Abstract
BACKGROUND AND PURPOSE:
Most imaging techniques used for the evaluation of obstructive epiphora, such as DS DCG, rely on undesired ionizing radiation. We evaluated the efficacy of topical contrast-enhanced MR DCG in comparison with DS DCG in patients with obstructive epiphora who underwent balloon DCG or stent placement.
MATERIALS AND METHODS:
Thirty-six LDSs of 21 patients treated with balloon DCG (n = 11) or stent placement (n = 11) were examined with MR DCG and DS DCG. Contralateral LDSs (n = 14) were also evaluated in patients with unilateral disease. A sterile 0.9% NaCl solution containing 1:100 diluted gadolinium chelate was instilled into conjunctival sacs. The 3D FSPGR sequence was used with a 1.5T scanner. MR and DS DCG findings were scored and compared according to morphology of the lacrimal sac, junction, and NLD and the presence of contrast media in the nasal cavity.
RESULTS:
Comparison of MR DCG and DS DCG findings showed no significant statistical differences in reference to anatomic locations according to the McNemar test (P > .05). Good or very good agreement (κ value > 0.61) was observed according to the κ statistics.
CONCLUSIONS:
Topical contrast-enhanced MR DCG is an effective and reliable noninvasive method for evaluation of the LDS in patients treated with IR procedures. This method avoids both cannulation and ionizing radiation and can, therefore, be repeated as often as is necessary in these complex patients.
Obstruction of the LDS, resulting in inadequate drainage of tears, can lead to intermittent or constant tearing, which is termed “epiphora.” It is an annoying condition representing 3%–5% of clinical consultations in ophthalmology.1 Most primary acquired lacrimal outflow obstructions are due to idiopathic inflammation, fibrosis, and scarring of the nasolacrimal duct.2 The classic treatment of epiphora resulting from LDS obstructions is external or endonasal endoscopic DCR. Transluminal balloon dilation of the LDS has been proposed as an alternative to surgical treatment.3,4 Placement of nasolacrimal polyurethane stents in the LDS is another less invasive approach to the treatment of epiphora.5
The cause of epiphora can be diagnosed by physical examination, diagnostic clinical tests, and imaging procedures. DS DCG is currently considered to be the criterion standard among imaging techniques. It has several drawbacks, however, including its inability to provide a functional evaluation, its use of ionizing radiation, a requirement for cannulation of the canaliculus, and the lack of data regarding the surrounding soft tissues.
Although MR DCG was first carried out by Goldberg et al in 1993,6 the MR DCG evaluation of the LDS in patients treated with either balloon DCG or nasolacrimal stent placement has not been previously reported, to our knowledge. In this study, we aimed to compare the findings of topical contrast-enhanced MR DCG with those of DS DCG in patients with LDS treated either with balloon DCG or stent placement and to determine the efficacy of the MR DCG technique in the evaluation of LDS obstructions treated by means of IR procedures.
Materials and Methods
Patients
This study was approved by our institutional review board, and informed consent was obtained from all patients.
The patient population included 17 women and 4 men (mean age, 50 years; range, 37–73 years) who were treated by interventional techniques for obstructive epiphora. In all cases, disease-free canaliculi were confirmed. Eleven balloon dilation procedures and 11 stent placements were performed in 22 LDSs of 21 patients. In 5 LDSs, formerly placed stents had been removed before MR DCG. The polyurethane nasolacrimal stent is 35 mm long and has a mushroom proximal tip (5 mm in diameter and length) like a Malecot catheter. The outer diameter of the stent is 2 mm, and the luminal diameter is 1.5 mm.1 Contralateral LDSs (n = 14) were also evaluated in patients with unilateral disease. In 6 patients, unilateral MR DCG examinations were performed. Grading of epiphora was determined on the basis of the Munk et al7 classification (0 = no epiphora, 1 = epiphora requiring wiping the eye less than twice a day, 2 = epiphora requiring wiping 2–4 times a day, 3 = epiphora requiring wiping 5–10 times a day, 4 = epiphora requiring wiping >10 times a day, 5 = constant tearing) at the time of MR DCG (Table 1).
