SUMMARY:
The beginning of neuroradiology can be traced to the early 1900s with the use of skull radiographs. Ventriculography and pneumoencephalography were introduced in 1918 and 1919, respectively, and carotid angiography, in 1927. Technical advances were made in these procedures during the next 40 years that lead to improved diagnosis of intracranial pathology. Yet, they remained invasive procedures that were often uncomfortable and associated with significant morbidity. The introduction of CT in 1971 revolutionized neuroradiology. Ventriculography and pneumoencephalography were rendered obsolete. The imaging revolution continued with the advent of MR imaging in the early 1980s. Noninvasive angiographic techniques have curtailed the use of conventional angiography, and physiologic imaging gives us a window into the function of the brain. In this historical review, we will trace the origin and evolution of the advances that have led to the quicker, less invasive diagnosis and resulted in more rapid therapy and improved outcomes.
Efforts to image the CNS began with skull radiographs shortly after Roentgen's discovery of x-rays.1–3 In the early 20th century, contrast studies of the brain, by using air for contrast, were developed with the introduction of ventriculography and pneumoencephalography.4,5 Shortly thereafter, cerebral angiography was described. Nearly 50 years later, the next great advances in imaging came with the introduction of CT and subsequently MR imaging.6,7 These discoveries have advanced the field of neuroradiology and improved the lives of patients via easier, more rapid diagnosis and treatment.
The Beginning: Skull Radiographs
Within the first decade after Roentgen's discovery of x-rays, several publications described the use of skull radiographs to diagnose brain tumors and other CNS abnormalities.1–3 In 1912, Röentgen-diagnostik der Erkrankungen des Kopfes (Radiology of Diseases of the Head) by Arthur Schüller8 was published in Austria and later translated into English in 1918. This is considered one of the most important contributions to skull radiography, being the first comprehensive study of intracranial disease by x-ray.3,9,10 For this, Schüller is considered the father of neuroradiology and is the one who coined the term “neuroradiology.”3,11 His contributions include describing displacement of pineal calcifications by masses, normal and pathologic intracranial calcifications, the differential diagnosis of intra- and extrasellar tumors, findings of increased intracranial pressure, and osteoporosis circumscripta.3 Other contributors to neuroradiology in its incipient period described widening of the internal auditory canal by vestibular schwannomas and the distribution of intracranial gas introduced by trauma, surgery, or infection.3 Skull radiographs were also used to diagnose fractures and foreign bodies.9
Obtaining a single skull radiograph took minutes, with the radiograph being produced on a glass plate with a slowly responding photographic emulsion. Patients needed to be immobilized while undergoing the x-ray process. Additionally there was no way to angle the x-ray tube or eliminate scattered radiation.3 Walter E. Dandy, the neurosurgeon, with his colleague George J. Heuer, published a review of the radiographic findings in 100 cases of brain tumors in 1916 and found abnormalities on the x-rays in 45% of patients.12 Nearly 60 years later, despite advances in technology and radiographic techniques, Bull reported specific and nonspecific findings on skull radiographs in only 50% of patients with known intracranial tumors.13
