SUMMARY:
Spinal DAVSs of the cervical level are rare lesions. The purpose of this study is to describe the clinical and angiographic characteristics of cervical spinal DAVSs. From a prospectively collected database including 449 cases of brain and spinal DAVSs, lesions located at the cervical level were selected. The clinical presentation, angiographic characteristics, and treatment outcome were assessed. Twelve cases of spinal DAVSs were identified at the level of the cervical spinal canal (male to female ratio = 8:4; mean age = 56.5 years). Five patients (41.7%) presented with hemorrhage including SAH (n = 4) and cerebellar hemorrhage (n = 1). Coincidental spinal DAVSs with cranial DAVSs or brain AVMs were noted in 5 cases (41.7%). The spinal DAVS was the symptomatic lesion in 10 cases and was incidentally discovered during evaluation for SAH from a coincidental lesion in 2 cases. Combined endovascular and surgical resection resulted in symptomatic improvement in 10 patients. In conclusion, DAVSs of the cervical spine are rare lesions which often present with hemorrhage and are frequently associated with complex coincidental vascular lesions. Combined endovascular and surgical treatment will result in good outcome.
Spinal DAVS is a disease in which an abnormal arteriovenous communication develops between the dural branch of a radicular artery and a radicular or leptomeningeal vein along the dura.1 It is by far the most common spinal arteriovenous shunt, typically characterized by presentation beyond the fourth or fifth decade with male predominance and venous congestive myelopathy. The most prevalent location is along the thoracolumbar spine, with rare occurrence in the cervical level.2 This disproportionate distribution has been attributed to the differences of venous drainage patterns. The thoracolumbar cord drains via small-caliber radiculospinal veins, which may make venous drainage somewhat tenuous and sensitive to hemodynamic alterations. In contrast, the venous drainage at the cervical level is more divergent and, therefore, may be less susceptible to the development of various vascular lesions.2 Literature regarding cervical spinal DAVSs is limited to a few case series.3–6
The purpose of this study was to assess the clinical and angiographic characteristics of cervical spinal DAVSs from a large prospectively collected single-center data base.
Materials and Methods
From a prospectively collected data base including 449 cases of brain (n = 358) and spinal (n = 91) DAVSs, lesions located at the cervical level were selected. The data base has been collected prospectively since 1989 by a team of both neuroradiologists and neurosurgeons in a multidisciplinary clinic. The evaluations included a detailed medical history, full neurologic examination, and imaging findings.
Cervical spinal DAVS is defined as a communication between a dural arterial feeder or converging arterial feeders and a radicular or leptomeningeal vein with an identifiable shunt surgery zone located between the C1 and C8 spaces. All diagnoses were confirmed with DSA.
Endovascular treatment was the first choice and was attempted in all patients with the aim of obliteration of the shunt and the initial venous segment. The treatment results were confirmed by angiography, preferably during the same hospital stay. The patients were followed clinically on a regular 3- to 6-month basis. Additional imaging was performed in any case of delayed recovery or clinical suspicion of recurrent disease.
The DSA images were evaluated for the feeders, shunt location, drainage routes, and accompanying angiographic features such as aneurysms or other vascular diseases. The age, sex, clinical presentation, treatment methods, and outcomes were assessed. Institutional review board approval was obtained with a waiver of informed consent.
Results
The characteristics of the patients are summarized in the On-line Table. Twelve cases of cervical spinal DAVSs were identified in 8 men and 4 women. The mean age was 56.5 years with a range of 36–76 years.
The main feeders were the vertebral (n = 10), thyrocervical (n = 1), and costocervical (n = 1) arteries. Ten cases were located at the upper cervical level (C1 through C2). Two cases were located at the lower cervical level (C8) with feeders arising from the segmental radicular branches of the costocervical (case 3) and thyrocervical (case 10) arteries. The main feeder of the lesion was located on the right side in 10 patients (83.3%). Five patients (41.7%) presented with SAH (n = 4) and cerebellar hemorrhage (n = 1). The spinal DAVS was the symptomatic lesion in 10 cases. In 2 cases, the spinal DAVS was incidentally discovered during evaluation for SAH from a coincidental vascular lesion (cases 1 and 2). Venous aneurysms associated with the DAVS were identified in 3 cases (cases 9, 10, and 11). All sources of hemorrhage were discovered on the initial DSA except in 1 patient (case 10). In this case, the DAVS associated with a venous aneurysm with the main feeder from the C8 radicular branch of the right thyrocervical trunk was discovered on the second angiogram after an initial negative finding on cerebral DSA.
