Abstract
BACKGROUND AND PURPOSE:
We have been performing the superselective transarterial infusion of high-dose cisplatin for advanced maxillary cancer since 1998 and the local control rate, disease free survival rate, and organ preservation have improved markedly compared with our former therapy. This study evaluates the effectiveness of superselective transarterial infusion therapy by using high-dose cisplatin on maxillary cancer with orbital invasion.
MATERIALS AND METHODS:
We treated 23 patients with maxillary cancer by using superselective transarterial infusion therapy with high-dose cisplatin and concomitant radiation therapy for 10 years. Of all patients, 15 showed orbital invasion, with 11 of these tumors fed by both internal maxillary and ophthalmic arteries. In all patients, we performed superselective transarterial infusion therapy via the internal maxillary artery and/or the other feeding branches from the external carotid artery. After the operation, we determined whether a pCR had occurred by checking for the presence of viable cells. In addition, we calculated the overall survival rate, preservation rate of the eyeball, and disease-free survival rate.
RESULTS:
For all 23 patients, pCR and overall survival rates were 95.7% and 78.4%, respectively. To date, 2 of these patients died of lung metastasis without local recurrence. For the 15 patients with orbital invasion, the respective pCR and disease-free survival rates were 93.3% and 87.5%. Eyeballs were preserved in all patients, and local recurrence occurred in only 1 patient, at the inferior wall of the maxillary sinus (not in the orbit).
CONCLUSIONS:
Superselective transarterial infusion therapy with high-dose cisplatin remarkably improved the local control rate and disease-free survival rate of maxillary cancer. Even in patients with orbital invasion, a high local control rate was achieved, with preservation of the eyeball, through infusion only into branches of the external carotid artery.
Maxillary cancer occurs in 6.4% of all head and neck cancers in Japan.1 Because the early symptoms are difficult to detect, tumors often invade the pterygopalatine fossa, infratemporal fossa, and/or orbit beyond the maxillary sinus before a diagnosis is made. Trimodal therapy, consisting of an operation, systemic chemotherapy, and irradiation, is generally used in the treatment of maxillary cancer. This form of therapy, however, often sacrifices vision, articulation, and mastication. In addition, it causes cosmetic problems due to inevitable facial deformity. The 5-year survival rates in patients with T4 receiving trimodal therapy have been found to be approximately 50%.2,3
Superselective transarterial infusion therapy using high-dose cisplatin was introduced as a novel intra-arterial infusion chemoradiotherapy regimen (radiotherapy and concomitant intra-arterial cisplatin) by Robbins et al.4–6 We began using this treatment in our patients with maxillary cancer in 1998. Since that time, both the local control rate and the disease-free survival rate have improved markedly; moreover, no patient has undergone eyeball removal, even patients with orbital invasion. The results of this study underscore the effectiveness of superselective transarterial infusion therapy on maxillary cancer by using high-dose cisplatin.
Materials and Methods
The protocol of this study was approved by the ethics committee of the Faculty of Medicine, Yamagata University. The initial subjects were 24 consecutive patients with maxillary squamous cell carcinoma examined between December 1998 and September 2007. All patients gave informed consent for superselective transarterial infusion therapy by using high-dose cisplatin with curative intent and underwent this method of treatment in our hospital. Before the study, none had leukopenia with a WBC of <2000/μL or showed renal dysfunction with a serum creatinine level of >1.5 mg/dL. Because 1 patient left the hospital of her own free will before completion of the treatment cycle, the final set of subjects consisted of 23 patients (16 men and 7 women) with an average of 64.7 years of age (range, 32–83 years).
Before treatment, all patients underwent surgical biopsy to provide samples for a pathologic diagnosis by otorhinolaryngologists. After the histopathology was confirmed, CT and MR imaging were performed to support decisions about the stage of the maxillary cancers. Of the 23 patients, 15 showed orbital invasions, which were confirmed by the destruction of the orbital wall and/or the existence of an intraorbital extension of the tumor. Tumors of 3 patients were classified as T3N0M0; 18, as T4aN0M0, and 2, as T4aN2M0.
