Abstract
BACKGROUND AND PURPOSE:
The atrophy of the caudate is considered the hallmark of HD-associated neurodegeneration and has high potential as a biomarker in structural MR imaging. This study aimed at comparing automated and manual caudate volumetry.
MATERIALS AND METHODS:
In this cross-sectional volumetric study in 40 patients with HD and 30 healthy controls, a fully automated caudate measurement by ABV was used for the first time in HD and was directly compared with manual delineation as the generally accepted criterion standard of volumetry.
RESULTS:
It could be shown that both techniques were able to separate patients and controls to a similar degree. The differences between the 2 volumetric measurements ranged within the limits of agreement; the systematically lower values by manual volumetry were caused by the different assessment of the dorsal caudate tail, which is hard to delineate manually.
CONCLUSIONS:
ABV may be used instead of manual volumetry to quantify caudate volume loss. Additionally, the ABV technique has the advantage of being much faster, is less laborious, and is free of a subjective region-of interest definition. ABV might serve as a tool in potential future clinical trials of disease-modifying treatments in HD.
In HD, neuroimaging is not required in the diagnostic work-up because molecular genetic testing is apt to provide the diagnosis. However, MR imaging−based techniques are increasingly used in the ongoing search for sensitive and reliable biomarkers of progressive neurodegeneration that could be used to assess the effect of therapeutic intervention on brain structure and function in potential clinical trials.1 With respect to brain structure, atrophy of the caudate and putamen as the hallmark of HD-associated neurodegeneration has been repeatedly described in region of interest−based volumetric and in morphometric approaches (compare Bohanna et al1 and Kloppel et al2 for reviews) and has been demonstrated to correlate with disease severity3 and other clinical functional parameters. Because it has been demonstrated for HD that effects of various user-specified parameters in neuroimaging approaches such as voxel-based morphometry can markedly alter results,4 MR imaging analyses that are not only valid but also as rater-independent and reproducible as possible are required. In addition, for an assessment of regional atrophy at the single-patient level, individual measurements with absolute quantification are necessary. We have recently presented a novel and fully automated MR imaging postprocessing technique (ABV) that has been successfully used to quantify striatal atrophy in patients with chorea-acanthocytosis5 and has been tested for intra- and interscanner reproducibility of results.6 In the present study, this method was applied to MR imaging of 40 patients with HD and 30 controls to quantify absolutely the atrophic process at the single-subject level. The results were compared with those obtained by the former criterion standard of volumetry (ie, manual region-of-interest measurement in the same patient and control sample).
Materials and Methods
The sample of patients with HD consisted of 40 patients with manifest HD (the demographic data are summarized in the Table), together with the number of trinucleotide (CAG) repeats and disease-related functional scales. No patients were taking medications suspected of influencing brain volume, and none of the patients had any concomitant neurologic illnesses. The age-matched control group consisted of 30 healthy individuals (Table) with no history of neurologic or psychiatric disorders and normal neurologic examination findings. The local ethics committee of the University of Ulm provided approval, and written informed consent from all subjects had been obtained before study initiation.
Demographic and disease-related data in patients and controlsa
| Controls | HD | |
|---|---|---|
| Number | 30 | 40 |
| Gender (M-F) | 15:15 | 19:21 |
| Age (yr) | 44.6 ± 12.5 yr | 45.1 ± 9.3 yr |
| CAG repeat length | NA | 44.7 ± 3.8 (range, 40–59) |
| Disease duration (yr since onset) | NA | 4.6 (2.5) |
| UHDRS motor score | NA | 24.1 ± 22.1 |
| UHDRS cognitive score | NA | 205.9 ± 72.2 |
| TFC | NA | 11.0 ± 1.5 |
All quantitative results are presented as mean ± 1 SD.
