Abstract
BACKGROUND AND PURPOSE:
Several studies suggest that various types of cellular therapies enhance recovery after stroke in animal models. IA-based delivery of cells to the brain is under investigation for stroke, but it is unknown whether cells are injured as a result of being injected through a catheter or exposed to iodinated contrast medium or solutions containing heparin.
MATERIALS AND METHODS:
We assessed the effect of catheterization with the Excelsior SL-10 catheter or exposure to heparin or iodine contrast on human bone marrow MNCs. Viability and cell injury were assessed by trypan blue exclusion, caspase-3 activity, and lipid peroxidation. Cellular function of MNCs was assessed by their production and release of VEGF, IL-10, and IGF-1.
RESULTS:
Flow rates of 10 million cells from 0.5 to 2 mL/min did not alter MNC viability; however, 5 mL/min of MNCs did reduce viability by 19%. Iodine and low-dose heparin exposure did not affect cell viability; however, high-dose heparin was cytotoxic. Catheter delivery at 2 mL/min did not affect levels of VEGF, IL-10, or IGF-1.
CONCLUSIONS:
MNCs do not appear to be damaged by heparin, iodine contrast, and the Excelsior SL-10 catheter at flow rates up to 2 mL/min. However, higher flow rates did reduce viability, and high-dose heparin did cause cell death.
Increasing experimental evidence suggests that cell transplantation can enhance recovery from stroke in animal models of focal cerebral ischemia.1 Many different types of cell-based therapies are under investigation, and clinical trials have already commenced testing the safety and feasibility of bone marrow cells in patients with ischemic stroke.2 The optimal delivery route for the administration of cells in patients with neurologic disorders is under debate. Prior clinical studies attempted direct intracranial delivery of neural cells in patients with chronic strokes, but some patients had complications related to the surgery.3,4 Intravenous delivery is less invasive, but the pulmonary circulation represents a significant barrier that traps many types of intravenously administered stem cells.5 Therefore, only a very small percentage of transplanted cells administered intravenously migrate to the injured area within the brain.5
An IA delivery into a cerebral artery such as the carotid or middle cerebral artery has the advantage of selective deposition of cells to the infarcted region without first having to pass through the venous system and peripheral organs.6 Such an approach would deliver cells more efficiently to the injured brain compared with an intravenous approach.6 One study has already shown that IA delivery of cells leads to better outcomes in a rodent model of stroke compared with an intravenous delivery.6 IA therapies in acute stroke require the use of catheters; however, a key translational question is whether cells are harmed by catheters and the associated chemicals, such as heparin, and contrast agents used in IA procedures.
In fact, the FDA has recently provided guidelines for somatic cell therapy in cardiac diseases regarding cell-delivery devices and cell viability.7 These guidelines recommend assessing the viability of cells postinfusion in a setting that accurately mimics a clinical protocol. We, therefore, tested whether catheters, routinely used for IA therapies in acute stroke, impact the viability and function of bone marrow MNCs, a cellular product that has been shown to improve recovery after stroke in animal models8,9 and is currently being tested in trials involving patients with acute stroke and various cardiac disorders.2,10,11 Proving that this method of delivery does not alter the viability and function of cells is a crucial step before its full-scale application in clinical safety and efficacy studies involving patients with neurologic disorders such as stroke.
Materials and Methods
Preparation of MNCs
MNCs were prepared following an established protocol12 from the bone marrow of a healthy volunteer in a good manufacturing process facility. In brief, the bone marrow was harvested from the posterior iliac crest of a healthy volunteer and purchased from Lonza (Walkersville, Maryland). The MNCs were then isolated from the marrow by using an attenuation gradient procedure on a SEPAX device (Biosafe, Geneva, Switzerland).