Air-Contrast Studies: Ventriculography and Pneumoencephalography
Limitations to skull radiographs were noted even in the early years after the discovery of the x-ray. Schüller8 was aware of the difficulty in diagnosing soft-tissue changes via skull radiography, while others noted that skull radiographs were most useful in tumors with calcification or bone destruction.3,14 Even in cases in which abnormalities were seen on skull radiographs, they were mainly in advanced stages of disease (Fig 1).4 Dandy theorized that the cerebral ventricles could be visualized if they were filled with a medium that produced a shadow on radiographs and that a change in the size or shape of the ventricles by intracranial lesions would permit the earlier diagnosis of intracranial pathology.4 In experiments on dogs, Dandy injected a variety of contrast agents into the ventricles that were used at the time for pyelography; all of these agents were lethal.4 Noting how air outlined normal and pathologic abdominal structures and how paranasal sinus and mastoid inflammatory and neoplastic processes replaced the normally air-filled structures, he attempted to outline the ventricles with air.4 In 1918, he reported his results by using air ventriculography in 20 pediatric patients. This technique necessitated a ventricular puncture through an open fontanel or via a small burr-hole if the fontanels were closed, removal of CSF, and injection of an equal amount of air.4 Ventriculography reportedly increased the diagnosis rate of tumors by 33%.10
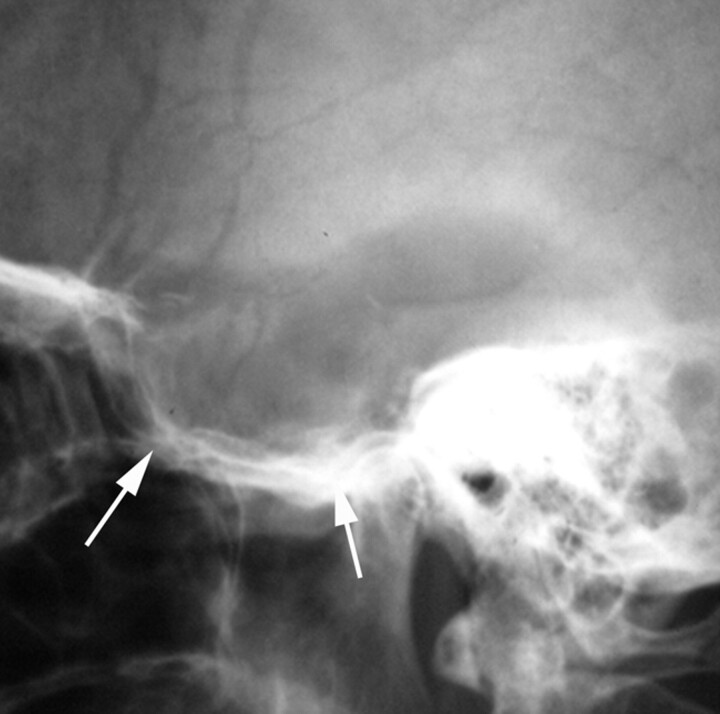
Fig 1.
Lateral skull radiograph obtained in a 36-year-old woman presenting with left-sided headache, right visual field defect, and hemiparesis shows marked enlargement and destruction of the sella and sphenoid sinuses (arrows). Subsequent surgery revealed a pituitary adenoma.
A year later, Dandy published the first description of pneumoencephalography and its use in diagnosing tumors and hydrocephalus. He discovered this somewhat fortuitously while performing ventriculography, observing that in some cases air had escaped the ventricular system into the subarachnoid space.5 Realizing that this could aid in diagnosis of disease directly or indirectly affecting the subarachnoid space, he postulated that the subarachnoid space could be visualized by directly injecting air into it via LP. Similar to ventriculography, CSF was removed by LP and replaced with an equal amount of air. However, due to concerns over complications, particularly increased intracranial pressure and herniation, he favored ventriculography.5
Around the same time, unaware of Dandy's work, a German internist, Adolf Bingel, incidentally observed air in the lateral ventricle of a patient following a LP.15,16 He too knew that air provided natural contrast on radiographs of air-filled organs. His initial experiments in cadavers and later in patients demonstrated air in the subarachnoid space and ventricles on skull radiographs following the injection of air by LP.15,16 Bingel was also concerned about the safety of the procedure and developed an instrument that assured that the amount of CSF drained equaled the amount of air injected in an attempt to keep intradural pressure constant.16 He also reported on the suboccipital technique of air introduction.16