Table 1:
Patients who underwent BD or nasolacrimal stent placement
| No. | Age (yr) | Sex | Epiphoraa (grade) |
Intervention |
Time Intervalb (yr) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| BD |
Stent |
|||||||||
| R | L | R | L | R | L | R | L | |||
| 1 | 65 | F | 0 | 0 | – | + | – | – | – | 12 |
| 2 | 39 | F | 0 | 1 | – | – | + | + | 4 | 4 |
| 3 | 44 | F | 0 | 0 | – | – | + | – | 5 | – |
| 4 | 40 | F | 1 | 0 | + | – | – | – | 9 | – |
| 5 | 59 | F | 1 | 0 | – | – | +c | – | 5 | – |
| 6 | 56 | M | 0 | 0 | + | – | – | – | 1 | – |
| 7 | 44 | M | 0 | 0 | – | + | – | – | – | 1 |
| 8 | 42 | F | 0 | 0 | – | + | – | 4 | – | |
| 9 | 38 | F | 0 | 0 | – | – | +c | – | 5 | – |
| 10 | 59 | F | 0 | 0 | – | – | – | +c | – | 4 |
| 11 | 43 | F | 0 | 5 | – | – | – | +c | – | 4 |
| 12 | 60 | F | 2 | 2 | + | – | – | – | 5 | – |
| 13 | 63 | F | 0 | 0 | + | – | – | – | 12 | – |
| 14 | 47 | F | 0 | 0 | + | – | – | – | 10 | |
| 15 | 47 | F | 0 | 0 | + | – | – | – | 11 | – |
| 16 | 37 | F | 1 | 0 | + | – | – | – | 11 | – |
| 17 | 63 | F | 0 | 0 | + | – | – | – | 7 | – |
| 18 | 53 | M | 0 | 0 | – | – | +c | – | 5 | – |
| 19 | 43 | F | 0 | 3 | – | – | – | + | – | 10 |
| 20 | 73 | M | 0 | 0 | – | + | – | – | – | 7 |
| 21 | 38 | F | 0 | 0 | – | – | – | + | – | 1 |
Note:—BD indicates balloon DCG; +, present; –, absent; R, right; L, left.
At the time of MR DCG.
The time interval between the last intervention and MR DCG.
Stent removed.
All patients had DS DCG 1 day to 3 months (average, 27.90 days) before MR DCG examinations. The interval between the last intervention and MR DCG was at least 1 year and at most 12 years (mean, 6.33 years; median, 5 years). Exclusion criteria included a history of allergic reaction, being younger than 18 years of age, pregnancy, and/or contraindications to MR imaging, such as severe claustrophobia and incompatible metallic implants.
MR Imaging
Eye drops containing 1:100 diluted gadobutrol (Gd-DO3A-butrol, Gadovist 1.0 mmol/mL; Bayer Healthcare, Berlin, Germany) in a sterile 0.9% NaCl solution were administered to 1 or both conjunctival sacs 4 times at 1-minute intervals while the patient was in a sitting position with his or her head in hyperextension. Then the patient was asked to lie down on the MR imaging table, and another 2 drops were administered to the conjunctival sacs before a 3-inch dual coil was placed on both orbits. After the localizer images were obtained, another 2 drops were administered without the need to reposition the coil.
MR imaging was performed with a 1.5T system (Signa Excite; GE Healthcare, Milwaukee, Wisconsin). The gradient system operates with a maximum gradient strength of 33 mT/m and a slew rate of 120 T/m/s. The 3D FSPGR sequence was used to obtain images in the axial and coronal planes. Following the second series, gentle massage was performed on the lacrimal sacs, and the third series was performed in the coronal plane. The total image acquisition time was <15 minutes in all cases, as shown in Table 2.
Table 2:
Parameters obtained during the study
| Series No. | View | FOV (cm) | TR (ms) | TE (ms) | Matrix | Thickness (mm) | NEX | FA (°) | Duration (min) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Coronal | 10 | 11 | 2.7 | 320 × 256 | 1 | 2 | 20 | 3 |
| 2 | Axial | 10 | 11 | 2.7 | 320 × 256 | 1 | 2 | 20 | 4.5 |
| 3 | Coronal (postmassage) | 10 | 11 | 2.7 | 320 × 256 | 1 | 2 | 20 | 3 |
Note:—FA = flip angle.
Following the examination, patients were asked about the presence of discomfort due to contrast media installation or any adverse effects and their comments were noted.
DS DCG Imaging
All patients had DS DCG examinations 1 day to 3 months before the MR DCG examinations. Before the procedure, a topical anesthetic solution (0.4% benoxinate hydrochloride) was applied to the conjunctival sac. Following the dilation of the lower punctum, a flexible 23-ga lacrimal cannula was placed into the inferior canaliculus. After the acquisition of the mask images, 2–4 mL of nonionic contrast media was injected, and images (1 frame/s) were obtained in posteroanterior projection; the imaging procedure was stopped when it became apparent that either the imaging of the lacrimal drainage system was complete and the contrast media had reached the nasal cavity or when the reflux of the contrast media toward the superior punctum was observed.