Assessment of Cellular Injury after Passage of MNCs at Different Flow Rates
Ten million cells per milliliter were passed at flow rates of 0.5, 1, 2, and 5 mL per minute. These flow rates were chosen on the basis of a planned phase I study involving IA administration of 100 million cells for 5–10 minutes in patients with acute stroke. The cell suspension was aspirated in saline into a 5-mL syringe and then infused through a 25-ga needle. The viability of MNCs through different needle sizes has already been assessed in a different report.13 The MNCs were passed through the Excelsior SL-10 catheter (Boston Scientific, Natick, Massachusetts), which is routinely used for endovascular treatments in acute stroke.14
Because a clinical study would involve cells traveling at body temperature in a catheter, we placed the catheter for these experiments in the incubator at 37°C for 30 minutes before cell-delivery experiments were performed. After the sample had been infused through the catheter, half was checked for postinfusion viability by trypan blue exclusion or lipid peroxidation, and a portion of the remaining cells were stored for 24 hours and then assessed for caspase-3 activation as a marker of apoptosis. Caspase activation was used to assess any evidence of delayed cellular injury. Lipid peroxidation and caspase-3 activation were estimated by using microtiter colorimetric methods. The lipid peroxidation product, malondialdehyde, was measured as described.15 Caspase activation and lipid peroxidation were compared in cells before and after catheter passage.
Exposing Bone Marrow MNCs to Contrast Agent
Ten million MNCs were exposed to 10% iohexol contrast agent (Omnipaque; Nycomed, Princeton, New Jersey) in PBS for 1 hour and then were placed in PBS for 24 hours. Ten percent contrast is an approximation to which MNCs might be exposed within the vasculature in a clinical study involving IA delivery of these cells. The cell suspension was centrifuged, and the cell pellet was dissolved in caspase-3 lysis buffer. One hundred microliters of the cell lysate was used for caspase-3 activation assay by using the caspase-3 substrate, AC-DEVD-ANC (Invitrogen, Carlsbad, California).
Effect of Heparin on Human MNCs
Two doses of heparin were examined. In the low-dose heparin group, 10 million MNCs were suspended in 2.5 U/mL of heparin for 1 hour at an ambient temperature. This concentration of heparin is commonly used for IA procedures at our institution and is what we intend to use for a planned phase I study on the safety of MNCs. In the high-dose group, 10 million MNCs were exposed to 500 U/mL of heparin for 1 hour. A third group of MNCs were exposed to 1× PBS as a control. After 1 hour's treatment, the cells were first assessed for viability with trypan blue and then stored in PBS for 24 hours and centrifuged. The supernatant was removed, and the pellet was dissolved in caspase-3 lysis buffer and assayed for caspase-3 activation.
Functional Assessment of MNCs after Passage Through a Catheter
Ten million MNCs per milliliter were passed through the Excelsior SL-10 catheter at a flow rate of 2 mL/min. After catheterization, the cells were subjected to 3 hours of hypoxia, which is known to increase cytokine secretion from these cells,16 followed by incubation in DMEM media under normoxic conditions for 24 hours at 37°C. A separate group of MNCs at the same concentration was passed through the catheter and was placed under normoxic conditions for 3 hours and then cultured in DMEM for 24 hours. A corresponding set of control cells that were from the same vials did not undergo catheterization but were divided into groups that underwent either hypoxia or normoxia for 3 hours and then were cultured for 24 hours in DMEM. Supernatants from all the groups described were collected at 24 hours and centrifuged at 4°C for 10 minutes at 3000 G to remove particulate material. One hundred microliters of supernatant was used to estimate different cytokines (IGF-1, IL-10, and VEGF) by using colorimetric microtiter sandwich ELISA. These cytokines are known to be secreted by MNCs.16
Statistical Analysis
Means and SDs were calculated. A P value < .05 was considered significant. Analysis of variance and t tests were used when appropriate.