MR Imaging Acquisition
High-resolution T1-weighted volume datasets of the whole head were acquired on a 1.5T scanner (Symphony; Siemens, Erlangen, Germany) by using a T1-weighted magnetization-prepared rapid acquisition of gradient echo sequence in the sagittal plane with the following parameters: 160–180 partitions depending on head size; TR, 9.7 ms; TE, 3.93 ms; flip angle, 15°; matrix, 256 × 256 mm2; FOV, 250 mm; and 1-mm isotropic voxel size. Scanning was consistent among all subjects. All data were thoroughly checked for movement artifacts due to the hyperkinetic movement disorder, and only those MR imaging data without gross movement artifacts were included in the MR imaging data base for further analysis.
MR Imaging Data Processing and Automated ABV
The MR imaging data processing and volumetry have been described in detail elsewhere.6 The method is based on algorithms of SPM5 (Wellcome Department of Imaging Neuroscience, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm) and masks derived from a probabilistic brain atlas provided by the LONI at the University of California, Los Angeles, California (http://www.loni.ucla.edu/Atlases/).7 The analysis is fully automated by use of a Matlab (MathWorks, Natick, Massachusetts) batch script and requires approximately 1 hour per MR imaging scan on an AMD Opteron 2.0-GHz PC (Silicon Mechanics, Bothell, Washington) with 2 dual cores, or approximately 12 minutes on an Xeon 5620 2.4-GHz PC (Intel, Santa Clara, California), with 2 quad cores and Matlab multithreaded computation-enabled.
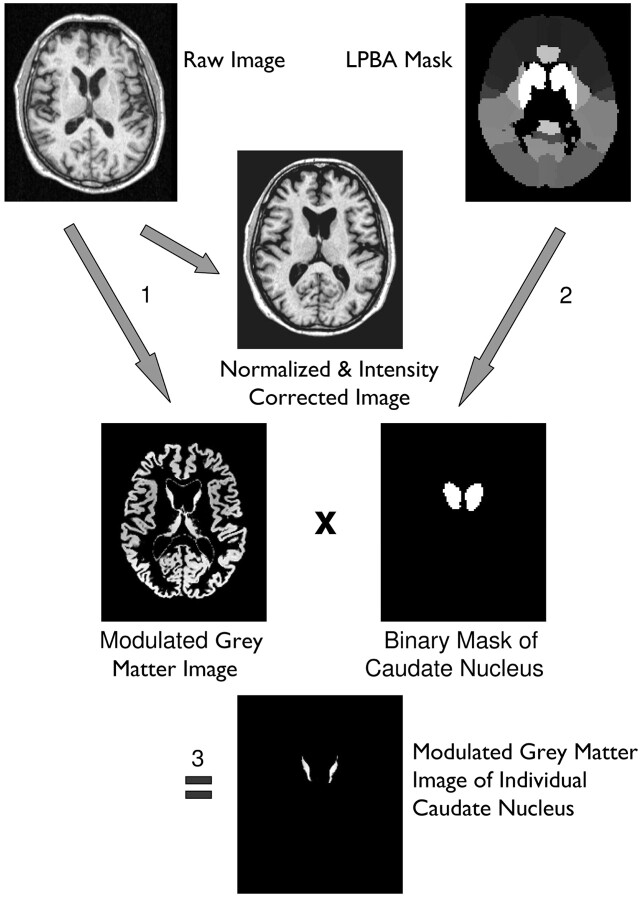
In short, each T1-weighted volume dataset was normalized to the standard brain of the Montreal Neurologic Institute included in the SPM5 distribution and segmented into different brain compartments (ie, gray matter, white matter, and CSF). This was done by using the Unified Segmentation tool of SPM5 with its default settings. The segmentation resulted in modulated and unmodulated images for the different tissue compartments. Modulation compensates for dilation or shrinkage during spatial normalization and has the effect of preserving the total amount of signal intensity from the respective tissue class in the normalized partitions.8 To determine the volume of the caudate nucleus, the corresponding mask derived from the LONI Probabilistic Brain Atlas (http://www.loni.ucla.edu/Atlases/LPBA40) was multiplied by the modulated image of the desired tissue class. The values of all voxels in the resulting image were summed up and divided by 1000 to get the volume of the investigated structure in milliliter units. Due to modulation of the tissue images, the effect of normalization (ie, extension or shrinkage of the investigated structure) was compensated for so that the computed volume represented the volume of the original structure in native space (Fig 1).