Image Evaluation
MR images were reconstructed with the MIP algorithm by using a computer workstation (Advantage Windows Workstation 4.1, GE Healthcare). The images obtained by MR DCG and DS DCG were assessed by an experienced radiologist who was blinded to the clinical findings of the patients. The MR DCG evaluation was reviewed in the following order: axial, coronal, and postsaccal massage–coronal images and MIP reconstructed images. The findings of the MR and DS DCG examinations were randomly evaluated and scored in 4 consecutive locations including the morphology of the lacrimal sacs, junctions, NLD, and nasal cavity for the presence of contrast media (Fig 1). For patients who had stents in their LDSs during MR DCG, the presence of contrast media in the stent and nasal cavity was evaluated. The evaluation of both the MR DCG and DS DCG images was based on a scoring system defined in Table 3.
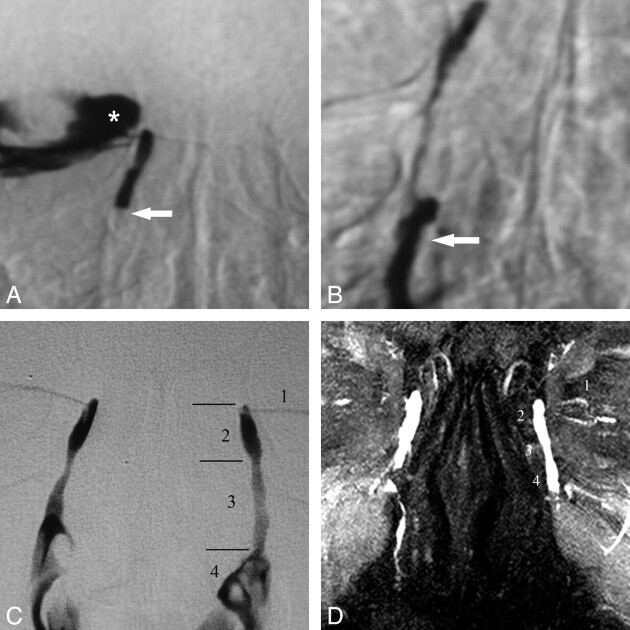
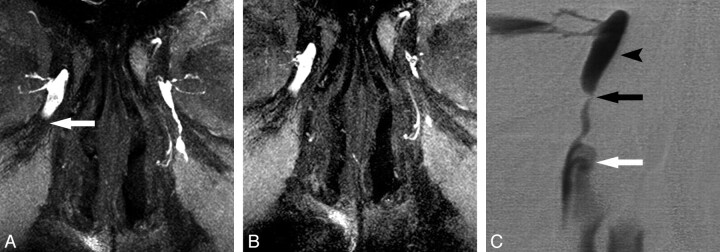
Fig 1.
DS and MR DCG 12-year follow-up after balloon DCG in an asymptomatic patient. A, DS DCG reveals occlusion of the distal NLD (arrow) and reflux of iodinated contrast material to the conjunctival sac (asterisk). B, DS DCG immediately after transluminal balloon dilation shows passage of the contrast media to the inferior meatus of the nasal cavity (arrow). Note that there is no reflux to the conjunctival sac after successful balloon DCG. C, Twelve-year DS-DCG follow-up with bilateral simultaneous contrast media injection reveals a completely normal LDS. The anatomic regions of the normal left LDS are the following: 1) inferior canaliculus, 2) lacrimal sac, 3) NLD, and 4) contrast media in the nasal cavity. D, Bilateral topical contrast-enhanced coronal MIP DCG image from 3D FSPGR sequence demonstrates patency of the LDSs both on the intervened right side and normal left side. 1 indicates the canaliculi; 2, lacrimal sac; 3, nasolacrimal duct; 4, contrast media in the nasal cavity.
Table 3:
Scoring system used to analyze and compare MR DCG and DS DCG images
| Lacrimal sac | Small (1) | Normal (2) | Dilated (3) | – |
| Sac-NLD junction | Obstructed (1) | Stenotic (2) | Normal (3) | Dilated (4) |
| Stent | Obstructed (1) | Stenotic (2) | Normal (3) | – |
| NLD | Obstructed (1) | Stenotic (2) | Normal (3) | Dilated (4) |
| Contrast media in the nasal cavity | Nonexistent (0) | Existent (1) | – | – |
Note:– indicates absent.