The development of ventriculography and pneumoencephalography marked the beginning of contrast studies for evaluations of the CNS and allowed diagnosis of more intracranial pathology than skull radiographs did. These techniques initially were relatively crude with no ability to manipulate the air. The wide use and acceptance of these techniques did not occur rapidly.9 Part of the reticence in accepting these procedures was related to their invasive nature and potentially severe complications. In 1925, a colleague of Dandy reported 3 deaths in a series of 500 patients in whom ventriculography was performed, while other neurosurgeons reported mortality as high as 30%.3
Development of Cerebral Angiography
Spurred in part by dissatisfaction with ventriculography and pneumoencephalography and the development of Lipiodol (iodized oil; Andre Guerbet, Aulnay-sous-Bois, France) myelography in 1921 and cholecystography in 1924, Portuguese neurologist Egas Moniz sought a method to opacify either the brain or the cerebral arteries with a radiopaque contrast agent.3,17 After failing at directly opacifying the brain, Moniz moved on to the arteries. He experimented extensively in animals and cadavers before his first attempts in humans. Initially he attempted to inject strontium bromide into the carotid artery percutaneously but later switched to sodium iodide injected via a “cutdown” on the carotid artery. He succeeded in visualizing the intracranial ICA via x-ray in his ninth patient, publishing the results in 1927.3,18,19 There were multiple complications in these initial patients including fever, dysphagia, Horner syndrome, aphasia, and death in 1 patient due to thrombosis.19 Moniz later described ICA stenosis and thrombosis and visualization of the vertebral artery via subclavian artery injection. Moniz used thorium dioxide as the contrast agent for angiography and was the first to take serial images, 1 per second for 6 seconds, due to the development of a radiocarousel by a radiology associate.9,10 He also wrote 2 books on cerebral angiography in the 1930s.3,9 Cerebral angiography also was only slowly accepted due to its invasiveness, discomfort, and high rate of significant complications (Fig 2).18
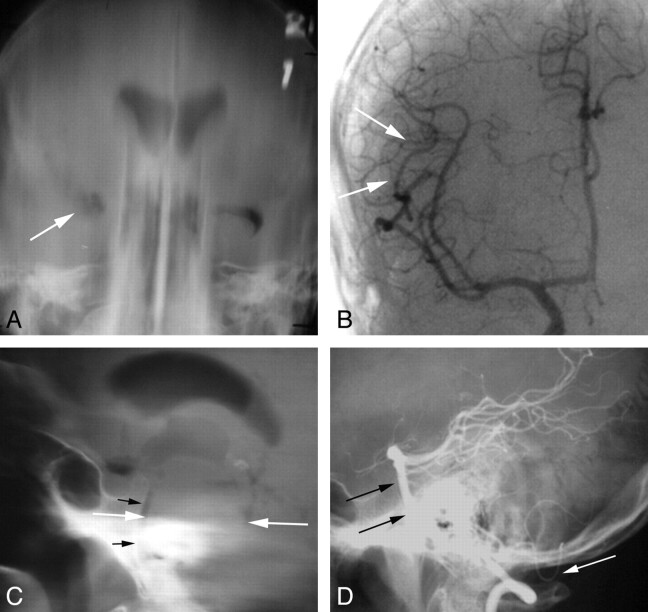
Fig 2.
Imaging of tumors with pneumoencephalography and angiography. A 48-year-old man with new-onset seizure. A, Frontal film from a pneumoencephalogram shows deformity and displacement of the right temporal horn (arrow), indicating a nonspecific mass in the anterior, basal, and lateral right temporal lobe. B, Frontal view from a right ICA angiogram shows minimal displacement of the right MCA branches in the Sylvian fissure (arrows). An infiltrating glioma was found at surgery. A 45-year-old man who presented with left-sided sensory changes of the face and body. C, Lateral film from a pneumoencephalogram shows marked enlargement of the pons (white arrows), which is nearly in contact with the clivus (black arrows). D, Lateral film from a vertebral angiogram shows anterior displacement of the basilar artery to the clivus (black arrows) and posterior inferior displacement of the posterior inferior cerebellar artery (white arrow). Findings are compatible with a brain stem lesion such as glioma, metastasis, or vascular malformation with hemorrhage. A glioma was found at surgery.
