SUMMARY:
Temozolomide, an oral alkylating agent, is a commonly used medicine in the treatment of anaplastic astrocytoma and glioblastoma multiforme. This paper will present the mechanism of action as well as the clinical role for this chemotherapeutic drug.
Temozolomide (Temodar) is an oral alkylating agent approved by the FDA for use in the first-line treatment of glioblastoma multiforme as well as for recurrent anaplastic astrocytoma.1 Alkylating agents are some of the oldest drugs in the chemotherapy arsenal and were originally developed as chemical weapons during the early part of the twentieth century. Their anti-neoplastic aspects were only further investigated after World War II.2 Traditional alkylating agents act by producing DNA cross-linkages, thus inhibiting DNA and cellular replication. Other similarly acting drugs, such as temozolomide, work by methylating DNA, which also results in inhibited DNA and cellular replication.2 All such agents act nonspecifically and affect both cancerous and normal cells alike. However, cancer cells divide more rapidly than normal tissue and thus should be more sensitive to these effects.
Proposed Mechanism of Action
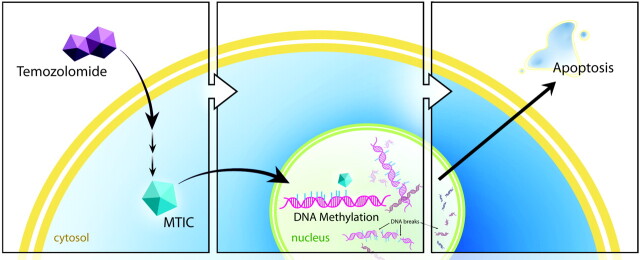
Temozolomide is an oral alkylating agent, first developed in the early 1980s at Aston University in Great Britain.3 The proposed mechanism of action is based on the ability of its metabolites to deposit methyl groups on DNA guanine bases. After oral administration, the prodrug temozolomide is readily absorbed in the small intestine, with good penetration of the blood-brain barrier due to its small size (194 Da). It then undergoes spontaneous intracellular conversion via hydrolysis into a potent methylating agent, MTIC.4 MTIC methylates a number of nucleobases, most important, the guanine base. This results in the formation of nicks in the DNA, followed by apoptosis, because cellular repair mechanisms are unable to adjust to the methylated base (Fig 1).5
Fig 1.
Schematic illustration of the proposed mechanism of temozolomide. Temozolomide is converted intracellularly into MTIC, which methylates DNA. Cellular repair mechanisms cannot adjust, resulting in DNA nicks and ultimately apoptosis.
Clinical Indications
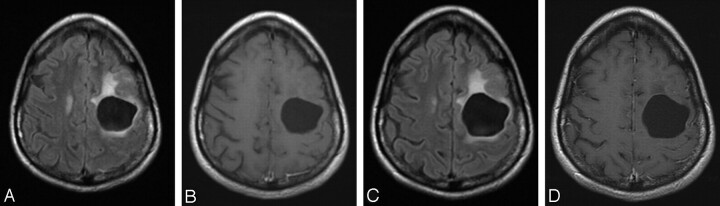
Temozolomide was granted FDA approval in the treatment of recurrent anaplastic astrocytoma in 1999, with subsequent approval for the first-line therapy of glioblastoma multiforme (Fig 2).1 The agent has also shown some activity in patients with metastatic melanoma.6,7
Fig 2.
Imaging findings associated with a good response to treatment in a patient with recurrent astrocytoma. Fluid-attenuated inversion recovery (A) and postcontrast T1-weighted sequences (B) demonstrate a postsurgical cavity with associated mild surrounding T2 prolongation and lack of enhancement. C and D, Follow-up examination 18 months later shows no disease progression.
Administration and Effects
Temozolomide is an oral prescription-only drug (though an intravenous form is available) administered once daily. The half-life of temozolomide is approximately 1.8 hours, with the active half-life of the metabolite (MTIC) being slightly longer. Temozolomide and its metabolites are excreted via the kidneys. Nausea and vomiting are common side effects and are usually mild to moderate. Seizure and thrombocytopenia are noted in less than 7% of patients.8,9
Economic Issues
Like other chemotherapeutic agents, temozolomide is quite expensive, and drug costs can vary widely from region to region.10 Temozolomide therapy costs run in the tens of thousands of dollars. These costs, though, are largely comparable with other such treatments.11 Temozolomide annual worldwide sales total approximately 1 billion US dollars. However, these costs may be dramatically reduced with the possible introduction of generic versions in the near future.12
Abbreviations
- FDA
US Food and Drug Administration
- MTIC
methyl triazeno imidazole carboxamide
References
- 1. Villano JL, Seery TE, Bressler LR. Temozolomide in malignant gliomas: current use and future targets. Cancer Chemother Pharmacol 2009;64:647–55 [DOI] [PubMed] [Google Scholar]
- 2. Colvin M. Alkylating agents and platinum antitumor compounds. In: Kufe DW, Frei E, Holland JF, et al. eds. Holland-Frei Cancer Medicine. 8th ed. Shelton, Connecticut: People's Medical Publishing House; 2010 [Google Scholar]
- 3. Newlands ES, Stevens MF, Wedge SR, et al. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev 1997;23:35–61 [DOI] [PubMed] [Google Scholar]
- 4. Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist 2000;5:144–51 [DOI] [PubMed] [Google Scholar]
- 5. Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res 2000;6:2585–97 [PubMed] [Google Scholar]
- 6. Nagasubramanian R, Dolan ME. Temozolomide: realizing the promise and potential. Curr Opin Oncol 2003;15:412–18 [DOI] [PubMed] [Google Scholar]
- 7. Quirt I, Verma S, Petrella T, et al. Temozolomide for the treatment of metastatic melanoma: a systematic review. Oncologist 2007;12:1114–23 [DOI] [PubMed] [Google Scholar]
- 8. McEvoy GK, Snow EK, Jane Miller J, et al. eds. AHFS Drug Information. Bethesda, Maryland: American Society of Health-System Pharmacists; 2009 [Google Scholar]
- 9. Singhal N, Selva-Nayagam S, Brown MP. Prolonged and severe myelosuppression in two patients after low-dose temozolomide treatment: case study and review of literature. J Neurooncol 2007;85:229–30 [DOI] [PubMed] [Google Scholar]
- 10. Klepper B, Pauker D. Medicare's drug plan: huge price disparities for common cancer drugs. Community Oncology 2006;3:753–55 [Google Scholar]
- 11. Crott R. The economics of temozolomide in brain cancer. Expert Opin Pharmacother 2007;8:1923–29 [DOI] [PubMed] [Google Scholar]
- 12. Pierson R. Teva wins patent battle over Merck's Temodar. Reuters. January 26, 2010. Available at http://www.reuters.com/article/idUSN2611057820100126. Accessed May 31, 2010. [Google Scholar]