SUMMARY:
CNS WD is fatal if antibiotics are not begun early, but knowledge regarding the variety of presentations on MR imaging is limited. In order to more effectively recognize this entity on MR imaging, the Mayo Clinic medical records were reviewed for subjects diagnosed with CNS WD from 1992-2006 who had also undergone MR imaging of the neuraxis. Seven subjects were identified and their imaging findings were reviewed by the authors. Four of 7 had head MR imaging findings indicative of WD. Two subjects demonstrated high T2 signal within the corticospinal tracts. CNS WD may demonstrate high T2 signal with minimal enhancement and no restricted diffusion, primarily in the midline of the midbrain, hypothalamus, and mesial temporal lobes and occasionally the corticospinal tracts. MR imaging may also be normal. Radiologists should be aware of these presentations and be prepared to mention CNS WD as a diagnostic possibility since early antibiotic therapy may significantly impact morbidity and mortality.
WD is a rare disorder that was first described in 1907 and subsequently found to be caused by a bacterium. Although WD is a systemic illness, it may present with signs and symptoms isolated to gastrointestinal, cardiac, or central nervous systems.1 Approximately 33% of patients with WD experience neurologic symptoms, including encephalopathy, ophthalmoplegia, myoclonus, ataxia, upper motor neuron signs, and hypothalamic manifestations, among others.
WD is invariably fatal without treatment, but even with antibiotics, 2%–33% of patients relapse, most often with a neurologic manifestation.2 CNS involvement of WD carries a grim prognosis with approximately 25% of patients dying within 4 years of diagnosis and another 25% having major neurologic sequelae.3 Approximately 60% of patients with CNS WD experience some improvement in their symptoms during antibiotic therapy; thus, early diagnosis and treatment initiation are paramount for survival.1 Diagnostic efforts may be hampered by the plethora of presenting signs and symptoms as well as the lack of sensitivity and specificity of laboratory tests.
CNS WD is a subset of a rare disease. Only approximately 1000 cases of all types of WD have been reported, and our understanding of head MR imaging findings is based only on case reports; thus, each new case imaged with modern equipment has the potential to expand our understanding significantly as long as the diagnosis has been carefully established.
Materials and Methods
This retrospective study was approved by the Mayo Clinic institutional review board. The Mayo Clinic medical records from 1992 to 2006 were searched for terms including “Whipple disease” and “encephalopathy.” Review of an initial 140 subjects identified with some form of WD revealed 20 patients thought to have CNS WD. Of those with strong evidence for CNS WD based on their signs and symptoms, CSF and serum studies, and small bowel biopsies performed via upper endoscopy, 7 had head MR images and 4 of these had MR imaging findings thought to be directly due to WD. One patient had isolated CNS WD, 1 had asymptomatic gastrointestinal disease, and 5 had a CNS recurrence following an initial gastrointestinal presentation.
Results
Subject 1
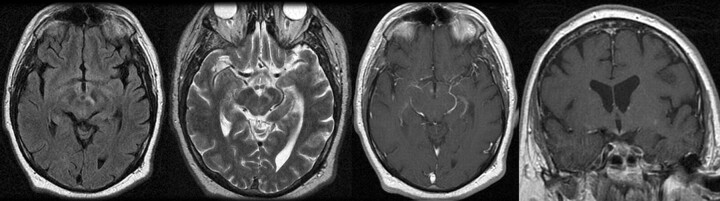
A 54-year-old man presented with hypersomnia, weight gain, lower extremity weakness, dysarthria, confusion, and gait ataxia. Head MR imaging without and with contrast demonstrated an approximately 9-mm focus of enhancing abnormal T2 signal intensity without mass effect within the inferomedial aspect of the hypothalamus bilaterally and extending slightly into the left cerebral peduncle (Fig 1). In addition, there was a 3-mm ovoid focus of nonenhancing T2 signal intensity within the subcortical white matter of the right frontal lobe. No restricted diffusion was seen. Diagnostic considerations at the time for the hypothalamic region included infectious or noninfectious inflammatory or granulomatous processes, including sarcoidosis, histiocytosis X, or neoplasia, such as lymphoma or a hypothalamic/optic glioma.
Fig 1.
Axial noncontrast FLAIR and fast spin-echo T2 MR images as well as postgadolinium axial and coronal T1 images demonstrate a 9-mm focus of enhancing abnormal T2 signal intensity without mass effect within the inferomedial aspect of the hypothalamus bilaterally, extending into the left cerebral peduncle.
