Abstract
BACKGROUND AND PURPOSE:
Most DTI studies in ALS have been limited to the assessment of the CST damage. In this study, we used DTI tractography to investigate whether microstructural abnormalities occur in the major motor and extramotor WM tracts in mildly disabled patients with ALS.
MATERIALS AND METHODS:
Brain conventional MR imaging and DTI were performed in 24 patients with probable or definite ALS and mild disability (ALSFRS score, ≥20) and 20 healthy controls. The mean disease progression rate was 0.62 (range = 0.08–2.50). DTI tractography was used to segment the CST, the corpus callosum, and the major WM association tracts (ie, cingulum, uncinate fasciculus, inferior fronto-occipital, inferior longitudinal, and superior longitudinal fasciculi).
RESULTS:
Compared with healthy controls, patients with ALS showed significantly decreased FA and significantly increased MD and radial D of the CST bilaterally (P values from .005 to .01). Patients with ALS also had a significantly increased axial D of the right uncinate fasciculus relative to controls (P = .04). CST FA significantly correlated with the rate of disease progression (right CST: r = −0.50, P = .02; left CST: r = −0.41, P = .05).
CONCLUSIONS:
Patients with ALS and mild disability have preferential damage to the CST. The association of CST damage with the rate of disease progression suggests that DTI has the potential to provide in vivo markers of ALS evolution. The subtle involvement of the uncinate fasciculus may precede the appearance of behavioral symptoms in patients with ALS.
ALS is the most common adult-onset motor neuron disease.1 The pathologic hallmark of sporadic ALS is the loss of upper motor neurons in the motor and premotor cortices and lower motor neurons in the brain stem and spinal cord.2 ALS is characterized by an extramotor cerebral pathology that, to a variable extent, overlaps the clinicopathologic features of frontotemporal lobar degeneration.3 To date, the only specific marker of sporadic ALS is the presence of inclusions staining positively for ubiquitin and TAR deoxyribonucleic acid−binding protein 43 (TDP-43) in degenerating neurons.4
By quantifying the magnitude and directionality of water diffusion within a tissue, DTI allows inferences about WM microstructure in vivo.5,6 Most DTI studies in ALS have been limited to the assessment of the CST damage.7 Whether on the basis of regions of interest,8–16 voxel-based analysis,17–20 or tractography,21–23 DTI studies in ALS have shown reduced FA along the CST.
More recently, voxel-based DTI studies have assessed the integrity of extramotor brain regions in patients with ALS.17–20 In agreement with pathologic findings,24,25 FA decrease was found in the anterior corpus callosum18–20 and in the prefrontal18,20 and temporal20 WM regions. Because of the intrinsic limitations of region of interest− and voxel-based analyses, however, such studies could only infer the specific WM tracts involved. On the other hand, DTI tractography allows one to gain quantitative information on the localization of damage to specific neuronal pathways.26 To the best of our knowledge, no studies have yet assessed the integrity of WM tracts, other than the CST, in patients with ALS.
In this study, we used DTI tractography to assess the structural integrity of the CST, the corpus callosum, and the major WM association tracts (ie, cingulum, uncinate fasciculus, inferior fronto-occipital, inferior longitudinal, and superior longitudinal fasciculi) in patients with ALS with mild disability, relative to age- and sex-matched healthy controls.
Materials and Methods
The study was conducted with institutional review board approval. Written informed consent was obtained from each participant.
Patients
From June 2005 to March 2006, we recruited 24 patients (13 men and 11 women; mean age = 55 years, range = 27–75 years; mean disease duration = 35 months, range = 6–58 months) with probable or definite ALS,27 and mild disability, defined as a score ≥20 on the ALSFRS.28 Six patients had a bulbar-onset and 18 patients had a limb-onset disease. Within 48 hours of study entry, patients were assessed clinically by a single physician who was unaware of the MR imaging results. Disease severity was assessed using ALSFRS.28 The mean ALSFRS score was 29 (range = 21–38). Patients with evidence of cognitive deficit or overt dementia were excluded. The rate of disease progression was calculated by using the following formula: 40 − ALSFRS score) / disease duration.23 The mean disease progression rate was 0.62 (range = 0.08–2.50). Twenty sex- and age-matched healthy subjects (11 men and 9 women, mean age = 53 years, range = 28–73 years) served as controls.