Statistical Analysis
Statistical analysis was performed by using commercially available statistical software (Statistical Package for the Social Sciences, Version 10.0 for Windows; SPSS, Chicago, Illinois). MR DCG and DS DCG findings were compared by using the McNemar test and κ statistics. For the McNemar test, a P value of < .05 was considered to indicate a significant difference. κ values for the κ statistic were interpreted as follows: <0.00 represented poor, between 0.00 and 0.20 represented slight, between 0.21 and 0.40 represented fair, between 0.41 and 0.60 represented moderate, between 0.61 and 0.80 represented good agreement, and between 0.81 and 1.00 represented very good agreement.
Results
MR DCG was performed after topical contrast administration and diagnostic images were obtained successfully for each patient.
A total of 36 LDSs were evaluated with MR imaging in 21 patients who had undergone DS DCG examinations. Balloon DCG was performed in 11 LDSs (Fig 2), stent placement was performed in 11 LDSs (Fig 3), and contralateral LDSs were evaluated in 14 patients with unilateral diseases. No side effects occurred during or after the instillation of diluted eye drops.
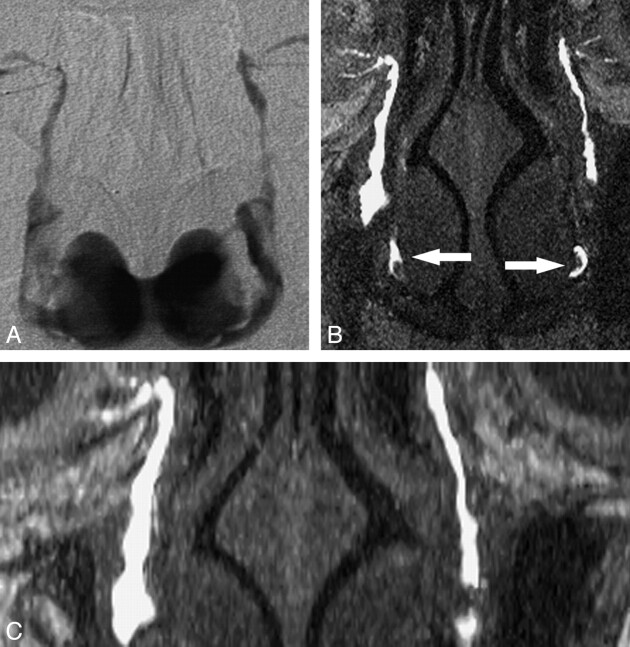
Fig 2.
Evaluation of both LDSs with recurrent epiphora treated with multiple balloon DCGs. A, DS DCG shows patency of the both LDSs in the fifth year of follow-up after the last intervention. B and C, Coronal MIP MR DCG from coronal (B) and axial (C) images shows patent LDSs with luminal irregularities and contrast media in the nasal cavity (arrows).
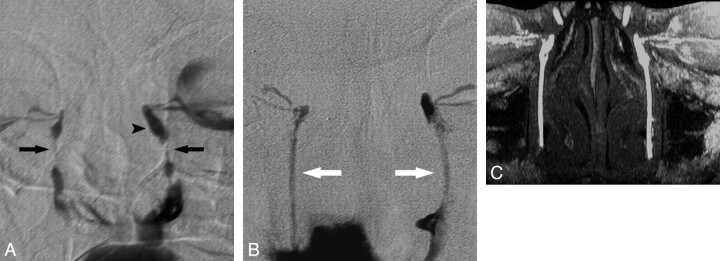
Fig 3.
MR DCG evaluation of bilateral LDSs treated with nasolacrimal stents 4 years before the examination. A, Bilateral DS DCG reveals severe nasolacrimal stenoses on both sides (arrows) with prominent dilation of the left lacrimal sac (arrowhead). B, The fourth-year follow-up DS DCGs show the patent lumens of polyurethane nasolacrimal stents (white arrows) in the LDSs with free flow of contrast media to the nasal cavity on both sides. C, Coronal MIP DCG image from an axial FSPGR sequence demonstrates the patency of the stent lumens on both sides.
The MR DCG and DS DCG findings were statistically compared with the above-referenced scoring system. In all LDSs (n = 36), no significant difference was determined for the morphology of the lacrimal sacs, junctions, NLDs, and presence of the contrast media in the nasal cavity according to the McNemar test (P > .05). According to the κ statistics, MR DCG and DS DCG analysis of the morphology of the lacrimal sacs, junctions, and NLDs showed very good agreement (κ > 0.80); the observation of contrast media presence in the nasal cavity showed good agreement (κ = 0.786).