Follow-up imaging 3 months later showed a decrease in the amount of T2 signal-intensity abnormality and abnormal gadolinium enhancement in the hypothalamus and left mesencephalon without any new lesions. These findings remained stable on MR imaging 2 months later.
Work-up included a positive PCR for Whipple bacterial nucleic acid in the CSF and serum and positive histopathology from a small bowel biopsy. The patient had no prior history of WD. Clinically, he stabilized briefly after initiation of antibiotics but was lost to follow-up.
Subject 2
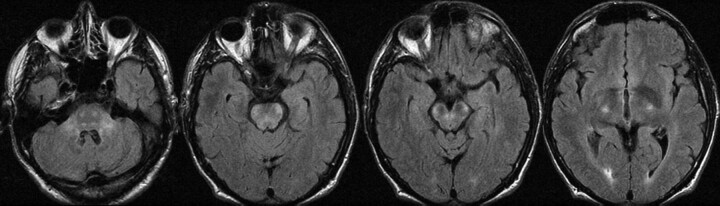
This patient presented at 59 years of age with a progressive brain stem syndrome of gait ataxia, dysarthria, dysphagia, diplopia, quadriparesis, cognitive deficits, and emotional lability. MR imaging findings included bilaterally symmetric T2 signal-intensity abnormality without mass effect or restricted diffusion involving the corticospinal tracts, brain stem, and brachium pontis with faint gadolinium enhancement along the ventral aspect of the cerebral peduncles (Fig 2). On presentation, he had mild weakness and mild-to-moderate spasticity in all 4 limbs. The enhancement resolved, but the T2 changes remained stable on repeat annual imaging for 4 years.
Fig 2.
Axial noncontrast FLAIR MR images demonstrate bilaterally symmetric T2 signal-intensity abnormality without mass effect, involving the corticospinal tracts, brain stem, and brachium pontis.
Work-up included PCRs positive for Whipple bacillus from the CSF and a small bowel biopsy. Small bowel biopsy was otherwise negative for PAS findings of WD. He had no prior history of WD. Although initially wheelchair-bound, his neurologic signs/symptoms improved remarkably, though gradually, with antibiotics for 6 years. He remains on antibiotics indefinitely, uses a cane to ambulate, but has normal speech and cognition.
Subject 3
A 38-year-old man presented with bifrontal headache, fatigue, intermittent horizontal diplopia, and olfactory hallucinations thought to represent partial seizures 10 years after being diagnosed with gastrointestinal WD with a positive small bowel biopsy after developing diarrhea and generalized arthralgias.
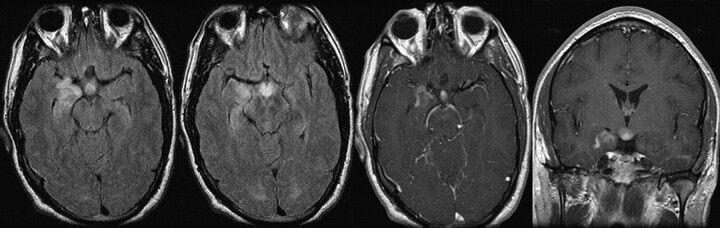
MR imaging demonstrated enhancing abnormally increased T2 signal intensity in the hypothalamus and anteromedial aspect of the right temporal lobe, including the hippocampal head (Fig 3). These areas appeared somewhat bulky, suggesting mass effect, but there was no surrounding vasogenic edema. Differential considerations based on imaging included WD, as well as sarcoidosis, histiocytosis X, Wegener granulomatosis, and lymphoma.
Fig 3.
Axial noncontrast FLAIR and axial and coronal T1 postgadolinium images demonstrate enhancing abnormally increased T2 signal intensity in the hypothalamus and anteromedial aspect of the right temporal lobe, including the hippocampal head.
PCR from the CSF was positive for Whipple bacillus nucleic acid, but serum PCR was negative. Symptoms improved dramatically following initiation of antibiotics, as did his imaging findings on follow-up MR imaging 6 months later. He remained neurologically improved during the 3-year follow-up period.
Subject 4
A 40-year-old man was diagnosed at 35 years of age with WD involving only the gastrointestinal system. He then presented with a supranuclear gaze palsy, hypothermia, and at least a 6-month history of cognitive decline. Physical examination also revealed rhythmic contractions of the jaw and tongue with coincident convergent oscillations of the eyes, classic for oculomasticatory myorrhythmia, a form of myoclonus pathognomonic for CNS WD.4 His strength was preserved, but he had diffuse hyperreflexia except at the ankles. Histopathology of the small bowel biopsy revealed PAS-positive material, and the small bowel PCR was positive for Whipple bacillus nucleic acid. The serum and CSF PCR test results were negative.