The main intradural venous drainage routes were rostral in 6 cases, caudal in 4 cases, and bidirectional in 2 cases. All the SAHs in the upper cervical spine were associated with a rostral intracranial venous drainage.
Coincidental vascular lesions associated with the cervical spinal DAVSs were noted in 5 patients (41.7%). These included a spinal pial AVF (Fig 1, case 1), a cerebellar pial AVM (case 2), an intracranial DAVS (cases 6 and 9), and a coincidental contralateral spinal DAVS and an epidural AVF (Fig 2, case 7). The intracranial DAVS lesions were not associated with cortical venous drainage.
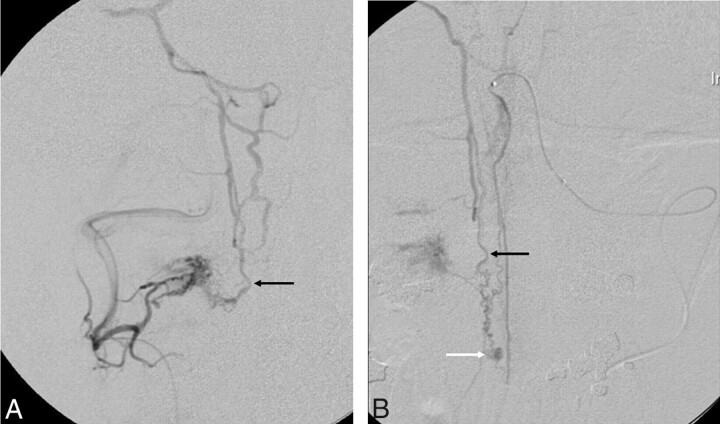
Fig 1.
Case 1. Complex spinal dural and pial arteriovenous shunt. A 43-year-old man presented with SAH. A, The superselective anteroposterior view of the C2 dural feeder from the right vertebral artery reveals a DAVS that drains rostrally (black arrow). B, The superselective anteroposterior view of the anterior spinal artery from the left vertebral artery also reveals a pial arteriovenous fistula with an aneurysmal sac (white arrow). The rostral venous drainage route of the pial arteriovenous fistula shares the same drainage route of the DAVS (black arrows in A and B). Surgical resection was performed for both lesions with the patient showing good recovery on 19-month clinical follow-up.
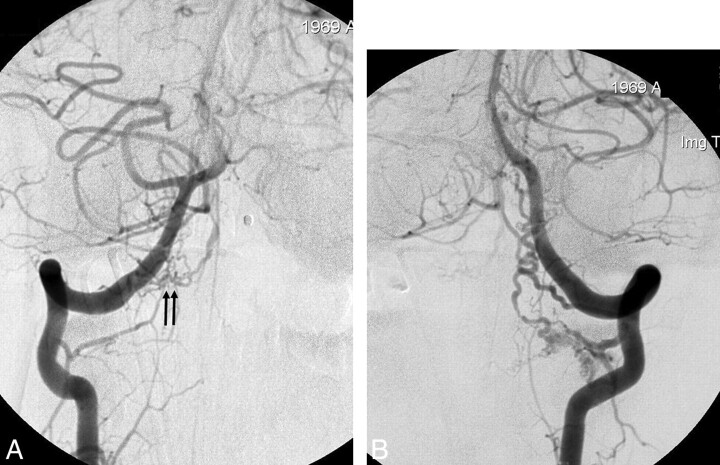
Fig 2.
Case 7. Coincidental bilateral spinal DAVS. A 39-year-old man presented with SAH. A, The anteroposterior view of the right VA angiogram shows a small dural shunt with the feeder from the right C1 dural artery (arrows). Venous drainage is rostral. B, The anteroposterior view of the left VA angiogram also reveals a separate dural shunt from the C2 dural artery, which also shows rostral venous drainage. Embolization was unsuccessful due to superselection failure; thus, surgical resection was performed for the respective lesions. The patient showed good recovery on 20-month clinical follow-up.
Angiographic cure of the lesion was achieved with n-BCA in 2 patients (16.7%, cases 3 and 11). One patient showed spontaneous occlusion of the main vertebral artery feeder; thus, a small residual feeder from the occipital artery lesion was then occluded with PVA. However, recanalization was demonstrated on 2-month follow-up DSA; the recurrence was then treated by surgery (case 5). No significant complications were noted related to the embolization procedures. Surgical resection was performed in 7 patients because of embolization failure (n = 4; cases 1, 4, 7, and 8) or residual/recurrent shunt (n = 3; cases 5, 6, and 10). Clinical follow-up showed symptomatic improvement in 10 patients (mean, 15.9 months; range, 2–53 months).