Results
Cell Viability with Different Flow Rates
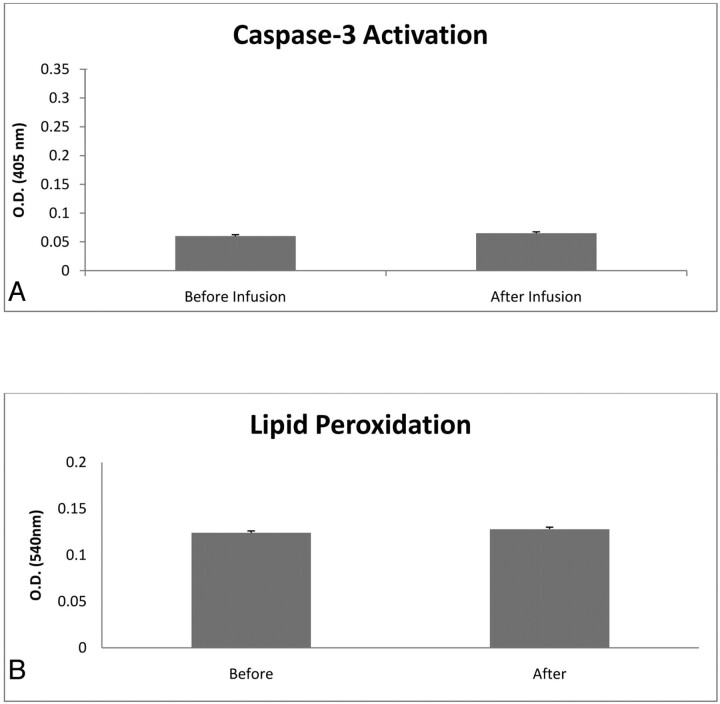
After passage through the Excelsior SL-10 catheter, there was no change in cell viability in any of the infusion-rate groups: 0.5, 1, and 2 mL/min. There was >99% viability after passage through the catheters (Table) at ≤2 mL/min. However, when the cells were passed at 5 mL/min, there was a significant decrease by 19% in viability in cells postinfusion compared with cells preinfusion (Table). There was no difference in caspase-3 activation (Fig 1 A) and lipid peroxidation (Fig 1B) before and after the cell infusion by using a rate of 2 mL/min.
Percentage viability of MNCs after passage through a catheter
| Flow Rate (mL/min) | Viability (%) |
|---|---|
| 0.5 | 99 ± 2 |
| 1 | 99 ± 2 |
| 2 | 99 ± 2 |
| 5 | 80 ± 5 |
Fig 1.
Histograms illustrating caspase-3 activation (A) and lipid peroxidation (B) before and after catheter infusion. MNCs were infused at 2 mL/min through an Excelsior SL-10 catheter and then assessed for lipid peroxidation at 3 hours and caspase-3 activity at 24 hours after catheterization. Data are mean ± SD of 5 determinations in each experimental group.
MNC Viability after Exposure to Iodine Contrast Agent
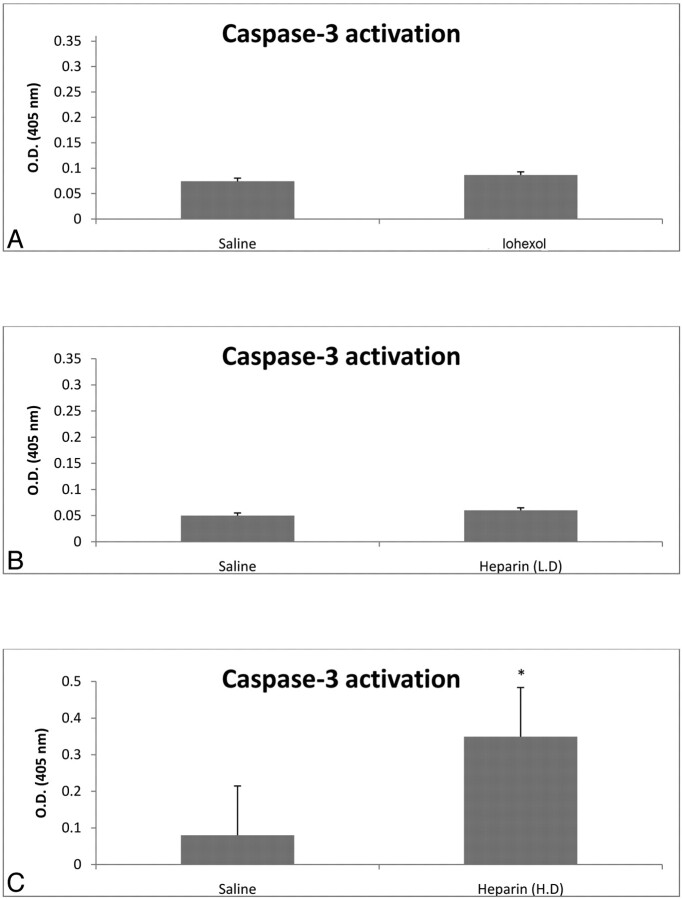
There were no significant differences in trypan blue exclusion (99% viability) or caspase-3 activation between saline-treated and Omnipaque-treated cells (Fig 2 A).