In all patients, both internal and external carotid angiographies were performed on the affected side by using a DSA system. We used a femoral approach under local anesthesia by using a 4F or 5F sheath and a 4F or 5F catheter. We focused our evaluation on tumor stain, tumor vessels, and feeding arteries. In 11 patients, the maxillary tumor was found to be fed by both the internal maxillary artery and the ophthalmic artery. On the basis of the DSA evaluation, the tumor volume fed with the ophthalmic artery was determined to be 10%–30% of the total tumor volume. Additionally, we confirmed the choroid crescent of the eyeball through the lateral view of the internal carotid angiograms. In cases in which no choroid crescent was present, infusion from the internal maxillary artery resulted in a loss of vision because the retina was being fed with branches of the external carotid artery.7
All patients received generalized heparinization at 1000 U per 10 kg body weight to prevent clot formation just before insertion of the 2.1F microcatheter, which was passed through the 4F or 5F catheter. The microcatheter was introduced to the internal maxillary artery, and its tip was always placed at the distal portion of the origin of the accessory meningeal artery. In all patients except 1, CT angiography was performed to simulate the distribution of the drug to the tumor before the first infusion therapy session.8,9 After the first patient was treated, we decided to perform CT angiography as a form of pretreatment evaluation. Our protocol was as follows: a total of 10 mL of iodine-based contrast medium (300 mg/mL) was injected for 100 seconds, and the CT scan was started 10 seconds before completion of the injection.
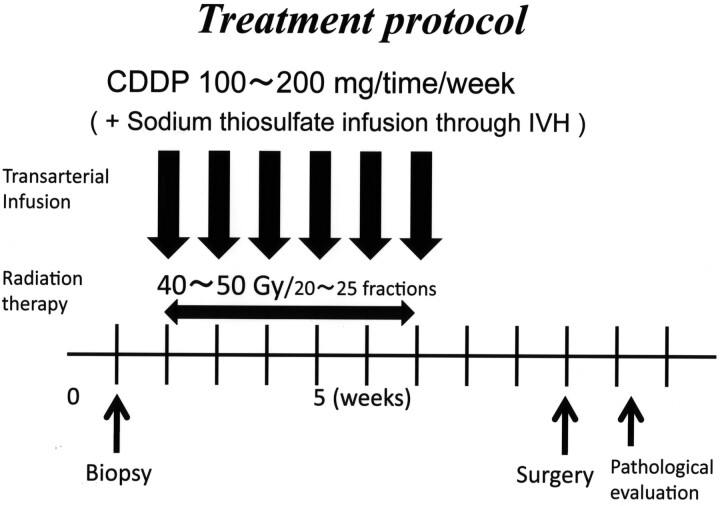
Immediately before the first infusion therapy session, provocation tests were performed on all patients by using 3–5 mg of lidocaine to confirm that there were no neurologic deficits. We performed high-dose cisplatin infusion therapy from the internal maxillary artery combined with an intravenous infusion of sodium thiosulfate, a cisplatin neutralizer, to reduce the systemic toxicity (8 g of sodium thiosulfate/150 mg of cisplatin). When the other feeding arteries, such as the facial artery (n = 3), the transverse facial artery (n = 1), and the superficial temporal artery (n = 1), were confirmed, a portion of the cisplatin was infused into them on the basis of the size of the tumor stain determined by DSA. However, we never infused cisplatin directly into the ophthalmic artery, even when feeding vessels were confirmed as originating from this artery. This intra-arterial infusion therapy was performed once per week, 4–9 times (average, 6.0 times), with 100–200 mg of cisplatin administered each time at a rate of 50 mg per 10 minutes. For 5 patients, 20 mg of docetaxel (Taxotere) was administered in conjunction with the cisplatin. Just after each infusion therapy session, we infused 4 mg of betamethasone sodium phosphate into the feeding arteries to prevent angitis resulting in vessel obstruction. All patients were irradiated with 40–50 Gy concomitantly (40 Gy in 4 patients, 48 Gy in 1 patient, and 50 Gy in 18 patients) (Fig 1).
