Abstract
BACKGROUND AND PURPOSE:
The STP has been regarded as the most plausible neural tract responsible for pathogenesis of central poststroke pain. The VPL nucleus has been a target for neurosurgical procedures for control of central poststroke pain. However, to our knowledge, no DTI studies have been conducted to investigate the somatotopic location of the STP at the VPL nucleus of the thalamus. In the current study, we attempted to investigate this location in the human brain by using a probabilistic tractography technique of DTI.
MATERIALS AND METHODS:
DTI was performed at 1.5T by using a Synergy-L SENSE head coil. STPs for both the hand and leg were obtained by selection of fibers passing through 2 regions of interest (the area of the spinothalamic tract in the posterolateral medulla and the postcentral gyrus) for 41 healthy volunteers. Somatotopic mapping was obtained from the highest probabilistic location at the ACPC level.
RESULTS:
The highest probabilistic locations for the hand and leg were an average of 16.86 and 16.37 mm lateral to the ACPC line and 7.53 and 8.71 mm posterior to the midpoint of the ACPC line, respectively. Somatotopic locations for the hand and leg were different in the anteroposterior direction (P < .05); however, no difference was observed in the mediolateral direction (P > .05).
CONCLUSIONS:
We found the somatotopic locations for hand and leg of the STP at the VPL nucleus; these somatotopies were arranged in the anteroposterior direction.
Detailed anatomic information on the human brain is mandatory in both basic and clinical neuroscience. Identification and elucidation of the anatomic location of a neural tract in the live human brain can provide useful information for clinicians, even in cases in which detailed anatomy of the neural tract is already well-known; it can also provide information for prediction of clinical outcomes, establishment of rehabilitative management strategies, or anatomic guidance for invasive procedures for patients with brain lesions. Furthermore, anatomic information according to somatotopy can provide more useful information than for a whole neural tract.
The VPL nucleus of the thalamus is a synaptic area of the STP and the medial lemniscothalamocortical pathway. The STP carries information on pain and touch from the contralateral limbs and body and has been regarded as the most plausible neural tract responsible for the pathogenesis of central poststroke pain.1–5 The VPL nucleus has been a target for neurosurgical procedures for control of central poststroke pain.6,7 Therefore, many studies have been conducted for elucidation of the somatotopy at the VPL nucleus,7–13 conducted by using microelectrode brain-stimulation techniques.7–12
In the past, identification and visualization of a neural tract in the live human brain has been impossible. The recent development of DTT, which is derived from DTI, allows anatomic study in the live brain by visualization and localization of neural tracts at the subcortical level in 3D.14 Moreover, DTT is now being used for study of the somatotopic location of the corticospinal tract and the somatosensory tract.13,15–17 However, little is known about the somatotopic location of the STP at the VPL nucleus of the thalamus.13 In the current study, we attempted to investigate this location in the human brain by using DTT.
Materials and Methods
Subjects
Forty-one healthy right-handed subjects (26 men, 15 women; mean age, 38.39 ± 13.12 years; range, 20–65 years) with no previous history of neurologic, psychiatric, or physical illness were enrolled in this study. Handedness was evaluated by using the Edinburgh Handedness Inventory.18 All subjects understood the purpose of the study and provided written informed consent before participation. The study protocol was approved by the local institutional review board.
Data Acquisition
DTI data were obtained by using a Synergy-L SENSE head coil on a 1.5T Gyroscan Intera system (Philips, Best, the Netherlands) equipped with single-shot echo-planar imaging. We acquired 67 contiguous sections parallel to the ACPC line. Imaging parameters were as follows: matrix = 128 × 128, FOV = 221 × 221 mm2, TR/TE = 10,726/76 ms, SENSE factor = 2, echo-planar imaging factor = 49 and b = 1000 mm2s−1, NEX = 1, and thickness = 2.3 mm, for each of the 32 noncollinear diffusion-sensitizing gradients. Signal intensity–to-noise SENSE was measured in non-diffusion-weighted images in the thalamus for each subject. The mean was 26.46 ± 8.34.