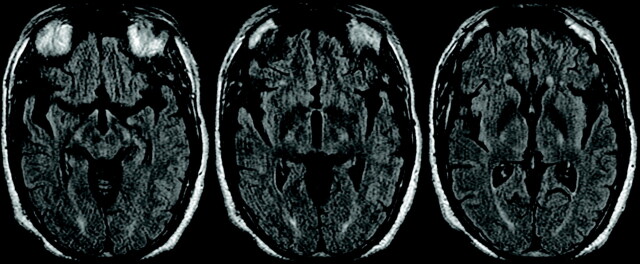
MR imaging demonstrated mild symmetric nonenhancing high T2 signal intensity in the hypothalami and corticospinal tracts (Fig 4). These lesions did not restrict diffusion. The mean apparent diffusion coefficient for this lesion (b = 1000) was elevated at 993 (10−6 mm/s).
Fig 4.
Axial noncontrast FLAIR images demonstrate symmetric high T2 signal intensity in the hypothalami and corticospinal tracts.
Subjects with Negative Head Findings on MR Images
Review of multiple MR images obtained with and without gadolinium during times when the last 3 subjects were manifesting their clinical symptoms failed to demonstrate any abnormalities that could be reasonably attributed to CNS WD.
Table 1 summarizes the diagnostic evaluations of each subject. Table 2 outlines the relationship between the interval from diagnosis to treatment and eventual clinical outcome.
Table 1:
Diagnostic evaluation summary
| Subject No. | Age (yr)/Sex | Primary | MR Imaging | PCR CSF | PCR Serum | PCR GI | GI Biopsy (PAS) |
|---|---|---|---|---|---|---|---|
| 1 | 54/M | CNS | + | + | + | NP | – |
| 2 | 59/M | CNS (asxs GI) | + | + | – | + | – |
| 3 | 38/M | GI→CNS | + | + | – | NP | + |
| 4 | 40/M | GI→CNS | + | – | – | + | + |
| 5 | 40/M | GI→CNS | – | + | NP | + | – |
| 6 | 52/M | GI→CNS | – | + | – | – | + |
| 7 | 63/M | GI→CNS | – | + | – | – | + |
Note:—+ indicates positive result; –, negative result.
Table 2:
Relation of treatment initiation and outcome
| Subject No. | Interval Symptoms→Rx | Outcome |
|---|---|---|
| 1 | 9 months | Stabilized on antibiotics but died 15 months later |
| 2 | 4 months | From wheelchair-bound to a cane on antibiotic therapy |
| 3 | 1 month | Complete recovery on antibiotics |
| 4 | 6 months | Lost to follow-up |
| 5 | ∼yrs? | Severe neurologic impairment |
| 6 | ∼1 month | Mild attention and concentration deficits |
| 7 | ∼3 months | Mild symptoms when lost to follow-up |
Discussion
There is no pathognomonic pattern of radiologic involvement for WD, though most imaging findings correlate with pathologic studies showing predominant involvement of the basilar telencephalon, thalamus, hypothalamus, quadrigeminal plate, and periaqueductal gray matter.5 The reasons Tropheryma whippelii is trophic to the gray matter in these regions is not understood. Furthermore, the exact pathophysiology is poorly understood, though it is suspected that damage is caused by direct bacterial replication, more so than the associated host's immune response with inflammatory damage.6 Histologic specimens of WD from the small bowel or brain demonstrate PAS-positive rods and sickle-shaped inclusions in macrophages, which infiltrate diffusely with central areas of necrosis and hemorrhage.7 Review of the literature demonstrates a wide array of MR appearances and clinical presentations in WD ranging from similar midline abnormalities as presented here to nodular parenchymal lesions or leptomeningeal enhancement to stroke-like presentations of focal tumorlike lesions.8–12
Most commonly, researchers report that MR images reveal diffuse unilateral or bilateral abnormal T2 signal intensity most evident on FLAIR sequences within the mesial temporal lobe, midbrain, hypothalamus, and thalamus, at times including transient enhancement similar to the cases presented in this series.13–15 Less frequently, patients with WD may present with scattered lesions of high T2 signal intensity in a peripheral array involving the gray-white junction with or without associated vasogenic edema, which does not involve the typical midline diencephalic structures.7,16 In 1 case, these areas of T2 signal intensity became confluent and were associated with hemorrhage.17,18
WD does not typically produce restricted diffusion.19 Enhancement is usually minimal. Most authors believe that mild-to-moderate atrophy occurs in approximately half of cases.