In the intervened (balloon DCG and stent placement) LDSs (n = 22), no significant difference was determined in lacrimal sacs, junctions, NLDs, and the presence of the contrast media in the nasal cavity according to the McNemar test (P > .05). Very good agreement (κ = 0.842) was observed in the lacrimal sacs, and good agreement (κ = 0.80) was observed in the junctions, NLDs, and presence of contrast media in the nasal cavity with regard to the κ statistics. The results of the statistical analysis are summarized in Table 4.
Table 4:
Results of the statistical analysis in terms of κ values for all LDSs and intervened LDSs
| All LDSs (n = 36) | Intervened LDSs (n = 22) | |
|---|---|---|
| Lacrimal sac | 0.844 | 0.842 |
| Junction | 0.826 | 0.752 |
| NLD | 0.814 | 0.768 |
| CM in nasal cavity | 0.786 | 0.776 |
Note:—CM indicates contrast media.
In 5 of the LDSs, discrepancies were noted between the findings of the 2 methods. All patients had been treated by balloon DCG, and the discrepancies are summarized in Table 5.
Table 5:
Discrepancies between DS and MR DCG findings
| Patient No. | Diagnosis Method | Lacrimal Sac | Junction | NLD | CM in the NC |
|---|---|---|---|---|---|
| 1 | DS DCG | Normal | Stenotic | Normal | + |
| MR DCG | Normal | Stenotic | Stenotic | + | |
| 6 | DS DCG | Dilated | Normal | Normal | + |
| MR DCG | Dilated | Stenotic | Normal | + | |
| 12 | DS DCG | Normal | Normal | Normal | + |
| MR DCG | Dilated | Normal | Normal | + | |
| 15 | DS DCG | Dilated | Stenotic | Stenotic | + |
| MR DCG | Normal | Normal | Normal | + | |
| 16 | DS DCG | Dilated | Stenotic | Stenotic | + |
| MR DCG | Dilated | Obstructed | NA | – |
Note:—CM indicates contrast media; NC, nasal cavity; +, existent; –, nonexistent.
Discussion
For many years, the treatment of obstructive epiphora was surgical external DCR. Despite its high success rate, its drawbacks included being an invasive procedure, often requiring general anesthesia, and the development of facial scar tissue.2,8–12 In recent years, endonasal endoscopic DCR has been developed as a less invasive treatment. In addition, IR methods such as balloon dilation and nasolacrimal polyurethane stent placement have been effectively used. Both endoscopic and IR-based methods are more easily tolerated by the patient compared with external DCR.4,5,13
It is important to determine the level of the obstruction before any surgical or radiologic treatment procedure. In addition to DS DCG, numerous imaging modalities have been used to evaluate the site and type of obstructive epiphora; these imaging techniques have a variety of advantages and limitations and are discussed in detail below. The lens of the eye is the most sensitive tissue to ionizing radiation in the region being studied, with a risk of subcapsular opacities and cataracts with a threshold-dependent deterministic effect.14,15 Therefore, the absence of ionizing radiation would be a great advantage for an imaging technique.
DS DCG is the criterion standard for the diagnosis of LDS obstruction, given its high spatial resolution, but it is not a functional method of analysis if performed with cannulation.16 It also relies on ionizing radiation. Galloway et al17 observed that the exposure of the eye lens to radiation is approximately 1.2 mGy during DS DCG. Meric et al18 have measured the radiation dose as 1.1 mGy for DS DCG. Ilgit et al19 measured the mean absorbed radiation doses of 4.6 mGy ± 2.2 to the lens of the treated side and 38.5 mGy ± 17.5 to the contralateral lens during DCS, with the dose to the contralateral lens related to the specific technique used in this study. Wilhelm et al20 reported the mean radiation dose as 5.43 mGy to the treated side and 1.37 mGy to the contralateral lens in a similar intervention. Additionally, the radiation dose increases with each additional DS DCG indicated for further treatment and/or follow-up of the previously treated patients. Finally, DS DCG requires cannulation of the lacrimal punctum and may be a potential cause of iatrogenic trauma, with consequent scarring of the canaliculus resulting in new or worsening epiphora.21
Other imaging methods that have been used to assess the lacrimal system include lacrimal scintigraphy and CT DCG, with the latter especially beneficial in cases in which bone structure is evaluated before the surgical procedure. Both of these techniques have the disadvantage of exposing the lens to significant doses of ionizing radiation.22,23
Since the introduction of MR DCG with the administration of diluted gadolinium solution by Goldberg et al,6 several studies have been reported with different imaging parameters and sequences, including topical or intracanalicular administration of gadolinium or saline solutions (Table 6). In our study, a 3D FSPGR imaging technique was used, due to the fact that reformation of images with MIP is possible with this technique. In a previous study of Karagülle et al,24 this imaging technique was performed successfully and reliably for the evaluation of obstruction in the LDS.