MR Imaging Acquisition
MR images were obtained on a 1.5T Avanto system (Siemens, Erlangen, Germany) by using a 4-channel head coil. MR imaging sequences included the following: 1) DE turbo spin-echo (TR = 3460 ms, TE = 27/109 ms, echo-train length = 5, FOV= 250 mm2, matrix size = 512 × 512, 35 contiguous 4-mm-thick axial sections, acquisition time = 5.30 minutes); 2) T2-weighted turbo spin-echo (TR = 3460 ms, TE = 109 ms, echo-train length = 13, number of averages = 2, FOV = 240 × 180 mm2, matrix size = 240 × 320, 24 coronal 4-mm-thick sections with a distance factor of 30%, acquisition time = 5.40 minutes); and 3) pulsed gradient spin-echo single-shot echo-planar (TR = 2900 ms, TE = 84 ms, flip angle = 90°, FOV = 240 mm2, matrix size = 128 × 128, nominal pixel size = 1.87 mm2, interecho spacing = 0.77 ms, 18 contiguous 4-mm-thick axial sections, acquisition time = 4.30 minutes) with diffusion-encoding gradients applied in 12 noncollinear directions, coded as the default in the scanner. The maximum b factor in each direction was set to 900 s/mm2 and only 2 b factors were used (b1=0, b2=900 s/mm2). The maximum amplitude of the diffusion gradients was 33 mT/m, and a multiple-channel head coil was used for signal-intensity reception. Two averages were acquired, with no parallel acquisition. The central section of this sequence was positioned to match exactly the central section of the DE set.
MR Imaging Analysis
All MR imaging analysis was performed by an experienced observer, unaware of subject's identity. Axial DE and coronal T2-weighted images were analyzed to assess the presence and location of areas with increased signal intensity.
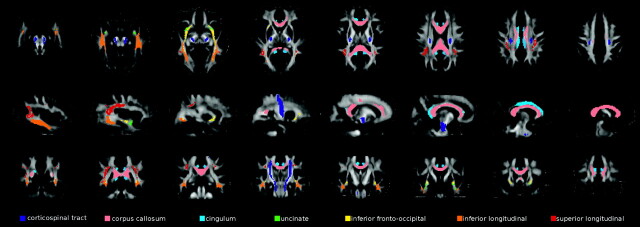
DTI analysis was performed by using in-house software. Pulsed gradient spin-echo single-shot echo-planar images were first corrected for distortion induced by eddy currents,29 then the diffusion tensor was estimated by linear regression5 and MD and FA maps were computed.30 Maps of axial D, which is equivalent to the magnitude of the largest eigenvalue of the tensor, and radial D, which is the average of the 2 smallest eigenvalues of the tensor, were also calculated.30 An FA atlas was obtained by using DTIs from healthy subjects ranging from 21 to 40 age years of age with no history of neurologic or psychiatric disorders (reference group), as previously described.31 Briefly, their DE scans were registered to the standard Montreal Neurologic Institute space32 with affine transformation by using the VTK CISG Registration Toolkit (http://vtk.org).33 This transformation was then applied to FA images to correct for differences in head size between controls. FA maps were then nonlinearly transformed with an iterative procedure to produce an average shape and intensity image atlas.31 On reference FA maps, fiber tracking34 was performed to segment the major brain WM tracts, bilaterally.35 These included the following: the CST, the corpus callosum, the cingulum, the uncinate fasciculus, the inferior fronto-occipital fasciculus, the inferior longitudinal fasciculus, and the superior longitudinal fasciculus. A single subject's WM tracts were then registered to the standard space by using the transformation matrices previously computed (see above) and were averaged to produce WM tract probability maps. These maps were thresholded at 40%. WM tract probability maps are shown in Fig 1. Finally, the nonlinear transformation between the FA atlas and the FA maps of each subject was estimated36 and applied to each subject's MD, FA, axial D, and radial D maps. WM tract probability maps were used as masks, and average MD, FA, axial D, and radial D values of each tract were measured.