The corticospinal tract high T2 signal intensity reminiscent of that seen in some cases of amyotrophic lateral sclerosis observed in subjects 2 and 4 has not, to our knowledge, been previously described as a finding in WD. It should be emphasized that 3 of 7 patients with CNS WD had no MR imaging findings to suggest the diagnosis, despite a typical history and positive PCR study results. The variables that affect the sensitivity of MR imaging are unknown but may relate to the attenuation of bacterial replication as well as the associated inflammatory response. Also, while the sensitivity of PCR for WD in the CSF and serum is high, the exact negative predictive value of PCR is not known.20,21
A negative PCR result or negative finding on small bowel biopsy histology do not completely exclude the diagnosis; thus, a complete work-up requires MR imaging, PCRs, and a small bowel biopsy in addition to a careful history and physical examination.
WD may occur in anyone at any age but typically is seen in middle-aged white men; thus, it is not unusual that all 7 of our subjects were men.
Two subjects improved dramatically, and 1 stabilized with antibiotic therapy.
The current treatment recommendation for CNS WD is ceftriaxone, 2 g, intravenously every 12 hours for 2 weeks followed by oral trimethoprim-sulfamethoxazole (1 tablet double-strength twice daily) for 1–2 years.22 T whippelii can be been found within the CSF in patients years after a prolonged course of antibiotics, but it is not known if this is due to incomplete eradication or re-infection.23
Because CNS WD is both fatal if untreated and, at times, a diagnostic challenge, familiarity with the range of MR imaging presentations is key to alerting the clinician that WD is a diagnostic possibility. Further extensive and invasive tests may yield false-negatives (as in the case of subject 1); thus, the MR imaging interpretation may be the prime variable in deciding to offer the patient empiric antibiotic therapy. Certainly, adverse pharmacologic effects and the risk of creating drug-resistant bacterial strains are justified in the proper clinical context of a potentially fatal illness. Also, some authorities have recommended possible lifelong antibiotic therapy for patients with CNS WD; thus, careful diagnosis and monitoring of the disease are justified.
Only 1 of the 4 patients (subject 3) with imaging findings had WD mentioned in the body of the radiologist's report. Subject 3 had a slowly progressive course and was started on antibiotics early; this decision likely produced his full recovery. Of course, WD may recur in previously affected individuals; thus, clinicians need to be vigilant in identifying possible recurrences.
Although findings in this series certainly expand our knowledge of how WD may appear on MR imaging, generalizations are limited by the small number of cases and future case documentation will remain important.
Conclusions
CNS WD may present with a variety of MR imaging lesions or no lesions at all. Often these lesions tend to be fairly symmetric with midline high T2 signal intensity involving the hypothalamus, midbrain, or mesial temporal lobes with minimal enhancement and no restricted diffusion. In our series, 2 of the 4 positive cases showed mild mass effect. Findings were most conspicuous on FLAIR sequences. Mesial temporal lobe involvement may simulate findings of herpes encephalitis or autoimmune/limbic encephalitis. High signal intensity in the corticospinal tracts has, to our knowledge, not been previously reported and may simulate findings seen in patients with amyotrophic lateral sclerosis. The longer interval between symptoms and treatment seems to correlate with morbidity and mortality; however, the extent of MR imaging lesions does not necessarily imply higher morbidity.
Familiarity with the range of possible MR imaging appearances of WD enables the radiologist to more effectively place WD on the differential diagnosis and thus spurs the clinician to consider both the diagnosis and early initiation of treatment, which may significantly impact outcome.
Abbreviations
- asxs
asymptomatic
- CNS
central nervous system
- FLAIR
fluid-attenuated inversion recovery
- GI
gastrointestinal
- NP
not performed
- PAS
periodic-acid-Schiff
- PCR
polymerase chain reaction
- Rx
therapy
- WD
Whipple disease
Footnotes
Paper previously presented in part at: Annual Meeting of the American Society of Neuroradiology, May 3–9, 2009; Vancouver, British Columbia, Canada.