Fiber Tracking
Diffusion-weighted imaging data were analyzed by using the Oxford Centre for FMRIB Software Library (www.fmrib.ox.ac.uk/fsl). Head-motion effect and image distortion due to eddy currents were corrected by affine multiscale 2D registration. Fiber tracking was performed by using a probabilistic tractography method based on a multifiber model and applied in the present study using tractography routines implemented in FMRIB Diffusion Toolbox (5000 streamline samples, 0.5-mm step lengths, curvature thresholds = 0.2).19–21 STPs for the hand and leg were determined by selection of fibers passing through both regions of interest for each subject. The seed region of interest was given at the area of the spinothalamic tract in the posterolateral medulla (posterior to the inferior olivary nucleus and anterior to the inferior cerebellar peduncle) for both STPs, as described in a brain atlas.22–26 Target regions of interest for the hand and leg were selected according to known anatomy (region of interest for the hand was the postcentral gyrus posterior to the precentral knob; and region of interest for the leg was the postcentral gyrus posterior to the leg somatotopy of the precentral gyrus) on the non-diffusion-weighted (b0) image (Fig 1).
Fig 1.
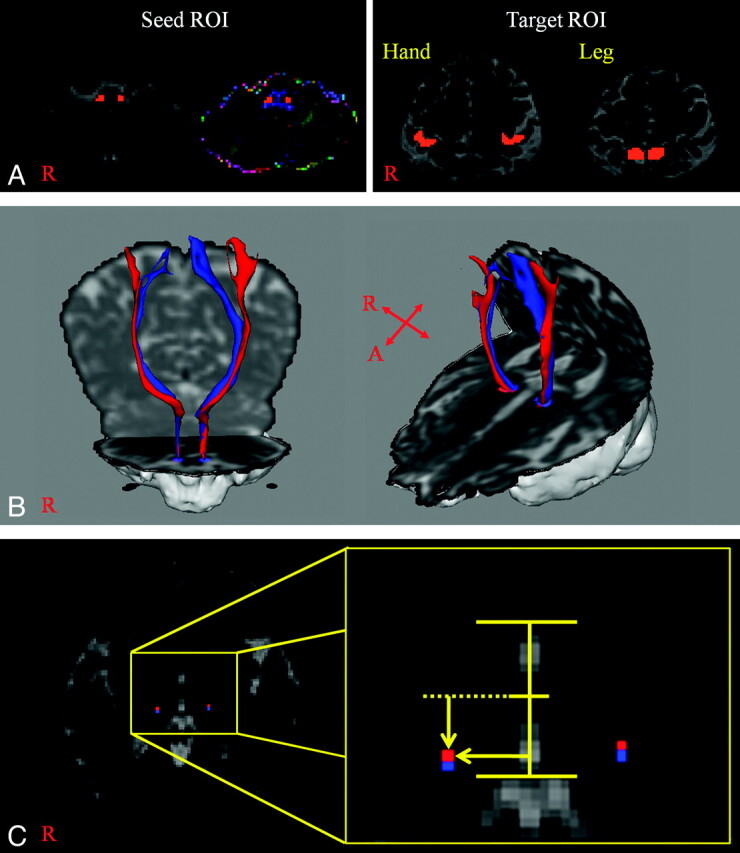
A, A seed region of interest is placed on the posterolateral medulla. Target regions of interest are shown at the postcentral gyrus posterior to the precentral knob for the hand and the postcentral gyrus posterior to the leg somatotopy of the precentral gyrus for the leg. B, The STP of a healthy subject (a 65-year-old woman) (red is the STP for the hand; blue is the STP for the leg). C, The highest probabilistic locations are measured laterally from the midline of the ACPC line in the mediolateral direction and posteriorly from the midpoint of the ACPC line in the anteroposterior direction (red is the highest probabilistic location of the STP for the hand; blue is the highest probabilistic location of the STP for the leg).