Discussion
The results of our study revealed some unique distinguishing features of cervical spinal DAVSs compared with the typical lesions located in the thoracolumbar area. Five of the 12 patients (42%) presented with hemorrhage as the initial feature. In 3 cases, the spinal DAVS was the main lesion responsible for the hemorrhage. All the SAHs in the upper cervical spine (C1-C2) were associated with a rostral intracranial venous drainage. Hemorrhagic presentation of a thoracolumbar DAVS is considered extremely rare.7 However, previous reports have shown that cervical spinal DAVSs may be more frequently associated with hemorrhage.3,4,6,8–10 According to a review of the literature by Aviv et al,4 approximately 45% of patients with cervical DAVSs presented with SAH as their initial symptom. However, many of these patients had DAVSs at the foramen magnum level (37%; 15 of 41 reviewed cases), which were not true cervical spinal DAVSs. The higher flow rate in the lesions of the cervical area compared with the thoracolumbar area has been proposed as a mechanism for increased bleeding tendency.11 Also, due to the anastomosis of medullary and pontomesencephalic veins, rostral venous flow of the shunt may drain the shunted flow into the cranium.3,4,7 Thus, the relatively high flow of shunted blood into the pial veins of the posterior fossa may be a cause of SAH.
Another distinguishing feature in our series of cervical spinal DAVSs, which has not been elaborated previously, was the high incidence of association with a coincidental vascular lesion in the vicinity of the cervical spinal DAVS. Multiplicity is known to occur in approximately 6%–8% of brain and 2%–3% of spinal DAVSs.12–14 However, in our series, 5 patients (41.7%) showed an additional complex vascular abnormality associated with a cervical spinal DAVS. Hemorrhagic presentation was noted in 3 of these patients. The types of coincidental lesions consisted of a spinal/brain AVM (n = 2) and a dural/epidural AVF (n = 3). In case 1, the coincidental spinal pial AVF, which was fed from the anterior spinal artery axis from the left VA, harbored an aneurysm, which was most likely the bleeding focus. A brain AVM located in the cerebellum with an associated arterial aneurysm was likely the cause of hemorrhage in case 2.
An incidental intracranial DAVS (transverse sinus and superior petrosal sinus) was identified in 2 cases, 1 of which presented with hemorrhage from the spinal DAVS (case 9). In case 7, 2 discrete coincidental DAVS nidi with feeders from the right C1 VA and left C2 VA were seen with hemorrhagic presentation in addition to a separate epidural shunt at the right C3 level. The hemorrhagic site could not be localized in this case, and both spinal DAVS lesions were surgically treated. The pathogenetic mechanism for these coincidental presentations is uncertain, but the association of both the pial AVM and the dural/epidural AVF type of lesion with the spinal DAVS seems to suggest a hemodynamic relationship. Venous sump effect from the sinus, induced by a dural shunt, may have recruited the development of an additional pial shunt.15 Alternatively, hemodynamic alterations including venous hypertension associated with venous thrombosis caused by an arteriovenous shunt may have provided an environment conducive for the formation of a separate DAVS lesion.
The prevalence of the main fistula feeder originating from the right-sided vessel of the patient is a prominent finding, which was also noted in other series.3,4 Ten of 12 patients (83.3%) in our series showed the main feeding artery arising from the right-sided artery (vertebral/costocervical/thyrocervical artery), including 8 cases of the right vertebral artery. The reason for this persistent right-sided dominance of the cervical spinal DAVS remains speculative. The different anatomic courses of the bilateral innominate veins draining the vertebral veins and the frequent compression of the left innominate vein by the aorta causing differences in the upstream venous pressure may have implications in the right-sided dominance of cervical spinal DAVS.16 The most important implication of this finding is the necessity to include the right vertebral artery—which may be omitted when the contralateral vertebral artery is dominant—especially in cases of hemorrhagic presentation.6 Our protocol included bilateral vertebral arteries, especially in cases of hemorrhagic presentation; thus, none of the cases were missed on the initial DSA except for a patient who hemorrhaged from a radicular feeder from the right thyrocervical artery (case 10). Spinal angiography or high-resolution MR angiography should be performed in a patient with SAH and negative findings on cerebral angiography if the patient shows symptoms referable to the spinal cord.17,18
Previous reports on the treatment of the cervical spinal DAVSs have predominantly focused on surgical ligation of the draining vein due to concerns of recanalization and the difficulty of selecting fine dural feeders.3,4,6,8,9,19 In our experience, a combined multidisciplinary approach of treatment resulted in symptomatic improvement in 10 patients (83%) in this series. In 2 patients, angiographic cure was achieved with liquid embolic agents only. An initial endovascular attempt for patients with favorable vascular anatomy may be a safe and effective strategy. In cases of failure or residual lesion, the patient may be subsequently treated by surgical resection.20,21 For patients who present with hemorrhagic coincidental vascular lesions, the main goal of treatment should always include, first of all, eradication of the symptomatic lesion.