References
- 1. Fenollar F, Puechal X, Raoult D. Whipple's disease. N Engl J Med 2007;356:55–66 [DOI] [PubMed] [Google Scholar]
- 2. Keinath RD, Merrell DE, Vlietstra R, et al. Antibiotic treatment and relapse in Whipple's disease: long-term follow-up of 88 patients. Gastroenterology 1985;88:1867–73 [DOI] [PubMed] [Google Scholar]
- 3. Schneider PJ, Reisinger EX, Gerschlager W, et al. Long-term follow-up in cerebral Whipple's disease. Eur J Gastroenterol Hepatol 1996;8:899–903 [PubMed] [Google Scholar]
- 4. Schwartz MA, Selhorst JB, Ochs AL, et al. Oculomasticatory myorrhythmia: a unique movement disorder occurring in Whipple's disease. Ann Neurol 1986;20:677–83 [DOI] [PubMed] [Google Scholar]
- 5. Romanul FC, Radvany J, Rosales RK. Whipple's disease confined to the brain: a case studied clinically and pathologically. J Neurol Neurosurg Psychiatry 1977;40:901–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scheld WM. Whipple disease of the central nervous system. J Infect Dis 2003;188:797–800 [DOI] [PubMed] [Google Scholar]
- 7. Niekrash RE, Mclean CA, Kaye AH, et al. Rapidly progressive Whipple's disease of the central nervous system. J Clin Neurosci 1995;2:171–76 [DOI] [PubMed] [Google Scholar]
- 8. Peters G, du Plessis DG, Humphrey PR. Cerebral Whipple's disease with a stroke-like presentation and cerebrovascular pathology. J Neurol Neurosurg Psychiatry 2002;73:336–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Famularo G, Minisola G, De Simone C. A patient with cerebral Whipple's disease and a stroke-like syndrome. Scand J Gastroenterol 2005;40:607–09 [DOI] [PubMed] [Google Scholar]
- 10. Frazier JL, Quinones-Hinojosa A. Isolated Whipple disease of the brain resembling a tumour. Acta Neurochir (Wien) 2009;151:173–75 [DOI] [PubMed] [Google Scholar]
- 11. Leesch W, Fischer I, Staudinger R, et al. Primary cerebral Whipple disease presenting as Klüver-Bucy syndrome. Arch Neurol 2009;66:130–31 [DOI] [PubMed] [Google Scholar]
- 12. Löhr M, Stenzel W, Plum G, et al. Whipple disease confined to the central nervous system presenting as a solitary frontal tumor: case report. J Neurosurg 2004;101:336–39 [DOI] [PubMed] [Google Scholar]
- 13. Scholz KB, Henning S, Paulus W, et al. MRI findings in isolated cerebral manifestation of Whipple's disease: case report and review of the literature. Euro J Radiol 2006;59:1–5 [Google Scholar]
- 14. Panegyres PK, Edis R, Beaman M, et al. Primary Whipple's of the brain: characterization of the clinical syndrome and molecular diagnosis. Q J Med 2006;99:609–23 [DOI] [PubMed] [Google Scholar]
- 15. Schnider P, Trattnig S, Kollegger H, et al. MR of cerebral Whipple disease. AJNR Am J Neuroradiol 1995;16:1328–29 [PMC free article] [PubMed] [Google Scholar]
- 16. Duprez TP, Grandin CB, Bonnier C, et al. Whipple disease confined to the central nervous system in childhood. AJNR Am J Neuroradiol 1996;17:1589–91 [PMC free article] [PubMed] [Google Scholar]
- 17. Yu C, Jiang A, Yu Y. Serial imaging changes of cerebral Whipple's disease: from onset to the end. J Neuroimaging 2007;17:81–83 [DOI] [PubMed] [Google Scholar]
- 18. Wu L, Wang X, Wei H, et al. Diffuse cortical lesions with hemorrhage in cerebral Whipple's disease. Clin Neurol Neurosurg 2008;110:83–87 [DOI] [PubMed] [Google Scholar]
- 19. Nelson JW, White ML, Zhang Y, et al. Proton magnetic resonance spectroscopy and diffusion-weighted imaging of central nervous system Whipple disease. J Comput Assist Tomogr 2005;29:320–22 [DOI] [PubMed] [Google Scholar]
- 20. Lynch T, Odel J, Fredericks DN, et al. Polymerase chain reaction-based detection of Tropheryma whippelii in central nervous system Whipple's disease. Ann Neurol 1997;42:120–24 [DOI] [PubMed] [Google Scholar]
- 21. von Herbay A, Ditton HJ, Schuhmacher F, et al. Whipple's disease: staging and monitoring by cytology and polymerase chain reaction analysis of cerebrospinal fluid. Gastroenterology 1997;113:434–41 [DOI] [PubMed] [Google Scholar]
- 22. Matthews BR, Jones LK, Saad DA, et al. Cerebellar ataxia and central nervous system Whipple disease. Arch Neurol 2005;62:618–20 [DOI] [PubMed] [Google Scholar]
- 23. Maiwald M, von Herbray A, Fredricks DN, et al. Cultivation of Tropheryma whippelii from cerebrospinal fluid. J Infect Dis 2003;188:801–08 [DOI] [PubMed] [Google Scholar]