Measurement of STP Location
The somatotopic location of the STP at the VPL nucleus was evaluated as the highest probabilistic location in the 2 sections on the non-diffusion-weighted (b0) image (the bicommissural level was the first axial image that can be seen on both the AC and PC; the above-bicommissural level was 2.3 mm above the bicommissural level) for each subject. These locations for the hand and leg of the STP were measured laterally from the midline of the ACPC line in the mediolateral direction and posteriorly from the midpoint of the ACPC line in the anteroposterior direction. Each location was calculated as an individual pixel unit and converted to millimeters.
STP probabilistic maps for the hand and leg were obtained by overlapping the location of the highest probabilistic location. The probabilistic map was superimposed on a mean non-diffusion-weighted image, which was created as a mean of the non-diffusion-weighted images of all subjects by using SPM8 software (Wellcome Department of Cognitive Neurology, London, United Kingdom).
Statistical Analysis
The independent t test was used for determination of differences in the somatotopic locations for the hand and leg of the STP according to side (right/left), medio-lateral direction, and anteroposterior direction. The level of significance was set to P < .05.
Results
STPs for the hand and leg started with the posterolateral medulla, which was selected as a seed region of interest; ascended through the VPL nucleus of the thalamus; and then terminated at the postcentral gyrus posterior to the precentral knob and the postcentral gyrus posterior to the leg somatotopy of the precentral gyrus, respectively.
Probabilistic maps for somatotopies of the STPs are shown in Fig 2. In the mediolateral direction, the highest probabilistic location for the hand was at an average of 16.86 mm lateral to the ACPC line at the bicommissural level and 19.18 mm at the above-bicommissural level. In contrast, the highest probabilistic location for the leg was at an average of 16.37 mm at the bicommissural level and 18.85 mm at the above-bicommissural level. This location for the leg was significantly posterior to the hand location (P < .05) (Table).
Fig 2.
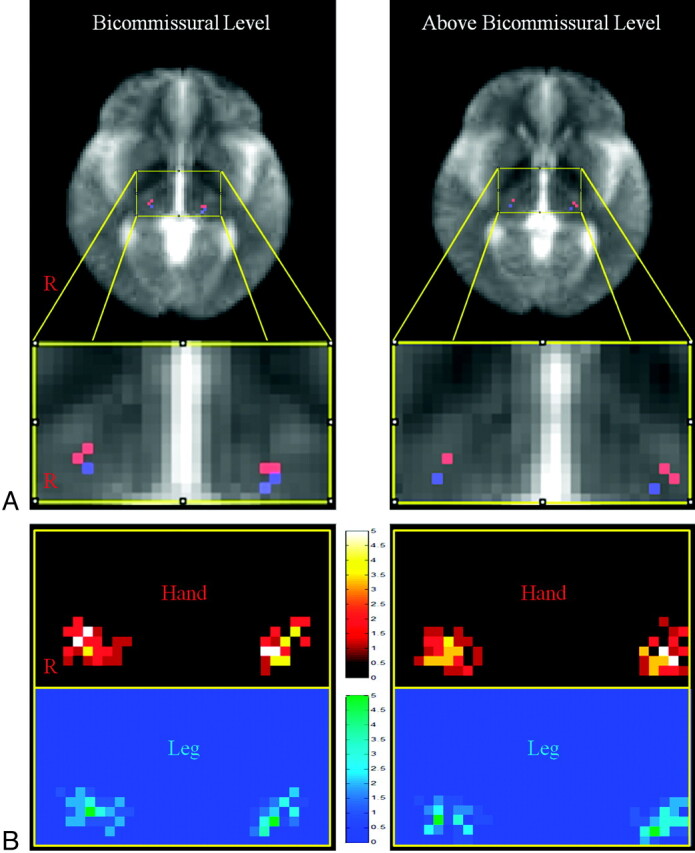
A, The most overlapping voxel of the probabilistic map for the STP superimposed on the mean of the non-diffusion-weighted image (red is the STP for the hand; blue is the STP for the leg). B, Probabilistic maps at the bicommissural level and above the bicommissural level. The probabilistic map shows overlapping voxels that are color-coded from 0 (black, blue) to 5 (white, green).