The angiographic differentiation of spinal DAVSs from radicular AVMs is a pertinent issue. Even though radicular AVMs are very rare, composing only 0.6% of the spinal cord vascular malformations, these lesions should be differentiated from spinal DAVSs.22 Radicular AVMs are arteriovenous shunts located on the nerve root and show a conglomerate of abnormal nidal vessels around the nerve root with relatively fast flow. Spinal DAVSs will have a shunting zone along the dura with radicular feeding vessels converging onto the draining radicular or leptomeningeal vein with relatively slow flow. Patients with radicular AVMs often present with radicular pain and only rarely show congestive venous myelopathy.23 In terms of the relationship between spinal and intracranial DAVSs, the spinal DAVS lesions probably are the embryologic homolog of the intracranial DAVSs draining into the petrosal vein or bridging veins of the medulla.11 They represent the lateral epidural group of DAVSs proposed by Geibprasert et al.14 These lateral epidural shunts are characterized by male predominance, later age of onset, and the presence of cortical venous reflux, which has also been shown in our series.14 However, the frequent association of the multiple coincidental vascular lesions in the cervical spinal DAVSs seems to be a feature distinguishing them from the intracranial lateral epidural type of lesion.
Conclusions
Cervical spinal DAVSs are rare lesions with distinguishing features, compared with the more common lesions located in the thoracolumbar area. The lesions are characterized by a high incidence of hemorrhagic presentation, right-sided location, and also frequent association with multiple coincidental vascular lesions. Multidisciplinary endovascular and surgical treatment should be directed to the symptomatic lesion and will result in good outcome.
Supplementary Material
Abbreviations
- APA
ascending pharyngeal artery
- ASA
anterior spinal artery
- AVF
arteriovenous fistula
- AVM
arteriovenous malformation
- br
branch
- Caud
caudal
- Cbll
cerebellar
- CostoCerv
costocervical artery
- DAVS
dural arteriovenous shunt
- DSA
digital subtraction angiography
- FU
follow-up
- hem
hemorrhage
- ICA
internal cerebral artery
- Lt
left
- min
minimal
- NA
not available
- n-BCA
n-butyl 2-cyanoacrylate
- OA
occipital artery
- PVA
polyvinyl alcohol
- Rost
rostral
- Rt
right
- SCA
superior cerebral artery
- SAH
subarachnoid hemorrhage
- SupPetSin
superior petrosal sinus
- ThCerv
thyrocervical artery
- Tsin
transverse sinus
- VA
vertebral artery
Footnotes
Indicates article with supplemental on-line table.