Fig 1.
Treatment protocol of the superselective high-dose cisplatin infusion therapy.
Several weeks after the completion of superselective high-dose cisplatin infusion therapy, all patients underwent surgery. On the basis of pathologic specimens obtained from the operation, pathologists classified viable cancer cells into 4 grades (Table).10 We classified grades 3 and 4 as pCR; evaluations equal to or below grade 2 were designated as non-pCR. We then calculated the pCR rate for all patients.
Postoperative histologic grading
| Grade | Histologic Characteristics |
|---|---|
| 0 | No therapeutic effects observed |
| 1 | Degenerative changes in tumor cells but no destruction of tumor nests |
| 2 | Destruction and disappearance of tumor nests but viable cells remain |
| 2a | Viable cells occupy large areas (>1/3) |
| 2b | Viable cells occupy small areas (<1/3) |
| 3 | Tumor cells remain but appear nonviable |
| 4 | No tumor cells remain |
After completion of the therapy, CT and MR imaging were performed every half year, and we checked for the existence of local recurrence and distant metastasis. We also calculated the overall survival rate, disease-free survival rate, local control rate, and the preservation rate of the eyeball by using the Kaplan-Meier method, with differences assessed by using the logrank test. Statistical analyses were performed by using the Statistical Package for the Social Sciences, Version 16.0 software package (SPSS, Chicago, Illinois). The mean follow-up period was 46.1 months (range, 7–102 months).
Results
Of the 23 patients, 22 were classified as pCR (95.7%), while a single patient was considered grade 2b on the basis of postoperative pathologic investigations. However, this patient functioned as a good local control at this point in the study. In patients with orbital invasion, 14 of 15 were considered as having pCR (93.3%). In patients with a large tumor fed by the ophthalmic artery, 10 of 11 were also found to have pCR (90.9%). In all cases, eyeballs were preserved without removal. Cataracts appeared after treatment in all patients, but vision was restored via lens replacement.
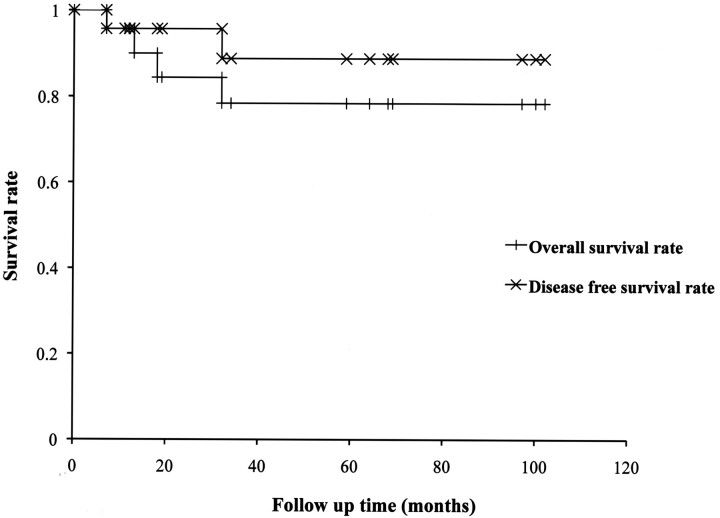
For all 23 patients, the survival rate was 78.4%, and the disease-free survival rate was 88.8%. The longest term of survival was 102 months (Fig 2 ). Four patients died during the follow-up period: 2 patients died of distant metastasis and 2 died of other cancers. Local recurrence was observed in only 1 patient at the inferior wall of the maxillary sinus, and this patient underwent a salvage operation and has survived for 59 months to date.
Fig 2.
Disease-free survival rate and overall survival rate of the superselective high-dose cisplatin infusion therapy for maxillary cancer.