Fig 1.
Image processing and volumetry of the caudate nucleus: 1, Unified segmentation of SPM5 (ie, simultaneous normalization, segmentation, and intensity correction) is performed on a T1-weighted volume dataset. 2, A binary caudate mask is derived from the LONI Probabilistic Brain Atlas by setting all voxels of the maximum likelihood map belonging to the caudate nucleus to a value of 1, while all other voxels are set to zero. 3, The modulated gray matter image resulting from unified segmentation is multiplied by the caudate mask, resulting in a modulated image of the individual caudate nucleus.
Manual Volumetry
Volumetric measurements were performed by 2 experienced observers (E.H.P., A.D.) by use of standardized measurements with the interactive software program MRreg (L. Lemieux, Epilepsy Imaging Group, Department of Clinical and Experimental Epilepsy, Institute of Neurology, University College London, London, United Kingdom; http://www.erg.ion.ucl.ac.uk/MRreg.html).9 The manual delineation of the caudate was performed separately in both hemispheres in reference to the established protocol by Looi et al.10,11 The volumes in each section (in-section volume) were calculated by multiplying the voxel number of each trace by the voxel volume and dividing this value by the magnification factor. The total volumes were calculated as the sum of all in-section volumes. The raters were blinded to the subject grouping.
In contrast to the fully automated measurement, manual volumetry is hardly dependent on computer performance. The time to conduct manual volumetry is about 1.5 hours per MR imaging scan for an experienced rater.
Statistical Analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences software (Version 13.0; SPSS, Chicago, Illinois). The volumetric data of the patient and control groups were compared by using the Mann-Whitney U test. A 2-sided significance level of P < .05 was used. The Spearman correlation coefficient was calculated for the volumetric results and the demographic and clinical data.
The comparison of automated and manual volumetry was performed by the Bland-Altman method to evaluate the extent of agreement and scatter range of measured data.12 As the limit of agreement, the average difference ± 1.96 × SD of the difference was used. The correlation between the 2 methods was calculated in accordance with the CCCLin,13 because this calculation considers a possible bias of the 2 measurements. The inter-rater reliability of manual volumetry was also assessed by the Bland-Altman method and by the ICC from repeated measurements.
Results
Volumetric Results
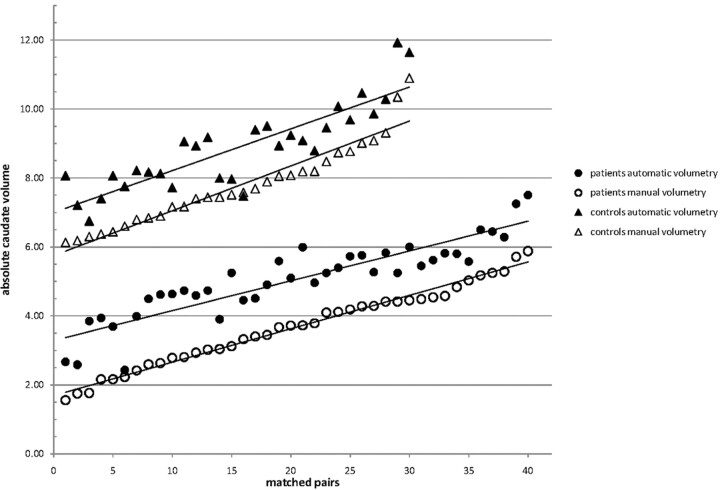
All values are reported as mean ± SD. The caudate volumes determined by automated ABV were 5.06 ± 1.13 mL for the HD group and 8.88 ± 1.23 mL for the control group (group comparison, P < .001). Manual volumetry obtained caudate volumes of 3.68 ± 1.14 mL for the HD sample and 7.77 ± 1.19 mL for the controls, also leading to a complete separation of the samples and a significant group difference at P < .001. The caudate volumes of all patients and controls, as measured by the automated and the manual approach, respectively, are depicted in Fig 2.