References
- 1. Dehdashti AR, Da Costa LB, terBrugge KG, et al. Overview of the current role of endovascular and surgical treatment in spinal dural arteriovenous fistulas. Neurosurg Focus 2009;26:E8. [DOI] [PubMed] [Google Scholar]
- 2. Berenstein A, Lasjaunias P, Ter Brugge KG. Surgical Neuroangiography. 2nd ed. Berlin, Germany: Springer-Verlag; 2004 [Google Scholar]
- 3. Kinouchi H, Mizoi K, Takahashi A, et al. Dural arteriovenous shunts at the craniocervical junction. J Neurosurg 1998;89:755–61 [DOI] [PubMed] [Google Scholar]
- 4. Aviv RI, Shad A, Tomlinson G, et al. Cervical dural arteriovenous fistulae manifesting as subarachnoid hemorrhage: report of two cases and literature review. AJNR Am J Neuroradiol 2004;25:854–58 [PMC free article] [PubMed] [Google Scholar]
- 5. Hurst RW, Bagley LJ, Scanlon M, et al. Dural arteriovenous fistulas of the craniocervical junction. Skull Base Surg 1999;9:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fassett DR, Rammos SK, Patel P, et al. Intracranial subarachnoid hemorrhage resulting from cervical spine dural arteriovenous fistulas: literature review and case presentation. Neurosurg Focus 2009;26:E4. [DOI] [PubMed] [Google Scholar]
- 7. Koch C, Gottschalk S, Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage: case report and review of the literature. J Neurosurg 2004;100:385–91 [DOI] [PubMed] [Google Scholar]
- 8. Hashimoto H, Iida J, Shin Y, et al. Spinal dural arteriovenous fistula with perimesencephalic subarachnoid haemorrhage. J Clin Neurosci 2000;7:64–66 [DOI] [PubMed] [Google Scholar]
- 9. Do HM, Jensen ME, Cloft HJ, et al. Dural arteriovenous fistula of the cervical spine presenting with subarachnoid hemorrhage. AJNR Am J Neuroradiol 1999;20:348–50 [PMC free article] [PubMed] [Google Scholar]
- 10. Ikeda H, Fujimoto Y, Koyama T. A rare case of high cervical spinal cord dural arteriovenous fistula presenting with intracranial subarachnoid hemorrhage [in Japanese]. No Shinkei Geka 1994;22:1045–48 [PubMed] [Google Scholar]
- 11. Mitsuhashi Y, Aurboonyawat T, Pereira VM, et al. Dural arteriovenous fistulas draining into the petrosal vein or bridging vein of the medulla: possible homologs of spinal dural arteriovenous fistulas. J Neurosurg 2009;111:889–99 [DOI] [PubMed] [Google Scholar]
- 12. van Dijk JM, TerBrugge KG, Willinsky RA, et al. Multiplicity of dural arteriovenous fistulas. J Neurosurg 2002;96:76–78 [DOI] [PubMed] [Google Scholar]
- 13. Barnwell SL, Halbach VV, Dowd CF, et al. Multiple dural arteriovenous fistulas of the cranium and spine. AJNR Am J Neuroradiol 1991;12:441–45 [PMC free article] [PubMed] [Google Scholar]
- 14. Geibprasert S, Pereira V, Krings T, et al. Dural arteriovenous shunts: a new classification of craniospinal epidural venous anatomical bases and clinical correlations. Stroke 2008;39:2783–94 [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Monaco R, Rodesch G, Terbrugge K, et al. Multifocal dural arteriovenous shunts in children. Childs Nerv Syst 1991;7:425–31 [DOI] [PubMed] [Google Scholar]
- 16. Braunwald E, Zipes DP, Libby P. Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. Philadelphia: Saunders; 2001 [Google Scholar]
- 17. Willinsky R, TerBrugge K, Lasjaunias P, et al. The variable presentations of craniocervical and cervical dural arteriovenous malformations. Surg Neurol 1990;34:118–23 [DOI] [PubMed] [Google Scholar]
- 18. Farb RI, Kim JK, Willinsky RA, et al. Spinal dural arteriovenous fistula localization with a technique of first-pass gadolinium-enhanced MR angiography: initial experience. Radiology 2002;222:843–50 [DOI] [PubMed] [Google Scholar]
- 19. Oishi H, Okuda O, Arai H, et al. Successful surgical treatment of a dural arteriovenous fistula at the craniocervical junction with reference to pre- and postoperative MRI. Neuroradiology 1999;41:463–67 [DOI] [PubMed] [Google Scholar]
- 20. Van Dijk JM, TerBrugge KG, Willinsky RA, et al. Multidisciplinary management of spinal dural arteriovenous fistulas: clinical presentation and long-term follow-up in 49 patients. Stroke 2002;33:1578–83 [DOI] [PubMed] [Google Scholar]
- 21. Chiba S, Nishioka H, Saitoh M, et al. Cervical dural arteriovenous malformation presenting with right-sided occipitalgia: before and after successful treatment by embolization. Headache 1994;34:234–36 [DOI] [PubMed] [Google Scholar]
- 22. Rodesch G, Hurth M, Alvarez H, et al. Classification of spinal cord arteriovenous shunts: proposal for a reappraisal—the Bicêtre experience with 155 consecutive patients treated between 1981 and 1999. Neurosurgery 2002;51:374–79, discussion 79–80 [PubMed] [Google Scholar]
- 23. Geibprasert S, Pongpech S, Jiarakongmun P, et al. Cervical spine dural arteriovenous fistula presenting with congestive myelopathy of the conus. J Neurosurg 2009;11:427–31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.