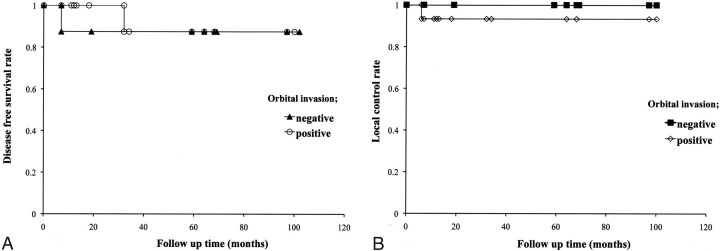
Among patients with orbital invasion (n = 15), the disease-free survival rate was 87.5% and the local control rate was 93.3%. For patients without orbital invasion (n = 8), the disease-free survival rate was 87.5%, and the local control rate was 100%. Regarding the disease-free survival rate or local control rate, no significant difference was found between patients with and without orbital invasion (P value for the disease-free survival rate = .745, P value for the local control rate = .465) (Fig 3).
Fig 3.
Disease-free survival rate (A) and local control rate (B) in patients with orbital invasion.
In patients with a tumor fed by the ophthalmic artery (n = 11), the disease-free survival rate was 75.0% and the local control rate was 90.9%. In patients without participation of the ophthalmic artery (n = 12), the disease-free survival rate was 91.7% and the local control rate was 100%. In terms of the disease-free survival rate or local control rate, no significant difference was found between patient groups on the basis of participation of the ophthalmic artery (P value for the disease-free survival rate = .726, P value for the local control rate = .296).
Minor complications were experienced by some subjects. Nausea and vomiting were often encountered before the end of the procedure, and in rare instances, patients complained of mild pain at the infusion site. These minor complications, which occurred in the same patients, were controlled by using appropriate drugs. All infusion procedures were run to completion. Focal skin necrosis was not observed in patients who received infusion therapy via a facial artery. We also used Taxotere in 5 patients, of whom 1 showed mild leukopenia with a WBC of <2000/μL immediately after the last intra-arterial-infusion therapy session, but the patient promptly improved when a granulocyte colony-stimulating factor agent was used.
Discussion
Our study revealed a remarkably higher local control rate and a remarkably higher overall survival rate of maxillary cancer than described in previous reports on conventional trimodal therapies2,3 and even in studies on intra-arterial infusion therapies performed in a manner similar to our procedures.10–14 In conventional trimodal therapy, the 5-year survival rate was found to be approximately 50% in patients with T4,2,3 while in previously reported intra-arterial infusion therapy studies, the 5-year survival rate was 56%–67% in patients with T4.10–14 Intra-arterial infusion therapy can enhance intratumoral concentration of cisplatin, compared with systemic chemotherapy, and even positively affects cisplatin resistance.15–17 Compared with the intra-arterial infusion therapy previously reported, our treatment was performed much more frequently and the location of the tip of the catheter may have been more appropriate and constant. Concomitant radiation therapy may have also contributed to the improvement of our results.
Good control of the primary tumor can have a strong influence on the prognosis18 because maxillary cancer is accompanied by only slight lymph node metastasis or distant metastasis, even in its advanced stages. Superselective transarterial infusion therapy of high-dose cisplatin can therefore play an important role in the control of maxillary cancer. Moreover, pathologic investigation after treatment can function as a good indicator in patients in whom a local recurrence is expected. Our data did not show viable cells in any patients with intraorbital invasion except for 1. This finding indicates that the superselective transarterial infusion therapy of high-dose cisplatin has a strong antitumor effect.
It is difficult to distinguish accurately grade 2b (non-pCR) from grade 3 (pCR) on the basis of the pathologic classification.10 There was only 1 patient with grade 2b, who functioned as a good local control in this study, which may suggest that grade 2b should be included in the pCR classification. Local recurrence was observed in only 1 of 22 patients with grade 3 (pCR). Local recurrence was accurately predicted in >90% of patients; thus, the pathologic classification10 used in this study may be a good clinical indicator.