Fig 1.
Illustration of the WM tract probability maps obtained from reference healthy subjects. Probability maps are superimposed on axial (top row), sagittal (middle row), and coronal (bottom row) sections of the FA atlas.
Statistical Analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences, Version 13.0 (SPSS, Chicago, Illinois). DTI variables were compared between patients with ALS and healthy controls by using a univariate analysis of variance. The subject's age was entered into the statistical analysis as a covariate. The same model was used to compare DTI changes between patients with and without hyperintensities along the brain CST. Univariate correlations were assessed by using the Spearman rank correlation coefficient. The significance threshold was set at P < .05.
Results
T2 hyperintensities along the CST were detected bilaterally in 13/24 patients with ALS (54%).17 No T2 hyperintensities were detected beyond the CST. On-line Tables 1 and 2 report the DTI metrics from patients with ALS and healthy controls. Compared with healthy controls, patients with ALS showed significantly increased MD and radial D values and significantly decreased FA of the CST bilaterally (P values ranging from .01 to .03). CST DTI metrics did not differ between patients with ALS with and without hyperintensities along the CST (data not shown). Patients with ALS also had a significant increased axial D of the right uncinate fasciculus relative to healthy controls (P = .04). No significant between-group difference was found in the other WM tracts (On-line Tables 1 and 2).
CST FA values significantly correlated with the rate of disease progression (right CST: r = −0.50, P = .02; left CST: r = −0.41, P = .05). No correlation was found between DTI metrics and the ALSFRS score.
Discussion
In this study, we investigated the structural integrity of the major cerebral WM tracts in mildly disabled patients with ALS. We found that patients with ALS with mild disability had preferential damage to the CST, which is associated with the rate of disease progression. In addition, the assessment of DTI eigenvalues in the investigation of WM damage revealed a subtle involvement of the right uncinate fasciculus, which may precede the appearance of behavioral symptoms in patients with ALS.
The finding of increased MD and radial D and reduced FA in the CST of patients with ALS is in agreement with previous studies,8–15,23 confirming that DTI is a valuable tool for assessing CST changes in ALS. An increased average MD can result from the enlargement of extracellular spaces, which indicates a breakdown of the barriers that restrict free water movement.6 The reduction of average FA might reflect both intracellular abnormalities of surviving axons and formation of “new” isotropic barriers, due to the presence of cell debris resulting from partially degenerated or disintegrated CST fibers, inflammatory infiltrates, and astrocytosis.6 From the evaluation of the results of the diffusion tensor eigenvalue analysis, it appears that MD and FA changes are secondary to an increased diffusion perpendicular to WM fibers (as indicated by an increased radial D) rather than decreased diffusion along the fiber bundles (as indicated by unchanged axial D values). It is usually assumed that radial D, which is the average diffusion perpendicular to fiber bundles, is modulated by the extracellular distance between membranes, axon diameter, and degree of myelination.37,38 As a consequence, it is thought that loss of fiber tracts and disruption of myelin sheaths in the course of Wallerian degeneration could explain enhanced transverse diffusion.37,38 The main pathologic changes in patients with ALS include loss of pyramidal motor neurons in the primary motor cortex and axonal degeneration of the CST.2 These abnormalities, together with the proliferation of glial cells, the extracellular matrix expansion, and the intraneuron abnormalities,2 may contribute to the observed CST changes in patients with ALS. Most interesting, no difference in CST DTI features was found between patients with ALS with and without CST hyperintensities on conventional MR images. These findings are in line with previous conventional MR imaging studies in ALS showing the low sensitivity and specificity of such abnormalities.39–46
In patients with ALS, the severity of CST damage correlated with the rate of disease progression, thus suggesting that the pathologic changes of the CST detected by DTI are likely to contribute to the rapidity of disease evolution in patients with ALS. In a previous tractography study,23 a strong association was found between disease-progression rate and left CST structural connectivity measures, while no association was found with CST FA. Differences in image preprocessing and tractography analysis may help explain the different results of the 2 studies and, in turn, do not allow a direct comparison of the results. In addition, Ciccarelli et al23 investigated DTI changes only in the CST above the internal capsule, while previous studies have reported that the most significant changes in FA are evident at the level of the cerebral peduncles47 and the internal capsule.9,10 Our failure to identify a correlation between CST damage and the ALSFRS score is in keeping with previous data obtained from patients with ALS.10,14,23,48 In addition, in our study, patients had a relatively small spread of ALSFRS scores, and this might have worked against the ability to detect a correlation between structural CST changes and disability.