Somatotopic locations of the highest probability point of the STP at the VPL nucleus of the thalamusa
| Level | Mediolateral Direction (mm) |
Anteroposterior Direction (mm) |
||||
|---|---|---|---|---|---|---|
| Hand | Leg | P Value | Hand | Leg | P Value | |
| Bicommissural | ||||||
| Right | 17.07 | 16.69 | 7.34 | 8.40 | ||
| (±2.63) | (±3.02) | (±1.96) | (±1.80) | |||
| Left | 16.65 | 16.06 | 7.72 | 9.03 | ||
| (±2.21) | (±2.42) | (±2.29) | (±1.92) | |||
| Total | 16.86 | 16.37 | .231 | 7.53 | 8.71 | .000 |
| (±2.42) | (±2.74) | (±2.13) | (±1.87) | |||
| Above-bicommissural | ||||||
| Right | 19.24 | 18.90 | 7.17 | 8.31 | ||
| (±2.48) | (±2.90) | (±1.96) | (±1.68) | |||
| Left | 19.11 | 18.80 | 7.72 | 8.73 | ||
| (±2.12) | (±2.42) | (±2.26) | (±1.97) | |||
| Total | 19.18 | 18.85 | .400 | 7.45 | 8.52 | .001 |
| (±2.29) | (±2.66) | (±2.12) | (±1.92) | |||
Values are means. Each location of the point was calculated as a pixel unit and converted to millimeters. Voxel size is 1.73 × 1.73 × 2.3 mm.
As for the anteroposterior direction, the highest probabilistic locations for hand and leg were posterior at an average of 7.53 mm from the midpoint of the ACPC line, 8.71 mm at the bicommissural level, and 7.45 mm and 8.52 mm at the above-bicommissural level, respectively. In terms of mediolateral direction, no significant difference was observed between somatotopic locations for the hand and leg (P > .05).
Discussion
In the current study, we investigated the somatotopic location for the hand and leg of the STP at the VPL nucleus of the thalamus in the human brain by using a probabilistic tractography technique. At the bicommissural level, the highest probabilistic locations for the hand and leg were at an average of 16.86 and 16.37 mm lateral to the ACPC line and 7.53 and 8.71 mm posterior to the midpoint of the ACPC line, respectively. Somatotopic locations for the hand and leg were significantly different in the anteroposterior direction; however, no significant difference was observed in the mediolateral direction. Many microelectrode stimulation studies have reported the location of STP, which is 12–18 mm lateral to the ACPC line and 5–8 mm posterior to the midpoint of the ACPC line at the bicommissural level.6,8,10,11,27,28 In addition, 1 postmortem brain dissection study also reported similar results.29 Therefore, our results coincide with those of previous studies.6,8,10,11,27–29 On the other hand, we found that somatotopies for the hand and leg of the STP were arranged in the anteroposterior direction at the VPL nucleus.
Many direct brain stimulation studies have reported the somatotopic arrangement at the VPL nucleus of the thalamus; however, these have been controversial.7–11,28 Some of these studies reported that somatotopies for the hand and leg were arranged in the mediolatatral direction at the VPL nucleus.10,11 By contrast, other studies reported an anteroposterior somatotopic arrangement similar to that of our results7,9 or a variable arrangement.8,28 This disagreement on results from previous studies appears to be attributed to the high individual variability of microelectrode stimulation studies.8
With the development of DTI, several studies have been conducted using DTI for identification of the thalamic nuclei and somatotopy of the somatosensory tracts.8,13,30–33 Some of these studies have attempted to identify the thalamic nuclei by using a different directionality, and other studies have attempted to identify them by using their connectivity with cortical areas.8,13,30,31,33 In 2003, Behrens et al20 attempted to identify the thalamic nuclei by using a probabilistic tractography algorithm. They reported the locations of each thalamic nucleus, including the VPL nucleus, by using connectivity with the functionally relevant cortices. Recently, Yamada et al32 (2010) constructed a DTT for the pyramidal tract, STP, and cerebellothalamocortical tract. They identified the VPL nucleus and ventrointermediate nucleus by using the relationship of the above 3 tracts. On the other hand, Yamada et al13 (2007) also reported the somatotopic arrangement of somatosensory pathways at the thalamus and the mediolateral arrangement at the thalamus. However, this study was conducted with only 7 subjects, and the authors analyzed whole somtosensory pathways without selection of the STP.