Advances in Radiography, Pnuemoencephalography, and Cerebral Angiography
Much of the initial work in what would develop into the field of neuroradiology was done by nonradiologists. As radiologists became more involved in not only interpreting studies of the CNS but also in performing the procedures, additional advances were achieved as radiologists sought to improve diagnostic methods. This change occurred first in Sweden where radiologist Erik Lysholm in collaboration with engineer Georg Schönander developed the skull table, with a rotating x-ray tube, which allowed reproducible angulation of the tube and greater precision in skull radiography than previously possible.20,21 Lysholm was the first to propose using at least 4 standard projections to adequately x-ray the skull.20,21 Additionally, he developed a method to more completely assess the ventricular system with smaller amounts of air than were typically used. With his skull table, radiographs were obtained with the patient's head in various positions, which permitted injected air to move throughout the ventricles. He published this work in the 3 volumes, Das Ventrikulogram, between 1935 and 1937.20,22–24 These developments led to improved accuracy in the diagnosis and localization of CNS lesions and improved surgical outcomes. Subsequently, radiologists from around the world went to study neuroradiology in Sweden.20
Around the same time at the Neurologic Institute of New York, Cornelius Dyke, the first full-time neuroradiologist in the United States, and neurosurgeon Leo Davidoff improved the technique of pneumoencephalography by injecting 20 mL of air in small fractions following the removal of an equal amount of CSF, obtaining a preliminary radiograph to assess ventricular size and injecting only enough additional air to obtain a satisfactory study. Similar to Lysholm, they placed the patient in various positions to move the air throughout the ventricles.25,26 Through their work, they were able to decrease the morbidity and discomfort and improve diagnostic information obtained from such procedures. This led to greater acceptance of pneumoencephalography, a decline in the use of ventriculography, and incremental knowledge of normal and abnormal pneumoencephalograms.25,27 In 1937, they published their classic text book, The Normal Encephalogram.28
The last major advance with respect to pneumoencephalography was in the early 1960s with the development of “somersaulting” chairs that rotated 360°, allowing greater movement of air with complete filling of the ventricles. Inventors of such chairs included Kurt Amplatz and Juan M. Taveras in collaboration with D. Gordon Potts.9,29,30
Occasionally, positive contrast ventriculography or cisternography was performed with a variety of contrast agents including Lipiodol, thorium dioxide, and iophendylate (Fig 3).31 These agents were quite irritating, in addition to thorium dioxide being carcinogenic and iophendylate causing severe arachnoiditis.31,32 Thus, air remained the contrast agent of choice. Positive contrast ventriculography was usually performed only if the air ventriculogram did not reveal or suboptimally delineated a lesion. Positive contrast cisternography was reserved for posterior fossa lesions, particularly those of the CPA cisterns.31,32 The organic water-soluble ionic contrast agents that were introduced in the late 1950s were not suitable for intrathecal or intraventricular injection due to severe side effects related to their hypertonicity.33,34
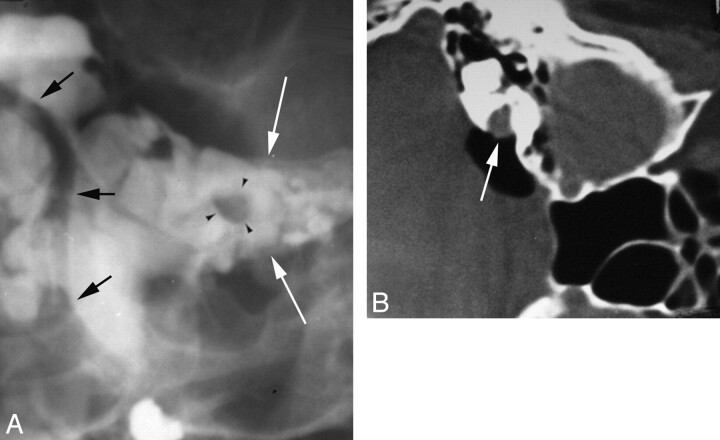
Fig 3.
Evolution of imaging for internal auditory canal CPA cistern masses. A, Frontal view from an iophendylate cisternogram shows a round filling defect (black arrowheads) within the left IAC (white arrows). The distal vertebral and basilar arteries can be seen outlined by contrast (black arrows). B, An air-CT cisternogram obtained with the patient in the decubitus position after injecting 5 mL of air via LP shows a mass in the right IAC extending into the CPA cistern (arrow).
