 patients) revealed carcinoid tumors that originated from an unknown site after a liver biopsy (patients 1 and 7) and from the pancreas after biopsy of a liver lesion (patient 5). In the remaining 4 cases, the patients had a known history of metastatic carcinoid tumor and had developed orbital lesions that could not reasonably be explained both clinically and on the basis of imaging findings by an alternative diagnosis, and responded clinically in a manner consistent with metastatic neuroendocrine tumor (eg, sensitivity to appropriate radiation therapy or chemotherapy). An illustrative case history is provided below:
Case History: Patient 1
This 64-year-old woman presented in late 2005 with abdominal pain. CT of the chest, abdomen, and pelvis revealed multiple liver lesions, hilar adenopathy, and diffuse sclerotic osseous lesions. Laboratory values revealed elevated serotonin levels. An indium-111 octreotide scan revealed multifocal lesions involving the liver, the lung hilum, and osseous structures. Sonographically guided biopsy of the liver lesion revealed metastatic well-differentiated carcinoid tumor, with the primary site unknown. The patient was treated with multiple rounds of chemotherapy with a waxing and waning response. In June 2007, the patient developed biopsy-proved metastatic neck lymphadenopathy from the carcinoid primary and still had evidence of extensive abdominal metastatic disease.
In December 2009, the patient presented with a left CN VI palsy but was otherwise asymptomatic from a neurologic perspective. MR imaging of the brain in December 2009 revealed multiple lesions of the EOMs thought most likely to represent carcinoid metastases, as well as a separate lesion involving the cavernous sinus. Given the absence of a competing diagnostic consideration, the decision was made to proceed with therapy without radionuclide scintigraphy or direct EOM biopsy. The patient was treated with neoadjuvant chemotherapy followed by external beam radiation therapy to the EOM with evidence of positive treatment response on follow-up MR imaging in February 2010.
Discussion
Our series examines the imaging appearance, on both CT and MR imaging, for EOM metastases in patients with carcinoid tumor. Comprising only about 0.5% of all malignancies, carcinoid tumor is an uncommon neoplasm.1 Metastases to the orbit do occur, generally in advanced disease, and are estimated to represent a disproportionately high percentage of all orbital metastases—approximately 5%.16 Despite this, to our knowledge, fewer than 40 cases have been reported in the ophthalmology/surgical literature.4,6,8,11,13,17 In the English radiology literature, only a single case report from 1987 by Braffman et al,14 of a single metastatic lesion to the orbit has been described. Our series demonstrates that these lesions can appear as well-circumscribed, fusiform, or round masses within the EOM muscle belly. In addition, although carcinoid metastases elsewhere in the body are typically thought of as hypervascular lesions, marked or avid postgadolinium enhancement was not a uniformly identified feature of these lesions in our series. The lesions were generally mildly hyperintense on T2-weighted imaging and mildly hyperattenuating to uninvolved EOM on contrast-enhanced CT.
EOM metastases are well-described in a host of primary neoplasms, including breast, prostate, kidney, lung, and melanoma.5,15,18,19 Our experience, combined with the existing ophthalmology literature, suggests a tendency for both intraorbital, extramuscular, and EOM metastatic disease in patients with carcinoid primary tumors.4,6,8,13,14,17 Reports suggest that uveal tract carcinoid metastases are more commonly noted in bronchial lesions, whereas EOM metastases more commonly occur in gastrointestinal primary carcinoids.7,8,13 Although our sample size was small and pathology was incomplete, our series is consistent with this distribution as gastrointestinal tract disease with liver metastases was demonstrated in those patients with available corroborating clinical data. The pathophysiology for carcinoid (or other neoplasms) to seed the EOMs versus other components of the orbit is unknown, though some authors speculate, and it seems likely, that an immunologic mechanism or tumor cell adhesion factors in the local cellular microenvironment may play a role.5,20
Regarding bilateral and multifocal disease, a study by Mehta et al13 of 13 patients with orbital carcinoid metastases showed that only 2 of 13 patients had bilateral lesions and no quadrant was favored. In contrast, our limited series showed a relatively higher proportion of bilaterality (5 of 7 patients) and a slight predominance of lesions in the lateral rectus. Also, 6 of our 7 patients were women, which is of interest in light of the known slight predominance of carcinoid tumor in women (55%),1 and the mild female bias noted in prior literature reviews for orbital metastases.13 However, given the small sample size of this and other series, the significance of such numeric comparisons is somewhat limited.
However, knowledge of the propensity of carcinoid tumors to spread to the EOM in patients with metastatic disease is of greater importance. MR imaging or CT remain excellent tools to evaluate the presence of these lesions, with focal nodular enlargement of the muscle without invasion of the orbital fat or adjacent osseous structures highly suggestive of EOM metastases in a patient with known malignancy.5 Furthermore, cross-sectional imaging can demonstrate bilateral disease even if only unilateral symptoms are present. Although other entities such as thyroid orbitopathy, infectious myositis, or idiopathic orbital inflammatory syndrome may produce a similar appearance,20 in the context of a known primary carcinoid, a metastasis should be high in the radiologic differential. Historically, confirmation of the diagnosis via tissue sampling, radionuclide scintigraphy with a somatostatin analog or I-131 meta-iodobenzylguanidine, or the appropriate combination of cross-sectional imaging findings and clinical history would result in orbital exenteration.8,13 However, with advances in chemotherapy and radiation treatment protocols, early or limited disease may not require radical surgical resection. Given the limited role of physical or ophthalmologic examination findings in confidently diagnosing EOM metastases, imaging may be the primary means of detecting this entity. As a consequence, suggestion of this entity by the radiologist may play an important role in the care of these patients.