Fig 2.
Caspase-3 activation after exposure to Omnipaque (iohexol) and heparin. MNCs were exposed to either Omnipaque, low-concentration heparin (2.5 U/mL) typically used for endovascular procedures, high-concentration heparin (500 U/mL), or saline for 1 hour. MNCs were then assessed for caspase-3 activation at 24 hours. There were no significant differences between saline-treated cells and Omnipaque-treated cells or low-dose-heparin–treated cells. However, high-dose heparin did significantly increase caspase-3 activation. The asterisk indicates P < .05. Data are mean ± SD of 5 determinations in each experimental group.
Viability after Exposure to 2 Different Concentrations of Heparin
In the low-dose heparin group, there was no significant difference in viability as determined by trypan blue exclusion (99%) or caspase-3 activation (Fig 2B) between saline-treated and low-dose heparin-treated MNCs. However, in the high-dose heparin group, viability was reduced by 30% and caspase-3 activation (Fig 2C) was significantly increased compared with saline controls.
Effect of Catheterization on Cytokine Release
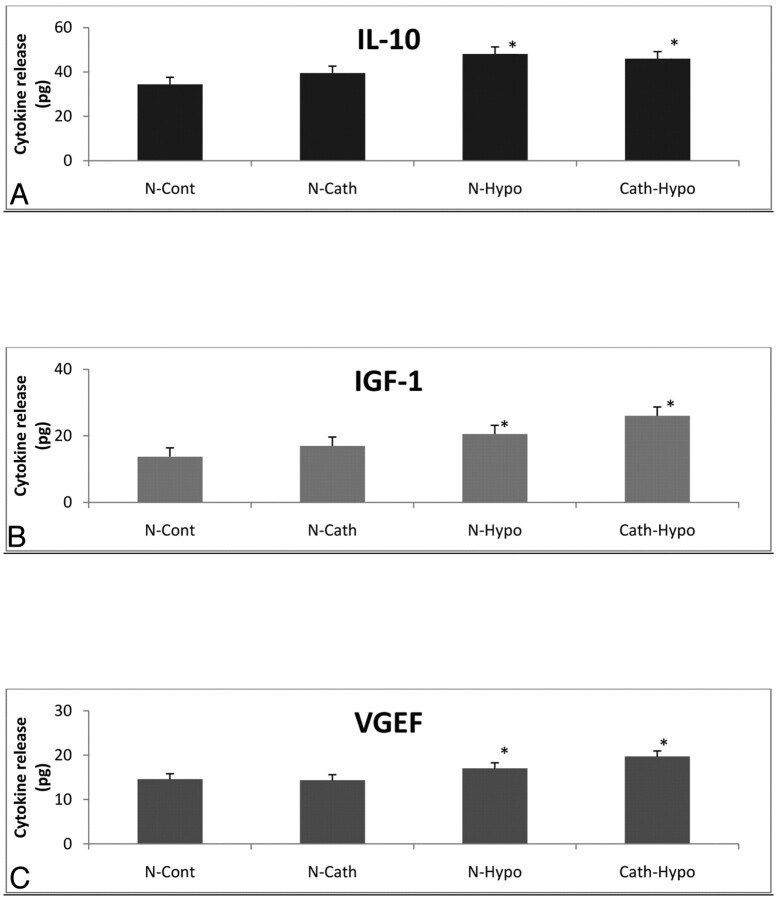
There was no significant difference in cytokine release of IGF-1, IL-10, and VEGF in nonconditioned MNCs between catheterized and noncatheterized groups. Cells that were conditioned with 3 hours of hypoxia released more cytokines than nonconditioned cells, as expected.16 There were no differences in cytokine release, however, in conditioned cells between the catheterized and noncatheterized cell groups (Fig 3).