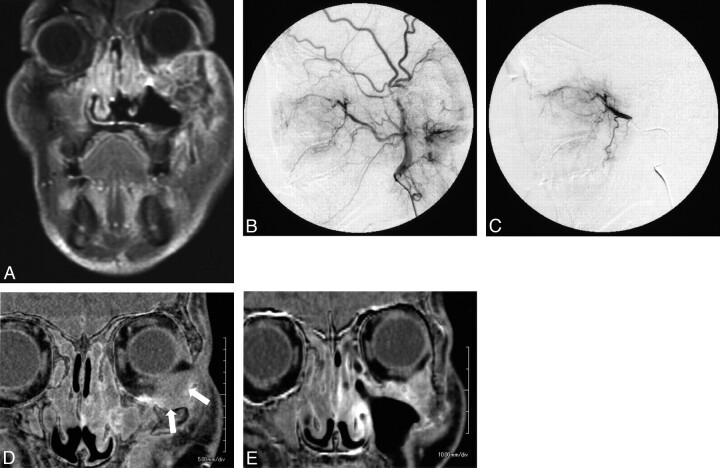
To preserve patients' vision, we did not infuse cisplatin into the ophthalmic artery. Pathologic studies showed no viable cells in >90% of patients with orbital invasion fed with the ophthalmic artery, so we were able to avoid eyeball removal; in all patients, eyeballs and vision were completely preserved. Previous reports described that the preservation rate of the orbital component was 67% by using intra-arterial infusion of 5-fluorouracil and cisplatin.13 The reason that the orbital tumor was controlled well, even though infusion therapy was not performed from the ophthalmic artery, was because feeding from the ophthalmic artery is a parasitic supply, which probably decreases gradually as the tumor shrinks following cisplatin infusion therapy from the internal maxillary artery. We believe that parasitic supplies scarcely contribute to feeding of tumors when the blood supply from the internal maxillary artery becomes sufficient for the tumor's needs. Eventually, the tumor is fed completely by the internal maxillary artery. In fact, we have actually noted some patients showing this change, as confirmed by CT angiography (Fig 4 ). In these cases, a part of the tumor with poor contrast enhancement before treatment showed clearly enhanced contrast at the end of therapy. We believe that most advanced maxillary cancers can be controlled through superselective transarterial infusion with high-dose cisplatin only into branches of the external carotid artery, with concomitant radiation therapy.
Fig 4.
Maxillary cancer with orbital invasion. A, This 59-year-old man was diagnosed with left maxillary cancer (T4N0M0) with orbital invasion. B and C, Before treatment, both an external carotid angiogram and a superselective internal maxillary angiogram reveal a tumor stain in the maxillary sinus. D and E, A subtraction image of the CT angiogram reveals the intraorbital component of the tumor as a hypoenhanced lesion (white arrows). After 4 sessions of intra-arterial infusion therapy, the intraorbital tumor has shrunk remarkably and is much more enhanced (E) than in the initial CT angiogram (D).
Homma et al11 have reported osteonecrosis, brain necrosis, and ocular/visual problems. In this study, our patients experienced neither major side effects nor complications. It may be because the radiation dose was relatively low compared with that in previous reports,10–14 and we infused a steroid agent into the feeding arteries to prevent angitis immediately following every cisplatin infusion session. Systemic toxicity, such as that resulting in myelosuppression and renal dysfunction, is also not an obstacle to this treatment. Sodium thiosulfate, which neutralizes cisplatin, enables us to administer massive cisplatin doses directly to feeding arteries of the tumor because it mitigates systemic side effects, such as myelosuppression and renal dysfunction.19–21 Fortunately, none of the patients presented with leukopenia or renal dysfunction before treatment in this study, and we could safely use cisplatin in all subjects. We observed mild leukopenia in only 1 patient who received Taxotere combined with cisplatin. We do, however, recommend watching for the occurrence of pancytopenia when using Taxotere. In this study, we also did not observe focal skin necrosis in patients who received infusion therapy via the facial artery.
Infusion only into the feeding arteries of the tumor is important in enhancing intratumoral concentration and in avoiding unexpected toxicity. Direct toxicity by arterial infusion cannot be avoided, even when sodium thiosulfate is used. Cisplatin should not be infused into an artery that is not supplying the tumor. In fact, CT angiography is a useful tool in precisely identifying these feeding arteries.8,9 It is important to be familiar with the connection between the external and internal carotid arteries. If the retina is supplied from the internal maxillary artery, infusion into the internal maxillary artery will result in a loss of vision. Fortunately, we have never experienced such cases in our treatments. Identification of the supply to the retina must be established to safely perform the infusion of toxic or embolic materials.