Another intriguing finding is that patients with ALS showed a subtle change (ie, increased axial D) of the right uncinate fasciculus compared with healthy controls. This finding is in agreement with pathologic,24,25 DTI,18,20 and voxel-based morphometry49 studies showing the involvement of the frontal lobes and the temporal and limbic areas in ALS. Regions of significant increase of axial D in the major corticocortical association tracts have been recently observed with normal aging50 and in other neurodegenerative diseases, such as Alzheimer disease.51,52 In a previous voxel-based DTI study of the same patients with ALS,17 we found clusters of extramotor increase of MD in the frontal, temporal, and occipital lobes, which only partially overlapped the uncinate fasciculus. One explanation for this would be the different spatial resolution (tract- versus voxel-based), because the presence of microstructural damage in a small region may not have affected the average DTI metrics of the WM tract. Although the functional role of the uncinate fasciculus is still debated, its termination in the anterior temporal lobe and in the ventral and orbital frontal regions35 suggests that its involvement is likely to contribute to the behavioral symptoms of patients with ALS.
ALS patients included in our study did not show clinical evidence of a frontal cognitive or behavioral syndrome.17 Thus, one may speculate that damage to the right uncinate may precede the appearance of such symptoms. According to Lomen-Horeth et al,53 approximately 25% of patients with ALS meet the Neary criteria for FTD and approximately another 25% manifest executive or behavioral dysfunction compatible with early FTD. This hypothesis is also supported by recent findings of a similarly damaged uncinate fasciculus in patients with behavioral FTD54,55 and semantic dementia54,56 and by the association between medial and ventral frontal WM damage and behavioral symptoms in patients with behavioral FTD.57 Longitudinal studies are now warranted to assess whether the extent and severity of the MR imaging−detectable extramotor damage is predictive of subsequent development of cognitive impairment in patients with ALS.
We should mention some limitations of our study: first, the relatively small sample size, which was nevertheless comparable with previous reports.7 Due to the exploratory nature of our study, we did not correct for multiple comparisons; therefore, some of the significances might have been overestimated. However, we observed DTI tractography changes only in brain areas that are known to be the preferential sites of ALS damage from neuropathologic studies.2 Finally, some methodologic issues related to DTI should be addressed. Regions of crossing fibers are characterized by low anisotropy and, thus, the determination of fiber orientation may be difficult in these voxels,58 leading to unreliable tensor estimates. However, the fasciculi that we investigated are major WM tracts associated with high anisotropy; therefore, the effect of crossing fibers should not have affected our results a great deal, especially in the case of CST.58 Despite such limitations, this study highlights the potential of DTI of the brain to provide in vivo markers of cerebral involvement in ALS.7
Supplementary Material
Abbreviations
- ALS
amyotrophic lateral sclerosis
- ALSFRS
ALS Functional Rating Scale
- axial D
axial diffusivity
- CST
corticospinal tract
- DE
dual-echo
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FTD
frontotemporal dementia
- MD
mean diffusivity
- radial D
radial diffusivity
- WM
white matter
Footnotes
Indicates article with supplemental on-line tables.