Table 6:
MR DCG technique and imaging parameters in previously published studies
| Study | Year | No. of Patients | Cannulation | Topical | Sequence Technique |
|---|---|---|---|---|---|
| Goldberg et al6 | 1993 | 11 | Gd-DTPA | Gd-DTPA | T1WI (fat-sat) |
| Caldemeyer et al21 | 1998 | 11 | – | Saline solution | FSE T2WI |
| Kirchhof et al28 | 2000 | 11 | – | Gd-DTPA | T1- and T2WI (fat-sat) |
| Manfre et el29 | 2000 | 36 | Gd-DTPA | Gd-DTPA | SE T1WI (fat-sat) |
| Yoshikawa et al25 | 2000 | 18 | Gd-DTPA | Saline solution | FSE T2WI; SE T1WI |
| Takehara et al31 | 2000 | 8 | Saline solution | – | Heavily T2WI |
| Karagülle et al24 | 2002 | 19 | Gd-DTPA | – | 3D FSPGR |
| Cubuk et al32 | 2010 | 35 | Saline solution | – | Single-shot SE T2WI (fat-sat) |
| Our study | 2011 | 21 | – | Gd-BT-DO3A | 3D FSPGR |
Note:—fat-sat indicates fat-saturated; SE, spin-echo; –, absent.
Yoshikawa et al25 have compared topical applications of saline solution and Gd-DTPA solution. They reported that the images obtained after the application of the gadolinium solution provided more accurate information than those obtained after the application of the saline solution. Slight burning, irritation, or dryness was reported for iodinated contrast material instillation,21 but there were no reported adverse events due to intracanalicular or topical administration of gadolinium-based contrast media.6,24–29 Topical application of 1:100 diluted gadobutrol solution was well tolerated by all the patients in our study and showed none of the adverse effects attributable to the contrast media during and immediately after the instillation.
MR DCG examinations with contrast media mentioned in the literature have used gadolinium concentrated at 0.5 mmol/mL.6,24–29 In our study, we administered nonionic gadolinium-based MR imaging contrast agent (gadobutrol, Gd-DO3A-butrol; Gadovist 1.0 mmol/mL), which is more viscous (viscosity, 4,96 mPa at 37°C) and has twice the gadolinium concentration (1.0 mmol/mL) of the other gadolinium-based contrast agents. During the DS DCG examinations, Priebe et al30 administered the same contrast media into the LDSs of 3 patients who had a history of severe allergic reactions to iodinated contrast media.30 No side effects or complications were reported in these patients.
Topical application is a more physiologic technique than the cannulation method. Contrast material, like tears, is propelled to the LDS by blinking, muscular contraction, and capillary action after topical administration21; thus, the LDS is not distended, unlike in DS DCG with intracanalicular injection. All 5 of the discrepancies between the 2 methods (illustrated in Figs 4 and 5) can be explained by this difference.
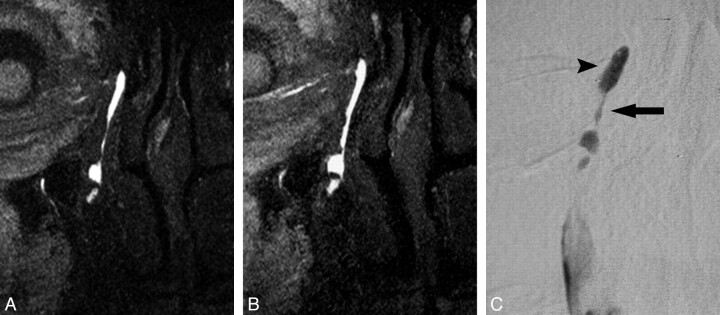
Fig 4.
Discrepancy of the MR and DS DCG in the asymptomatic patient who had been treated with balloon DCG. A and B, MR DCG reveals normal right LDS (A), and there is no obvious difference after the sac massage (B). C, MR DCG findings could not be confirmed at the DS DCG showing stenosis of the junction and NLD (arrow) with a dilated lacrimal sac (arrowhead).