Conclusions
We found the somatotopic locations for the hand and leg of the STP at the VPL nucleus, which were arranged in the anteroposterior direction by using a probabilistic tractography technique. This is the first DTI study to report on the somatotopic location for the hand and leg of the STP at the VPL nucleus of the thalamus in the human brain. We think that the methodology and data used in this study would be helpful in research on the thalamus and for invasive procedures for patients with brain lesions. The limitation of this study is that we adopted the highest probabilistic location for the STP, which indicates that the somatotopic location does not represent the whole somatotopy for the hand and leg, but the highest point of each somatotopy. Therefore, this work invites further study of the whole somatotopy and clinical correlation studies in the near future.
Abbreviations
- ACPC
anterior/posterior commissure
- DTI
diffusion tensor imaging
- DTT
diffusion tensor tractography
- FMRIB
Functional Magnetic Resonance Imaging of the Brain
- SENSE
sensitivity encoding
- STP
spinothalamocortical pathway
- VPL
ventroposterolateral
Footnotes
This work was supported by a National Research Foundation of Korea grant funded by the Korean Government (KRF-2008-314-E00173).
References
- 1. Boivie J, Leijon G, Johansson I. Central post-stroke pain: a study of the mechanisms through analyses of the sensory abnormalities. Pain 1989;37:173–85 [DOI] [PubMed] [Google Scholar]
- 2. Goto T, Saitoh Y, Hashimoto N, et al. Diffusion tensor fiber tracking in patients with central post-stroke pain; correlation with efficacy of repetitive transcranial magnetic stimulation. Pain 2008;140:509–18 [DOI] [PubMed] [Google Scholar]
- 3. Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol 2009;8:857–68 [DOI] [PubMed] [Google Scholar]
- 4. Seghier ML, Lazeyras F, Vuilleumier P, et al. Functional magnetic resonance imaging and diffusion tensor imaging in a case of central poststroke pain. J Pain 2005;6:208–12 [DOI] [PubMed] [Google Scholar]
- 5. Vestergaard K, Nielsen J, Andersen G, et al. Sensory abnormalities in consecutive, unselected patients with central post-stroke pain. Pain 1995;61:177–86 [DOI] [PubMed] [Google Scholar]
- 6. Owen SL, Green AL, Stein JF, et al. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain 2006;120:202–26 [DOI] [PubMed] [Google Scholar]
- 7. Tasker RR. Thalamotomy. Neurosurg Clin N Am 1990;1:841–64 [PubMed] [Google Scholar]
- 8. Bertrand G, Jasper H, Wong A. Microelectrode study of the human thalamus: functional organization in the ventro-basal complex. Confin Neurol 1967;29:81–86 [DOI] [PubMed] [Google Scholar]
- 9. Hassler RG, Walker AE, Albe-Fessard D. Trigeminal Neuralgia: Pathogenesis and Pathophysiology. Philadelphia: W.B. Saunders; 1970 [Google Scholar]
- 10. Hua SE, Garonzik IM, Lee JI, et al. Microelectrode studies of normal organization and plasticity of human somatosensory thalamus. J Clin Neurophysiol 2000;17:559–74 [DOI] [PubMed] [Google Scholar]
- 11. Lenz FA, Dostrovsky JO, Tasker RR, et al. Single-unit analysis of the human ventral thalamic nuclear group: somatosensory responses. J Neurophysiol 1988;59:299–316 [DOI] [PubMed] [Google Scholar]
- 12. Oye C, Fukamachi A, Narabayashi H. Spontaneous and evoked activity of sensory neurons and their organization in the human thalamus. Z Neurol 1972;203:219–34 [DOI] [PubMed] [Google Scholar]