We always use a femoral approach, though a superficial temporal artery approach is another viable alternative.22 The superficial temporal artery approach does not require frequent punctures of the artery, yet the possibility exists of catheter infection and catheter dislocation. This therapy has little effect on advanced tumors with multiple feeding arteries.23 In the transfemoral approach, it is possible to infuse antitumor drugs into multiple feeding arteries and there is no possibility of catheter infection because the catheter is removed each time. The only problem with this treatment is its inherently complicated technique, which is more labor-intensive and takes 2–3 hours for each session. Interventional radiologists, otorhinolaryngologists, nurses, and other staff must mutually cooperate to become familiar with its detailed procedures.
This study has several limitations. The number of patients was small, and the observation period was insufficiently short. Further consideration of the proper frequency of intra-arterial infusion therapy is necessary, along with consideration of the proper dosages of cisplatin and radiation.
Conclusions
Superselective transarterial infusion therapy with high-dose cisplatin for maxillary cancer remarkably improved both the local control rate and the disease-free survival rate. Even in patients with orbital invasion, a high local control rate was achieved, with preservation of the eyeball, by infusion only into branches of the external carotid artery.
Abbreviations
- DSA
digital subtraction angiography
- pCR
pathologic complete response
- WBC
white blood cell count
This work was supported by a Grant-in-Aid from the Global COE program of the Japan Society for the Promotion of Science.
Paper previously presented at: 67th Annual Meeting of Japan Radiological Society, April 4–6, 2008; Yokohama, Japan; and 33rd Annual Meeting of European Society of Neuroradiology, September 18–21, 2008; Krakow, Poland.
References
- 1. Saikawa S. Report of head and neck cancer registry of Japan clinical statistics of registered patients, 2002. Jpn J Head Neck Cancer 2006;32(suppl):1–14 [Google Scholar]
- 2. Persons JT, Mendenhall WM, Stringer SP, et al. Nasal cavity and paranasal sinuses. In: Perez CA, Brady LW, eds. Principles and Practice of Radiation Oncology. 3rd ed. Philadelphia: Lippincott-Raven; 1977:941–59 [Google Scholar]
- 3. Yoshimura R, Shibuya H, Ogura I, et al. Trimodal combination therapy for maxillary sinus carcinoma. Int J Radiat Oncol Biol Phys 2002;53:656–63 [DOI] [PubMed] [Google Scholar]
- 4. Robbins KT, Storniolo AM, Kerber C, et al. Rapid superselective high-dose cisplatin infusion for advanced head and neck malignancies. Head Neck 1992;14:364–71 [DOI] [PubMed] [Google Scholar]
- 5. Robbins KT, Storniolo AM, Kerber C, et al. Phase I study of highly selective supradose cisplatin infusion for head and neck cancer. J Clin Oncol 1994;12:2113–20 [DOI] [PubMed] [Google Scholar]
- 6. Robbins KT, Vicario D, Seagren S, et al. A targeted supradose cisplatin chemoradiation protocol for advanced head and neck cancer. Am J Surg 1994;168:419–22 [DOI] [PubMed] [Google Scholar]
- 7. Mames RN, Snady-McCoy L, Guy J, et al. Central retinal and posterior ciliary artery occlusion after particle embolization of the external carotid artery system. Ophthalmology 1991;98:527–31 [DOI] [PubMed] [Google Scholar]
- 8. Yokoyama J. Usefulness of CT-angiography for superselective intra-arterial chemotherapy for advanced head and neck cancers [in Japanese]. Gan To Kagaku Ryoho 2002;29:2302–06 [PubMed] [Google Scholar]