Fig 5.
Junctional stenosis in patient having grade 1 epiphora. A, MR DCG shows occlusion at the nasolacrimal junction (arrow) with a dilated lacrimal sac on the right. The left LDS is normal. B, After the lacrimal sac massage, there is no difference on the right and drainage of the left LDS with free flow of contrast media. C, DS DCG reveals severe junctional stenosis (black arrow) with dilation of the lacrimal sac (arrowhead). Passage of the contrast media to the nasal cavity is obvious (white arrow).
Conclusions
MR DCG is a useful imaging technique for the evaluation of LDS in patients treated with IR procedures, and it compares favorably with the criterion standard DS DCG. Our study is the first use of MR DCG in the evaluation of the LDS in a group of patients who underwent balloon DCS or stent placement with the topical application of a gadolinium-based contrast media with relatively high concentration. Among its advantages, this method requires no cannulation and avoids exposure of the radiosensitive lens to ionizing radiation.
Abbreviations
- DCG
dacryocystography
- DCR
dacryocystorhinostomy
- DS
digital subtraction
- FSPGR
fast-spoiled gradient-recalled
- Gd-DTPA
gadolinium-diethylene-triamine pentaacetic acid
- IR
interventional radiology
- LDS
lacrimal drainage system
- MIP
maximum intensity projection
- NaCl
sodium chloride
- NLD
nasolacrimal duct
References
- 1. Song HY, Jin YH, Kim JH, et al. Nonsurgical placement of a nasolacrimal polyurethane stents: long-term effectiveness. Radiology 1996;200:759–63 [DOI] [PubMed] [Google Scholar]
- 2. Mandeville JT, Woog JJ. Obstruction of the lacrimal drainage system. Curr Opin Ophthalmol 2002;13:303–09 [DOI] [PubMed] [Google Scholar]
- 3. Song HY, Ahn HS, Park CK, et al. Complete obstruction of the nasolacrimal system. Part I. Treatment with ballon dilatation. Radiology 1993;186:367–71 [DOI] [PubMed] [Google Scholar]
- 4. Ilgit E, Yuksel D, Unal M, et al. Transluminal balloon dilatation of the lacrimal drainage system for the treatment of epiphora. AJR Am J Roentgenol 1995;165:1517–24 [DOI] [PubMed] [Google Scholar]
- 5. Ilgit E, Önal B, Coskun B. Interventional radiology in the lacrimal drainage system. Eur J Radiol 2005;55:331–39 [DOI] [PubMed] [Google Scholar]
- 6. Goldberg RA, Heinz GW, Chiu L. Gadolinium magnetic resonance imaging dacryocystography. Am J Ophthalmol 1993;115:738–41 [DOI] [PubMed] [Google Scholar]
- 7. Munk PL, Lin DT, Morris DC. Epiphora: treatment by means of dacryocystoplasty with balloon dilation of the nasolacrimal drainage apparatus. Radiology 1990;177:687–90 [DOI] [PubMed] [Google Scholar]
- 8. Becker BB. Recanalization of the obstructed nasolacrimal duct system. J Vasc Interv Radiol 2001;12:697–99 [DOI] [PubMed] [Google Scholar]
- 9. Allen K, Berlin AJ. Dacryocystorhinostomy failure: association with nasolacrimal silicone intubation. Ophthalmic Surg 1989;20:486–89 [PubMed] [Google Scholar]
- 10. Dryden RM, Wulc AE. Surgery of the lacrimal system. In: Waltman SR, Keates RH, Hoyt CS. eds. Surgery of the Eye. New York: Churchill-Livingstone; 1988:607–28 [Google Scholar]
- 11. Glatt HJ. Dacryocystoplasty: an oculoplastic surgeon's perspective (letter). Radiology 1991;180:289–90 [DOI] [PubMed] [Google Scholar]
- 12. Patrinely JR, Gigantelli JW. Dacryocystorhinostomy. In: Linberg JV. ed Lacrimal Surgery. New York: Churchill-Livingstone; 1988;151–67 [Google Scholar]
- 13. Song HY, Jin YH, Kim JH, et al. Nonsurgical placement of a nasolacrimal polyurethane stent. Radiology 1995;194:233–37 [DOI] [PubMed] [Google Scholar]