References
- 1. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med 2001;344:1688–700 [DOI] [PubMed] [Google Scholar]
- 2. Hughes JT. Pathology of amyotrophic lateral sclerosis. Adv Neurol 1982;36:61–74 [PubMed] [Google Scholar]
- 3. Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol 2007;6:994–1003 [DOI] [PubMed] [Google Scholar]
- 4. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006;314:130–33 [DOI] [PubMed] [Google Scholar]
- 5. Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 1994;103:247–54 [DOI] [PubMed] [Google Scholar]
- 6. Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology 1996;201:637–48 [DOI] [PubMed] [Google Scholar]
- 7. Turner MR, Kiernan MC, Leigh PN, et al. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol 2009;8:94–109 [DOI] [PubMed] [Google Scholar]
- 8. Cosottini M, Giannelli M, Siciliano G, et al. Diffusion-tensor MR imaging of corticospinal tract in amyotrophic lateral sclerosis and progressive muscular atrophy. Radiology 2005;237:258–64 [DOI] [PubMed] [Google Scholar]
- 9. Ellis CM, Simmons A, Jones DK, et al. Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 1999;53:1051–58 [DOI] [PubMed] [Google Scholar]
- 10. Toosy AT, Werring DJ, Orrell RW, et al. Diffusion tensor imaging detects corticospinal tract involvement at multiple levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2003;74:1250–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abe O, Yamada H, Masutani Y, et al. Amyotrophic lateral sclerosis: diffusion tensor tractography and voxel-based analysis. NMR Biomed 2004;17:411–16 [DOI] [PubMed] [Google Scholar]
- 12. Iwata NK, Aoki S, Okabe S, et al. Evaluation of corticospinal tracts in ALS with diffusion tensor MRI and brainstem stimulation. Neurology 2008;70:528–32 [DOI] [PubMed] [Google Scholar]
- 13. Graham JM, Papadakis N, Evans J, et al. Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 2004;63:2111–19 [DOI] [PubMed] [Google Scholar]
- 14. Schimrigk SK, Bellenberg B, Schluter M, et al. Diffusion tensor imaging-based fractional anisotropy quantification in the corticospinal tract of patients with amyotrophic lateral sclerosis using a probabilistic mixture model. AJNR Am J Neuroradiol 2007;28:724–30 [PMC free article] [PubMed] [Google Scholar]
- 15. Senda J, Ito M, Watanabe H, et al. Correlation between pyramidal tract degeneration and widespread white matter involvement in amyotrophic lateral sclerosis: a study with tractography and diffusion-tensor imaging. Amyotroph Lateral Scler 2009;10:1–8 [DOI] [PubMed] [Google Scholar]
- 16. Wang S, Poptani H, Woo JH, et al. Amyotrophic lateral sclerosis: diffusion-tensor and chemical shift MR imaging at 3.0 T. Radiology 2006;239:831–38 [DOI] [PubMed] [Google Scholar]
- 17. Agosta F, Pagani E, Rocca MA, et al. Voxel-based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability. Hum Brain Mapp 2007;28:1430–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ciccarelli O, Behrens TE, Johansen-Berg H, et al. Investigation of white matter pathology in ALS and PLS using tract-based spatial statistics. Hum Brain Mapp 2009;30:615–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sach M, Winkler G, Glauche V, et al. Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 2004;127:340–50 [DOI] [PubMed] [Google Scholar]
- 20. Sage CA, Peeters RR, Gorner A, et al. Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage 2007;34:486–99 [DOI] [PubMed] [Google Scholar]
- 21. Aoki S, Iwata NK, Masutani Y, et al. Quantitative evaluation of the pyramidal tract segmented by diffusion tensor tractography: feasibility study in patients with amyotrophic lateral sclerosis. Radiat Med 2005;23:195–99 [PubMed] [Google Scholar]