Acknowledgments
We thank Wendy R. Smoker, MD, Laurie Loevner, MD, Deborah Shatzkes, MD, and A. John Tsiouris, MD, for their kind assistance in helping to identify potential cases for our series.
C. Douglas Phillips has a financial relationship with Amirsys, Inc.
- CN VI =
- cranial nerve VI
- EOM =
- extraocular muscle;
- IO =
- inferior oblique;
- IR =
- inferior rectus;
- LR =
- lateral rectus;
- MR =
- medial rectus;
- MRI =
- MR imaging;
- N/A =
- not applicable;
- SO =
- superior oblique;
- SR =
- superior rectus
References
- 1. Modlin IM, Lye KD, Kidd M.. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934–59 [DOI] [PubMed] [Google Scholar]
- 2. Pinchot SN, Holen K, Sippel RS, et al. Carcinoid tumors. Oncologist 2008;13:1255–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zuetenhorst JM, Taal BG.. Metastatic carcinoid tumors: a clinical review. Oncologist 2005;10:123–31 [DOI] [PubMed] [Google Scholar]
- 4. Borota OC, Kloster R, Lindal S.. Carcinoid tumour metastatic to the orbit with infiltration to the extraocular orbital muscle. APMIS 2005;113:135–39 [DOI] [PubMed] [Google Scholar]
- 5. Capone A, Jr, Slamovits TL.. Discrete metastasis of solid tumors to extraocular muscles. Arch Ophthalmol 1990;108:237–43 [DOI] [PubMed] [Google Scholar]
- 6. Fan JT, Buettner H, Bartley GB, et al. Clinical features and treatment of seven patients with carcinoid tumor metastatic to the eye and orbit. Am J Ophthalmol 1995;119:211–18 [DOI] [PubMed] [Google Scholar]
- 7. Harbour JW, De Potter P, Shields CL, et al. Uveal metastasis from carcinoid tumor: clinical observations in nine cases. Ophthalmology 1994;101:1084–90 [DOI] [PubMed] [Google Scholar]
- 8. Kiratli H, Yilmaz PT, Yildiz ZI.. Metastatic atypical carcinoid tumor of the inferior rectus muscle. Ophthal Plast Reconstr Surg 2008;24:482–84 [DOI] [PubMed] [Google Scholar]
- 9. Perri P, Bandello F, Police G, et al. Multiple bilateral choroidal metastatic tumor from bronchial carcinoid: case report. Retina 1998;18:385–86 [DOI] [PubMed] [Google Scholar]
- 10. Shetlar DJ, Font RL, Ordonez N, et al. A clinicopathologic study of three carcinoid tumors metastatic to the orbit: immunohistochemical, ultrastructural, and DNA flow cytometric studies. Ophthalmology 1990;97:257–64 [DOI] [PubMed] [Google Scholar]
- 11. Shields CL, Shields JA, Eagle RC, Jr, et al. Orbital metastasis from a carcinoid tumor: computed tomography, magnetic resonance imaging, and electron microscopic findings. Arch Ophthalmol 1987;105:968–71 [DOI] [PubMed] [Google Scholar]
- 12. Tunc M, Wendel R, Char DH.. Bilateral multifocal choroidal carcinoid: long-term follow-up without treatment. Am J Ophthalmol 1998;125:875–76 [DOI] [PubMed] [Google Scholar]
- 13. Mehta JS, Abou-Rayyah Y, Rose GE.. Orbital carcinoid metastases. Ophthalmology 2006;113:466–72 [DOI] [PubMed] [Google Scholar]
- 14. Braffman BH, Bilaniuk LT, Eagle RC, Jr, et al. MR imaging of a carcinoid tumor metastatic to the orbit. J Comput Assist Tomogr 1987;11:891–94 [DOI] [PubMed] [Google Scholar]
- 15. Souayah N, Krivitskaya N, Lee HJ.. Lateral rectus muscle metastasis as the initial manifestation of gastric cancer. J Neuroophthalmol 2008;28:240–41 [DOI] [PubMed] [Google Scholar]
- 16. Goldberg RA, Rootman J, Cline RA.. Tumors metastatic to the orbit: a changing picture. Surv Ophthalmol 1990;35:1–24 [DOI] [PubMed] [Google Scholar]
- 17. Khaw P, Ball D, Duchesne G.. Carcinoid tumour of the orbital muscles: a rare occurrence. Australas Radiol 2001;45:179–81 [DOI] [PubMed] [Google Scholar]
- 18. Alsuhaibani AH, Carter KD, Nerad JA, et al. Prostate carcinoma metastasis to extraocular muscles. Ophthal Plast Reconstr Surg 2008;24:233–35 [DOI] [PubMed] [Google Scholar]
- 19. Spitzer SG, Bersani TA, Mejico LJ.. Multiple bilateral extraocular muscle metastases as the initial manifestation of breast cancer. J Neuroophthalmol 2005;25:37–39 [DOI] [PubMed] [Google Scholar]
- 20. Sira M, Clauss RP, Maclean C, et al. Orbital metastases from neuroendocrine carcinoma, masquerading as Graves orbitopathy. Orbit 2010;29:94–96 [DOI] [PubMed] [Google Scholar]