Fig 2.
Scatterplot of absolute caudate volumes (ordinate) for matched pairs of patients (circles) and controls (triangles) in ascending order of the volumes for manual (outlined icons) and automated volumetric measurements (solid icons).
The inter-rater reliability measurements for manual volumetry of the total caudate volume were all within the limits of agreement. The ICC for inter-rater data was 0.989 (95% confidence interval, 0.975–0.997). The values corresponded well to what has been reported in the literature.14
Comparison of Volumetric Techniques
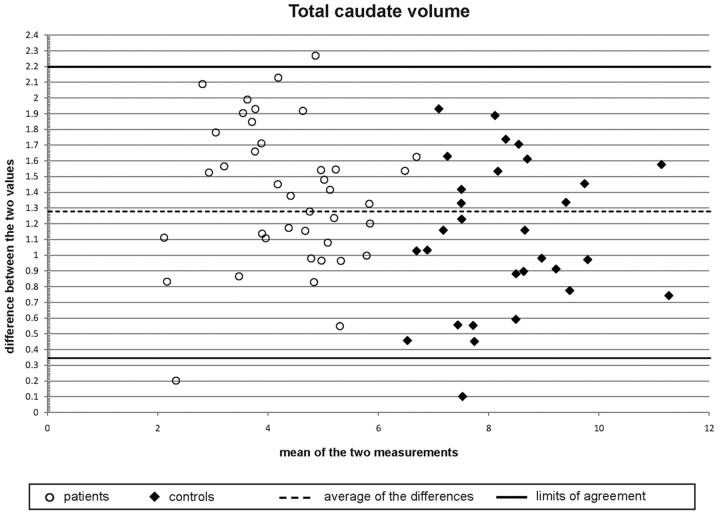
As depicted in Fig 2, the slopes of the regression lines of manual and automatic measurements are nearly the same. However, there is a systematic error with larger volumes obtained by the automatic method for each single measure, amounting to a mean difference of 1.27 mL between the results of manual and automated volumetry (for both patients and controls). Even with this systematic error, however, the correlation coefficient is 0.846 (CCCLin). From the Bland-Altman plots (Fig 3), one can conclude the following: 1) the differences of the automatic and manual measurement values are similarly symmetrically distributed, 2) the variance of the measurements is not linked with higher or lower caudate volumes, and 3) the comparison of the 2 volumetric approaches for caudate volume measurements show a range within the limits of agreement (except for 3 values).
Fig 3.
Bland-Altman scatterplot of the differences plotted against the means of the manual and automated volumetric measurements for patients (circles) and controls (diamonds). Horizontal lines show the limits of agreement (solid lines), defined as the mean difference ± 1.96 × SD of the differences and the average of the differences (dashed line).
Correlation with Clinical Data
The correlation analyses demonstrated an association between caudate atrophy and CAG repeat length, which was significant for both measurement approaches (ABV, P = .003; manual volumetry, P = .001), whereas there were no significant correlations for the results of both volumetric measurements and age and disease duration, respectively. However, it was remarkable that the caudate volumes were significantly correlated with all functional clinical measurements (ie, with total functional scale, UHDRS motor scale, and UHDRS cognitive scale, respectively, at P < .001 each).