- 13. Yamada K, Nagakane Y, Yoshikawa K, et al. Somatotopic organization of thalamocortical projection fibers as assessed with MR tractography. Radiology 2007;242:840–45 [DOI] [PubMed] [Google Scholar]
- 14. Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265–69 [DOI] [PubMed] [Google Scholar]
- 15. Holodny AI, Gor DM, Watts R, et al. Diffusion-tensor MR tractography of somatotopic organization of corticospinal tracts in the internal capsule: initial anatomic results in contradistinction to prior reports. Radiology 2005;234:649–53 [DOI] [PubMed] [Google Scholar]
- 16. Hong JH, Son SM, Jang SH. Somatotopic location of corticospinal tract at pons in human brain: a diffusion tensor tractography study. Neuroimage 2010;51:952–55. Epub 2010 Mar 4 [DOI] [PubMed] [Google Scholar]
- 17. Ino T, Nakai R, Azuma T, et al. Somatotopy of corticospinal tract in the internal capsule shown by functional MRI and diffusion tensor images. Neuroreport 2007;18:665–68 [DOI] [PubMed] [Google Scholar]
- 18. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 19. Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 2007;34:144–55. Epub 2006 Oct 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003;6:750–57 [DOI] [PubMed] [Google Scholar]
- 21. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(suppl 1):S208–19 [DOI] [PubMed] [Google Scholar]
- 22. Habas C, Cabanis EA. Anatomical parcellation of the brainstem and cerebellar white matter: a preliminary probabilistic tractography study at 3 T. Neuroradiology 2007;49:849–63 [DOI] [PubMed] [Google Scholar]
- 23. Hong JH, Son SM, Jang SH. Identification of spinothalamic tract and its related thalamocortical fibers in human brain. Neurosci Lett 2010;468:102–05 [DOI] [PubMed] [Google Scholar]
- 24. Naidich TP, Duvernoy HM, Delman BM, et al. Duvernoy's Atlas of the Human Brain Stem and Cerebellum: High-Field MRI: Surface Anatomy, Internal Structure, Vascularization and 3D Sectional Anatomy. New York: Springer-Verlag; 2009 [Google Scholar]
- 25. Salamon N, Sicotte N, Alger J, et al. Analysis of the brain-stem white-matter tracts with diffusion tensor imaging. Neuroradiology 2005;47:895–902 [DOI] [PubMed] [Google Scholar]
- 26. Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford University Press; 2006 [Google Scholar]
- 27. Kiss ZH, Dostrovsky JO, Tasker RR. Plasticity in human somatosensory thalamus as a result of deafferentation. Stereotact Funct Neurosurg 1994;62:153–63 [DOI] [PubMed] [Google Scholar]
- 28. Ohye C. Depth microelectrode studies. In: Schaltenbrand G, Walker AE. eds. Stereotaxy of the Human Brain: Anatomical Physiological and Clinical Applications. Stuttgart, Germany: Thieme; 1982:372–89 [Google Scholar]
- 29. Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol 1997;387:588–630 [DOI] [PubMed] [Google Scholar]
- 30. Unrath A, Klose U, Grodd W, et al. Directional colour encoding of the human thalamus by diffusion tensor imaging. Neurosci Lett 2008;434:322–27. Epub 2008 Feb 12 [DOI] [PubMed] [Google Scholar]
- 31. Wiegell MR, Tuch DS, Larsson HB, et al. Automatic segmentation of thalamic nuclei from diffusion tensor magnetic resonance imaging. Neuroimage 2003;19:391–401 [DOI] [PubMed] [Google Scholar]
- 32. Yamada K, Akazawa K, Yuen S, et al. MR imaging of ventral thalamic nuclei. AJNR Am J Neuroradiol 2010;31:732–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ziyan U, Tuch D, Westin CF. Segmentation of thalamic nuclei from DTI using spectral clustering. Med Image Comput Comput Assist Interv 2006;9:807–14 [DOI] [PubMed] [Google Scholar]