Fig 3.
The effect of catheterization on cytokine release of MNCs. Human MNCs were passed through an Excelsior SL-10 catheter at a rate of 2 mL/min and then were cultured in media for 24 hours. The media were assayed for cytokine levels (N-Cath). This group was compared with MNCs that were not catheterized (N-Cont). A second group of MNCs was catheterized as described, then exposed to hypoxia for 3 hours, and cultured in media for 24 hours. Cytokines in the media were quantified (Cath-Hypo). A−C, This group was compared with control MNCs not catheterized but exposed to hypoxia for 3 hours and cultured for 24 hours (N-Hypo). IL-10 (A), IGF-1 (B), and VEGF (C) were quantified by ELISA. Cytokines were estimated from 100-μL MNC supernatants. There was a significant increase (P < .05) in cytokine release from cells exposed to hypoxia compared with cells exposed to normoxia. Catheterization did not affect cytokine levels from normoxic- or hypoxic-treated cells. Data are mean ± SD of 5 determinations in each experimental group. The asterisk indicates P < .05.
Discussion
MNCs have been found, in multiple laboratories, to reduce neurologic deficits in animal models of stroke.6,8,17 An IA delivery of MNCs through the carotid artery confers more benefit in rats compared with an intravenous approach.6 While clinical studies outside the United States in patients with stroke have already commenced delivering MNCs into the cerebral arterial circulation,11 it is important to assess whether MNCs can be damaged with the devices commonly used in the endovascular suite. A recent study assessed the effects of catheter passage of mesenchymal stem cells to the heart and found that higher flow rates did impair gene expression of certain growth factors, which are believed to underlie, in part, the effects of these cells in promoting cardiac repair.18 Criteria have already been recommended by the FDA to determine the safety of endovascular delivery of stem cells in cardiac patients.7
We, therefore, performed ex vivo experiments to simulate a planned study involving an intracarotid infusion of MNCs in patients with ischemic stroke. We found that catheterization rates of 10 million cells/mL up to 2 mL/min did not affect viability. However, we did observe an increase in cell death (≤19%) when cells were injected at 5 mL/min. We also performed functional assays of the MNCs by quantifying secreted cytokines before and after catheterization. IGF-1, IL-10, and VEGF are 3 of the many cytokines that may underlie, in part, the benefits of MNCs observed to improve recovery after myocardial infarction.16 We found that catheterization did not affect the output of any of these cytokines. More important, a conditional stimulus, hypoxia, increased cytokine release from MNCs, as previously described,16 and catheterization did not affect the elevation in cytokines. This result suggests that catheter passage does not interfere with cytokine elaboration of MNCs. In addition, IA delivery might place MNCs directly into contact with heparin and iodine contrast, which are routinely used in endovascular procedures. However, in this study, heparin and iodine contrast did not cause cellular injury; they, therefore, appear safe and would not be expected to interfere with the potential beneficial effects of MNCs as demonstrated in animals.6,8 However, at concentrations that are at least 100-fold higher, heparin did cause injury to MNCs.
This study has limitations, including the use of only 1 catheter model and not assessing the effect of radiation exposure on MNCs.
Conclusions
Flow rates ≤2 mL/min at concentrations of 10 million cells/mL do not alter MNC viability or function. In addition, heparin and iodine contrast appear not to harm bone marrow MNCs. The results from this study suggest that assessing the impact of flow rates on viability and function may be needed before advancing any particular cellular product to clinical trial involving an endovascular delivery in patients with stroke or other neurologic disorders.
Abbreviations
- Cath-Hypo
hypoxia
- DMEM
Dulbecco's Modified Eagle's Medium
- ELISA
enzyme-linked immunosorbent assay
- FDA
US Food and Drug Administration
- H.D.
high dose
- H-Hypox
MNCs not catheterized but conditioned
- IA
intra-arterial
- IGF-1
insulin-like growth factor 1
- IL-10
interleukin 10
- L.D.
low dose
- MNC
mononuclear cell
- N-Cath
nonconditioned MNCs
- N-Cont
MNCs not catheterized
- O.D.
optical density
- PBS
phosphate-buffered saline
- VEGF
vascular endothelial growth factor
Footnotes
This study was supported by the National Institutes of Health grant R21 NS064316-02, the Howard Hughes Medical Institute, and the Notsew Orm Sands Foundation. The authors have no conflicts of interest. Marrow MNCs were manufactured by the Production Assistance for Cellular Therapies (PACT) group at Baylor College of Medicine (National Heart, Lung, and Blood Institute [NHLBI]) contract NO1-HB-37163).