- 9. Terayama N, Sanada J, Matsui O, et al. Feeding artery of laryngeal and hypopharyngeal cancers: role of the superior thyroid artery in superselective intraarterial chemotherapy. Cardiovasc Intervent Radiol 2006;29:536–43 [DOI] [PubMed] [Google Scholar]
- 10. Shiga K, Yokoyama J, Hashimoto S, et al. Combined therapy after superselective arterial cisplatin infusion to treat maxillary squamous cell carcinoma. Otolaryngol Head and Neck Surg 2007;136:1003–09 [DOI] [PubMed] [Google Scholar]
- 11. Homma A, Oridate N, Suzuki F, et al. Superselective high-dose cisplatin infusion with concomitant radiotherapy in patients with advanced cancer of the nasal cavity and paranasal sinuses. Cancer 2009;115:4705–14 [DOI] [PubMed] [Google Scholar]
- 12. Samant S, Robbins KT, Vang M, et al. Intra-arterial cisplatin and concomitant radiation therapy followed by surgery for advanced paranasal sinus cancer. Arch Otolaryngol Head Neck Surg 2004;30:948–55 [DOI] [PubMed] [Google Scholar]
- 13. Nibu K, Sugasawa M, Asai M, et al. Results of multimodality therapy for squamous cell carcinoma of maxillary sinus. Cancer 2002;94:1476–82 [DOI] [PubMed] [Google Scholar]
- 14. Fujishiro Y, Nakao K, Watanabe K, et al. A new aspect of tri-modal therapy with superselective intra-arterial chemotherapy in maxillary sinus carcinoma. Acta Otolaryngol Suppl 2007;559:151–56 [DOI] [PubMed] [Google Scholar]
- 15. Teicher BA, Holden SA, Kelley MJ, et al. Characterization of a human squamous cell carcinoma cell line resistance to cis-diamminedichloroplatinum (II). Cancer Res 1987;47:388–89 [PubMed] [Google Scholar]
- 16. Von Hoff DD, Clark GM, Weiss GR, et al. Use of in vivo dose response effects to select antineoplastics for high dose or regional administration regimens. J Clin Oncol 1986;4:1827–34 [DOI] [PubMed] [Google Scholar]
- 17. Robbins KT, Fontanesi J, Wong S, et al. A novel organ preservation protocol for advanced carcinoma of the larynx and pharynx. Arch Otolaryngol Head and Neck Surg 1996;122:853–57 [DOI] [PubMed] [Google Scholar]
- 18. Le QT, Fu KK, Kaplan MJ, et al. Lymph node metastasis in maxillary sinus carcinoma. Int J Radiat Oncol Biol Phys 2002;46:541–49 [DOI] [PubMed] [Google Scholar]
- 19. Los G, Blommaert A, Barton R, et al. Selective intraarterial infusion of high-dose cisplatin in patients with advanced head and neck cancer results in high tumor platinum concentrations and cisplatin-DNA adduct formation. Cancer Chemother Pharmacol 1995;37:150–54 [DOI] [PubMed] [Google Scholar]
- 20. Nagai N, Hotta K, Yamamura H, et al. Effect of sodium thiosulfate on the pharmacokinetics of unchanged cisplatin and on the distribution of platinum species in rat kidney: protective mechanism against cisplatin nephrotoxicity. Cancer Chemother Pharmacol 1995;36:404–10 [DOI] [PubMed] [Google Scholar]
- 21. Hirosawa A, Niitani H, Hayashibara K, et al. Effects of sodium thiosulfate in combination therapy of cis-dichlorodiammineplatinum platinum and vindesine. Cancer Chemother Pharmacol 1989;23:255–58 [DOI] [PubMed] [Google Scholar]
- 22. Nakasato T, Katoh K, Sone M, et al. Superselective continuous arterial infusion chemotherapy through the superficial temporal artery for oral cavity tumors. AJNR Am J Neuroradiol 2000;21:1917–22 [PMC free article] [PubMed] [Google Scholar]
- 23. Tsurumaru D, Kuroiwa T, Yabuuti H, et al. Efficacy of intra-arterial infusion chemotherapy for head and neck cancers using coaxial catheter technique: initial experience. Cardiovasc Intervent Radiol 2007;30:207–11 [DOI] [PubMed] [Google Scholar]