- 22. Hong YH, Sung JJ, Kim SM, et al. Diffusion tensor tractography-based analysis of the pyramidal tract in patients with amyotrophic lateral sclerosis. J Neuroimaging 2008;18:282–87 [DOI] [PubMed] [Google Scholar]
- 23. Ciccarelli O, Behrens TE, Altmann DR, et al. Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain 2006;129:1859–71 [DOI] [PubMed] [Google Scholar]
- 24. Wilson CM, Grace GM, Munoz DG, et al. Cognitive impairment in sporadic ALS: a pathologic continuum underlying a multisystem disorder. Neurology 2001;57:651–57 [DOI] [PubMed] [Google Scholar]
- 25. Mackenzie IR, Feldman HH. Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia, and frontotemporal dementia of the motor neuron disease type represent a clinicopathologic spectrum. J Neuropathol Exp Neurol 2005;64:730–39 [DOI] [PubMed] [Google Scholar]
- 26. Basser PJ, Pajevic S, Pierpaoli C, et al. In vivo fiber tractography using DT-MRI data. Magn Reson Med 2000;44:625–32 [DOI] [PubMed] [Google Scholar]
- 27. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis: Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci 1994;(124 suppl):96–107 [DOI] [PubMed] [Google Scholar]
- 28. The Amyotrophic Lateral Sclerosis Functional Rating Scale: assessment of activities of daily living in patients with amyotrophic lateral sclerosis—the ALS CNTF treatment study (ACTS) phase I-II Study Group. Arch Neurol 1996;53:141–47 [PubMed] [Google Scholar]
- 29. Studholme C, Hill DL, Hawkes DJ. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys 1997;24:25–35 [DOI] [PubMed] [Google Scholar]
- 30. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996;111:209–19 [DOI] [PubMed] [Google Scholar]
- 31. Pagani E, Agosta F, Rocca MA, et al. Voxel-based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuroimage 2008;41:657–67 [DOI] [PubMed] [Google Scholar]
- 32. Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci 2001;356:1293–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hartkens T, Rueckert JA, Schnabel DJ, et al. VTK CISG Registration Toolkit: An open source software package for affine and non-rigid registration of single- and multimodal 3D images. In: Meiler M, Saupe D, Kruggel F.et., ed. Bildverarbeitung für die Medizin 2002, Algorithmen - Systeme - Anwendungen. Workshop Proceedings, Leipzig, March 2002. Series: Informatik aktuell. Berlin: Springer-Verlag; 2002 [Google Scholar]
- 34. Pagani E, Filippi M, Rocca MA, et al. A method for obtaining tract-specific diffusion tensor MRI measurements in the presence of disease: application to patients with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage 2005;26:258–65 [DOI] [PubMed] [Google Scholar]
- 35. Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008;44:1105–32. Epub 2008 May 23 [DOI] [PubMed] [Google Scholar]
- 36. Rohde GK, Aldroubi A, Dawant BM. The adaptive bases algorithm for intensity-based nonrigid image registration. IEEE Trans Med Imaging 2003;22:1470–79 [DOI] [PubMed] [Google Scholar]
- 37. Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 2002;15:435–55 [DOI] [PubMed] [Google Scholar]
- 38. Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 2001;13:1174–85 [DOI] [PubMed] [Google Scholar]
- 39. Abe K, Fujimura H, Kobayashi Y, et al. Degeneration of the pyramidal tracts in patients with amyotrophic lateral sclerosis: a premortem and postmortem magnetic resonance imaging study. J Neuroimaging 1997;7:208–12 [DOI] [PubMed] [Google Scholar]
- 40. Cheung G, Gawel MJ, Cooper PW, et al. Amyotrophic lateral sclerosis: correlation of clinical and MR imaging findings. Radiology 1995;194:263–70 [DOI] [PubMed] [Google Scholar]