References
- 1. Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke 2009;40:510–15. Epub 2008 Dec 18 [DOI] [PubMed] [Google Scholar]
- 2. Savitz SI, Misra V. Launching intravenous bone marrow cell trials for acute stroke. Regen Med 2009;4:639–41 [DOI] [PubMed] [Google Scholar]
- 3. Wechsler LR. Clinical trials of stroke therapy: which cells, which patients? Stroke 2009;40:S149–151 [DOI] [PubMed] [Google Scholar]
- 4. Savitz SI, Dinsmore J, Wu J, et al. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: a preliminary safety and feasibility study. Cerebrovasc Dis 2005;20:101–07 [DOI] [PubMed] [Google Scholar]
- 5. Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first pass effect. Stem Cells Dev 2008;18:683–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamiya N, Ueda M, Igarashi H, et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci 2008;83:433–37 [DOI] [PubMed] [Google Scholar]
- 7. US Food and Drug Administration. Draft Guidance for Industry: Somatic Cell Therapy for Cardiac Disease. March 2009. US Department of Health and Human Services Web site. Vaccines, Blood, and Biologics. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm164265.htm. Accessed December 9, 2009
- 8. Brenneman M, Sharma S, Harting M, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab 2010;30:140–49. Epub 2009 Sep 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baker AH, Sica V, Work LM, et al. Brain protection using autologous bone marrow cell, metalloproteinase inhibitors, and metabolic treatment in cerebral ischemia. Proc Natl Acad Sci U S A 2007;104:3597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lipinski MJ, Biondi-Zoccai GG, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol 2007;50:1761–67 [DOI] [PubMed] [Google Scholar]
- 11. Correa PL, Mesquita CT, Felix RM, et al. Assessment of intra-arterial injected autologous bone marrow mononuclear cell distribution by radioactive labeling in acute ischemic stroke. Clin Nucl Med 2007;32:839–41 [DOI] [PubMed] [Google Scholar]
- 12. Harting MT, Cox CS, Day MC, et al. Bone marrow-derived mononuclear cell populations in pediatric and adult patients. Cytotherapy 2009;11:480–84 [DOI] [PubMed] [Google Scholar]
- 13. Tol M, Akar AR, Durdu S, et al. Comparison of different needle diameters and flow rates on bone marrow mononuclear stem cell viability: an ex vivo experimental study. Cytotherapy 2008;10:98–99 [DOI] [PubMed] [Google Scholar]
- 14. Yoon W, Park MS, Cho KH. Low-dose intra-arterial urokinase and aggressive mechanical clot disruption for acute ischemic stroke after failure of intravenous thrombolysis. AJNR Am J Neuroradiol 2010;31:161–64. Epub 2009 Aug 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sangchot P, Sharma S, Chetsawang B, et al. Deferoxamine attenuates iron-induced oxidative stress and prevents mitochondrial aggregation and alpha-synuclein translocation in SK-N-SH cells in culture. Dev Neurosci 2002;24:143–53 [DOI] [PubMed] [Google Scholar]
- 16. Takahashi M, Li TS, Suzuki R, et al. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol 2006;291:H886–93 [DOI] [PubMed] [Google Scholar]
- 17. Iihoshi S, Honmou O, Houkin K, et al. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res 2004;1007:1–9 [DOI] [PubMed] [Google Scholar]
- 18. Heng BC, Hsu SH, Cowan CM, et al. Trans-catheter injection induced changes in human bone marrow-derived mesenchymal stem cells. Cell Transplant 2009;18:1111–21. Epub 2009 Jun 22 [DOI] [PubMed] [Google Scholar]