- 14. Lipman RM, Tripathi BJ, Tripathi RC. Cataracts induced by microwave and ionizing radiation. Surv Ophthalmol 1988;33:200–10 [DOI] [PubMed] [Google Scholar]
- 15. Jackson A, Hardcastle MP, Shaw A, et al. Reduction of ocular lens dosage in dacryocystography. Clin Radiol 1989;40:615–16 [DOI] [PubMed] [Google Scholar]
- 16. Baert AL, Heuck FH, Youker JE. Medical radiology, diagnostic imaging. In: Mukherji SK, Castelijns JA. eds. Modern Head and Neck Imaging. Springer-Verlag; 2000:211–35 [Google Scholar]
- 17. Galloway JE, Kavie TA, Raflo GT. Digital subtraction macrodacryocystography: a new method of lacrimal system imaging. Ophthalmology 1984;91:956–62 [DOI] [PubMed] [Google Scholar]
- 18. Meric N, Bor D, Ilgit ET, et al. Comparison of eye lens dose measurement techniques in imaging and interventions of the lachrymal drainage system. Phys Medica 1998;14:95–100 [Google Scholar]
- 19. Ilgit ET, Meric N, Bor D, et al. Lens of the eye: radiation dose in balloon dacryocystoplasty. Radiology 2000;217:54–57 [DOI] [PubMed] [Google Scholar]
- 20. Wilhelm K, Kramer S, Textor J, et al. Radiation exposure of radiation-sensitive risk organs—ocular lens, parotid gland, thyroid gland—in dacryocystography and therapy [in German]. Rofo 1998;168:270–74 [DOI] [PubMed] [Google Scholar]
- 21. Caldemeyer KS, Stockberger SM, Jr, Broderick LS. Topical contrast-enhanced CT and MR dacryocystography: imaging the lacrimal drainage apparatus of healthy volunteers. AJR Am J Roentgenol; 1998;171:1501–04 [DOI] [PubMed] [Google Scholar]
- 22. Robertson JS, Brown ML, Colvard DM. Radiation absorbed dose to the lens in dacryoscintigraphy with 99mTcO4. Radiology 1979;133:747–50 [DOI] [PubMed] [Google Scholar]
- 23. Waite D, Whittet H, Shun-Shin G. Technical note: computed tomographic dacryocystography. Br J Radiol 1993;66:711–13 [DOI] [PubMed] [Google Scholar]
- 24. Karagülle T, Erden A, Erden I, et al. Nasolacrimal system: evaluation with gadolinium-enhanced MR dacryocystography with a three-dimensional fast spoiled gradient-recalled technique. Eur Radiol 2002;12:2343–48 [DOI] [PubMed] [Google Scholar]
- 25. Yoshikawa T, Hirota S, Sugimura K. Topical contrast-enhanced magnetic resonance dacryocystography. Radiat Med 2000;18:355–62 [PubMed] [Google Scholar]
- 26. Amrith S, Goh PS, Wang SC. Tear flow dynamics in the human nasolacrimal ducts: a pilot study using dynamic magnetic resonance imaging. Graefes Arch Clin Exp Ophthalmol 2005;243:127–31 [DOI] [PubMed] [Google Scholar]
- 27. Hoffmann KT, Hosten N, Anders N, et al. High-resolution conjunctival contrast-enhanced MRI dacryocystography. Neuroradiology 1999;41:208–13 [DOI] [PubMed] [Google Scholar]
- 28. Kirchhof K, Hahnel S, Jansen O, et al. Gadolinium-enhanced magnetic resonance dacryocystography in patients with epiphora. J Comput Assist Tomogr 2000;24:327–31 [DOI] [PubMed] [Google Scholar]
- 29. Manfre L, de Maria M, Todaro E, et al. MR dacryocystography: comparison with dacryocystography and CT dacryocystography. AJNR Am J Neuroradiol 2000;21:1145–50 [PMC free article] [PubMed] [Google Scholar]
- 30. Priebe M, Mohr A, Brossmann J, et al. Gadobutrol: an alternative contrast agent for digital subtraction dacryocystography. Eur Radiol 2002;12:2083–86. Epub 2002 Mar 19 [DOI] [PubMed] [Google Scholar]
- 31. Takehara Y, Isoda H, Kurihashi K, et al. Dynamic MR dacryocystography: a new method for evaluating nasolacrimal duct obstructions. AJR Am J Roentgenol 2000;175:469–73 [DOI] [PubMed] [Google Scholar]
- 32. Cubuk R, Tasali N, Aydin S, et al. Dynamic MR dacryocystography in patients with epiphora. Eur J Radiol 2010;73:230–33 [DOI] [PubMed] [Google Scholar]