- 41. Goodin DS, Rowley HA, Olney RK. Magnetic resonance imaging in amyotrophic lateral sclerosis. Ann Neurol 1988;23:418–20 [DOI] [PubMed] [Google Scholar]
- 42. Hecht MJ, Fellner F, Fellner C, et al. MRI-FLAIR images of the head show corticospinal tract alterations in ALS patients more frequently than T2-, T1- and proton-density-weighted images. J Neurol Sci 2001;186:37–44 [DOI] [PubMed] [Google Scholar]
- 43. Hecht MJ, Fellner F, Fellner C, et al. Hyperintense and hypointense MRI signals of the precentral gyrus and corticospinal tract in ALS: a follow-up examination including FLAIR images. J Neurol Sci 2002;199:59–65 [DOI] [PubMed] [Google Scholar]
- 44. Ishikawa K, Nagura H, Yokota T, et al. Signal loss in the motor cortex on magnetic resonance images in amyotrophic lateral sclerosis. Ann Neurol 1993;33:218–22 [DOI] [PubMed] [Google Scholar]
- 45. Mirowitz S, Sartor K, Gado M, et al. Focal signal-intensity variations in the posterior internal capsule: normal MR findings and distinction from pathologic findings. Radiology 1989;172:535–39 [DOI] [PubMed] [Google Scholar]
- 46. Thorpe JW, Moseley IF, Hawkes CH, et al. Brain and spinal cord MRI in motor neuron disease. J Neurol Neurosurg Psychiatry 1996;61:314–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hong YH, Lee KW, Sung JJ, et al. Diffusion tensor MRI as a diagnostic tool of upper motor neuron involvement in amyotrophic lateral sclerosis. J Neurol Sci 2004;227:73–78 [DOI] [PubMed] [Google Scholar]
- 48. Mitsumoto H, Ulug AM, Pullman SL, et al. Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology 2007;68:1402–10 [DOI] [PubMed] [Google Scholar]
- 49. Chang JL, Lomen-Hoerth C, Murphy J, et al. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology 2005;65:75–80 [DOI] [PubMed] [Google Scholar]
- 50. Burzynska AZ, Preuschhof C, Backman L, et al. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage 49:2104–12 [DOI] [PubMed] [Google Scholar]
- 51. Acosta-Cabronero J, Williams GB, Pengas G, et al. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease. Brain 2010;133(pt 2):529–39. Epub 2009 Nov 13 [DOI] [PubMed] [Google Scholar]
- 52. Pievani M, Agosta F, Pagani E, et al. Assessment of white matter tract damage in mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp 2010. February 16. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lomen-Hoerth C, Murphy J, Langmore S, et al. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 2003;60:1094–97 [DOI] [PubMed] [Google Scholar]
- 54. Matsuo K, Mizuno T, Yamada K, et al. Cerebral white matter damage in frontotemporal dementia assessed by diffusion tensor tractography. Neuroradiology 2008;50:605–11 [DOI] [PubMed] [Google Scholar]
- 55. Zhang Y, Schuff N, Du AT, et al. White matter damage in frontotemporal dementia and Alzheimer's disease measured by diffusion MRI. Brain 2009;132:2579–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Agosta F, Rocca MA, Valsasina P, et al. A longitudinal diffusion tensor MRI study of the cervical cord and brain in amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry 2009;80:53–55 [DOI] [PubMed] [Google Scholar]
- 57. Borroni B, Brambati SM, Agosti C, et al. Evidence of white matter changes on diffusion tensor imaging in frontotemporal dementia. Arch Neurol 2007;64:246–51 [DOI] [PubMed] [Google Scholar]
- 58. Henry RG, Oh J, Nelson SJ, et al. Directional diffusion in relapsing-remitting multiple sclerosis: a possible in vivo signature of wallerian degeneration. J Magn Reson Imaging 2003;18:420–26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.