Discussion
In this cross-sectional volumetric study of 40 patients with HD and 30 healthy controls by use of a novel fully observer-independent region of interest−based 3D MR imaging analysis (ABV), an absolute quantification of the caudate could be achieved for the investigated patients with HD and controls, demonstrating HD-associated volume reductions that are consistent with volume losses reported from other imaging studies or postmortem findings and that correlated with functional clinical measurements, as previously shown by various approaches. For the first time, a rater-independent caudate measurement such as ABV was directly compared with manual delineation as the generally accepted criterion standard of volumetry. It could be demonstrated that the differences between the 2 volumetric measurements ranged within the limits of agreement (ie, no severe discordant values could be detected) and that the difference values were symmetrically distributed, independent of higher or lower caudate volumes. In addition, both techniques were, to a similar degree, able to separate patients and controls (Fig 2).
There have been applications of the automatic technique to healthy brains previously,6 and the applicability to healthy and diseased brains was to be addressed by the current study. In this particular case of manual-versus-automatic measurements, it is important to evaluate the comparability of these methods also in atrophic brains because both the altered configuration of brain tissue and the altered configuration of subarachnoid/ventricular spaces may affect volumetric results.
The comparison of absolute volumetric results between ABV and manual measurements, however, demonstrated systematically smaller volumes in the manual assessment, with a relatively constant difference between the absolute volumes of automatic and manual volumetry for both patients with HD and controls and independent of absolute caudate volumes. The main reason for this systematic difference is most probably the different assessment of the caudate tail, which is rather hard to delineate manually in its dorsal parts and could not be completely included in the manual volume. In addition, there were 3 scans in which automated volumetry seemed to differ substantially from the manual measurements (Fig 3). Although all data were thoroughly checked for gross movement artifacts before volumetric analysis, retrospective evaluation of the 3 outliers revealed moderate movement artifacts in the MR imaging data of the control and the 2 patients, which might have influenced segmentation during automated volumetry.
On the basis of these comparison results, it is safe to conclude that ABV may be used as a replacement for manual volumetry in cross-sectional or longitudinal studies to quantify caudate volume loss. There are a variety of reasons that lead to this statement. From the pragmatic viewpoint, the ABV technique is much faster and puts much less strain on human resources. Compared with other automatic region-of-interest volume measurements in HD (1,2,15 for reviews), including a recent longitudinal investigation of global brain volumes over 2 years,16 these previous studies mostly either do not provide absolute quantification or partially include manual delineation techniques. ABV is free from subjective region-of-interest definition, can be applied to any brain area included in the LONI brain atlas, and has very good intrascanner reproducibility.6 Because it has been demonstrated previously that the cutoffs for significant volume changes between 2 measurements in the same subject amounted to approximately 1.4% for measurements on the same scanner,6 ABV may, in addition, serve as a surrogate marker in the investigation of disease progression in a longitudinal setup with repeated measurements of the same patient. Finally, by its absolute quantification approach and the calculation of absolute value differences between single datasets and groups, it can be used within the diagnostic work-up at the individual level when needed.5
Why are these methodologic MR imaging analysis issues of particular interest in the pathoanatomically rather well-defined neurodegenerative HD? Regional brain atrophy arguably leads to the eventual development of symptoms—any intervention in HD would ideally rescue striatal and/or cortical neurons and thereby attenuate atrophy.1 In light of this result, caudate MR imaging volume has been proposed as a biomarker and outcome measure for use in HD clinical trials.15,17 In a prospective international study aimed at identifying preclinical HD biomarkers (the Neurobiological Predictors of HD study), the potential of structural MR imaging as a biomarker was shown, because a reduction in striatal volume was demonstrated to be identifiable in up to 15 years before the estimated time of disease diagnosis.18 This suggests that imaging including striatal volumetry might be used as a tool in potential future clinical trials of putative disease-modifying treatments, both to select appropriate presymptomatic participants (as a result from the Neurobiological Predictors of HD data) and to serve as one possible quantitative surrogate marker.
Abbreviations
- ABV
atlas-based volumetry
- CAG
cytosine-adenine-guanine
- CCCLin
Lin concordance correlation coefficient
- HD
Huntington disease
- ICC
intraclass correlation coefficient
- LONI
Laboratory of Neuroimaging
- TFC
total functional capacity
- UHDRS
Unified Huntington's Disease Rating Scale
References
- 1. Bohanna I, Georgiou-Karistianis N, Hannan AJ, et al. Magnetic resonance imaging as an approach towards identifying neuropathological biomarkers for Huntington's disease. Brain Res Rev 2008;58:209–25 [DOI] [PubMed] [Google Scholar]
- 2. Kloppel S, Henley SM, Hobbs NZ, et al. Magnetic resonance imaging of Huntington's disease: preparing for clinical trials. Neuroscience 2009;164:205–19. Epub 2009 Jan 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kassubek J, Juengling FD, Kioschies T, et al. Topography of cerebral atrophy in early Huntington's disease: a voxel-based morphometric MRI study. J Neurol Neurosurg Psychiatry 2004;75:213–20 [PMC free article] [PubMed] [Google Scholar]
- 4. Henley SM, Ridgway GR, Scahill RI, et al. Pitfalls in the use of voxel-based morphometry as a biomarker: examples from Huntington disease. AJNR Am J Neuroradiol 2010;31:711–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huppertz HJ, Kroll-Seger J, Danek A, et al. Automatic striatal volumetry allows for identification of patients with chorea-acanthocytosis at single subject level. J Neural Transm 2008;115:1393–400. Epub 2008 Jul 22 [DOI] [PubMed] [Google Scholar]
- 6. Huppertz HJ, Kroll-Seger J, Kloppel S, et al. Intra- and interscanner variability of automated voxel-based volumetry based on a 3D probabilistic atlas of human cerebral structures. Neuroimage 2010;49:2216–24 [DOI] [PubMed] [Google Scholar]
- 7. Shattuck DW, Mirza M, Adisetiyo V, et al. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage 2008;39:1064–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage 2000;11(6 pt 1):805–21 [DOI] [PubMed] [Google Scholar]
- 9. Lemieux L, Wieshmann UC, Moran NF, et al. The detection and significance of subtle changes in mixed-signal brain lesions by serial MRI scan matching and spatial normalization. Med Image Anal 1998;2:227–42 [DOI] [PubMed] [Google Scholar]
- 10. Looi JC, Svensson L, Lindberg O, et al. Putaminal volume in frontotemporal lobar degeneration and Alzheimer disease: differential volumes in dementia subtypes and controls. AJNR Am J Neuroradiol 2009;30:1552–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Looi JC, Tatham V, Kumar R, et al. Caudate nucleus volumes in stroke and vascular dementia. Psychiatry Res 2009;174:67–75 [DOI] [PubMed] [Google Scholar]
- 12. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10 [PubMed] [Google Scholar]
- 13. Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989;45:255–68 [PubMed] [Google Scholar]
- 14. van Elst LT, Woermann FG, Lemieux L, et al. Affective aggression in patients with temporal lobe epilepsy: a quantitative MRI study of the amygdala. Brain 2000;123(pt 2):234–43 [DOI] [PubMed] [Google Scholar]
- 15. Rosas HD, Feigin AS, Hersch SM. Using advances in neuroimaging to detect, understand, and monitor disease progression in Huntington's disease. NeuroRx 2004;1:263–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wild EJ, Henley SM, Hobbs NZ, et al. Rate and acceleration of whole-brain atrophy in premanifest and early Huntington's disease. Mov Disord 2010;25:888–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aylward EH, Rosenblatt A, Field K, et al. Caudate volume as an outcome measure in clinical trials for Huntington's disease: a pilot study. Brain Res Bull 2003;62:137–41 [DOI] [PubMed] [Google Scholar]
- 18. Paulsen JS, Nopoulos PC, Aylward E, et al. Striatal and white matter predictors of estimated diagnosis for Huntington disease. Brain Res Bull 2010;82:201–07 [DOI] [PMC free article] [PubMed] [Google Scholar]