While air-contrast ventricular studies had evolved from Dandy's day, they still remained uncomfortable procedures for the patient and, in many instances, the neuroradiologist.35,36 At best, they mainly revealed mass effect produced by intracranial lesions. There was significant morbidity with patients frequently experiencing headache, nausea, and emesis during and up to 6 hours after the procedure. Other complications included transient meningeal irritation; blood pressure changes, particularly marked elevation; intracranial hemorrhage; and brain herniation. The mortality rate was estimated to be 0.25%.37
Nearly 10 years after Moniz failed at percutaneous carotid angiography, the technique was successfully described by Julius Loman and Abraham Myerson.14,38 A variety of techniques were developed for vertebral angiography, most consisting of cutdown or percutaneous puncture of brachial, subclavian, or vertebral arteries.39 In 1953, Sven Seldinger described catheter replacement of the needle in percutaneous angiography, though this technique was not routinely used for cerebral angiography for another decade.9,40,41 With few exceptions, cerebral angiography at this time was mainly performed by neurosurgeons and neurologists. In the early 1960s, more neuroradiologists started performing and reporting on their experience with cerebral angiography by direct puncture of the common carotid, vertebral, or brachial arteries, with contrast injection directly through the needle or with placement of a catheter through the needle.39,42 A complete evaluation of the cerebral vasculature involved multiple punctures, often with the patient under general anesthesia and performed on multiple days.43 Despite the development of the Seldinger technique and the description of transfemoral catheterization of the vertebral artery by Lindgren in 1954, transfemoral cerebral angiography did not gain acceptance until the mid-1960s. Per Amundsen of Norway44 was one of the major proponents of this technique, with Hans Newton and Kurt Amplatz advocating it in the United States.20,42,45 This finally allowed visualization of all the extra- and intracranial cerebral arteries via a single femoral puncture.42
The need for rapid serial imaging during cerebral angiography helped spur the development of film changers in the 1940s and 1950s, allowing multiple films to be obtained per second.9,41 The development of power injectors allowed contrast to be injected as a rapid bolus.44 Film subtraction techniques were introduced in the early 1960s, allowing the removal of unwanted shadows, such as the skull, leaving only vessel detail.46 Magnification techniques were also introduced in the 1960s, allowing visualization of subtle changes in small vessels.47 Early catheters for cerebral angiography were quite crude. The early commercial “cerebral” catheters, while opaque, were large (7F or 8F), stiff, not easily shaped, and often lost their curves intravascularly. Smaller catheters were available but were radiolucent and had limited torque control.42 Most angiographers shaped their own catheters.42
The development of safe effective intravascular contrast agents also advanced the development and use of cerebral angiography. The sodium iodide contrast used by Moniz for the first cerebral angiograms caused significant discomfort and often produced seizures and other neurologic symptoms. Thorium dioxide was used from the 1930s to the 1950s but was abandoned when it was found to be carcinogenic.9,48 Organic iodide compounds were introduced in the early 1940s but initially were irritating when injected and not very opaque.9 In the late 1950s, diatrizoate, an organic water-soluble ionic contrast agent, was introduced. This was a significant improvement for cerebral angiography and virtually replaced other contrast agents in the United States.9 The introduction of low-osmolar nonionic contrast agents in the 1970s resulted in fewer systemic side effects and allergic reactions with less discomfort.49,50
In the 1950s and 1960s as cerebral angiography evolved, new information was added to the medical literature on vascular neuroanatomy, dynamics, and pathology.22,44 Cerebral angiography was used to investigate a wide variety of cerebral pathology, including vascular abnormalities, tumors, and posttraumatic lesions. The presence of a lesion was often identified by its mass effect on the cerebral arteries and veins, with the location and contour of the shift characteristic of lesions in certain locations. Some tumors could be identified by their vascular pattern in different phases of the arteriography, such as glioblastomas and meningiomas, as could some ischemic lesions.51–53 Cerebral angiography had advanced from the days when it left visible scars on the neck to a point where it could be done as an outpatient procedure and relatively easily repeated. However, cerebral angiography was a long procedure because multiple views were usually necessary for each vessel and each injection required processing of a series of films in a darkroom with subsequent assessment before proceeding.34 One can imagine that such a lengthy procedure could be difficult for acutely ill or injured patients to tolerate (Fig 4).
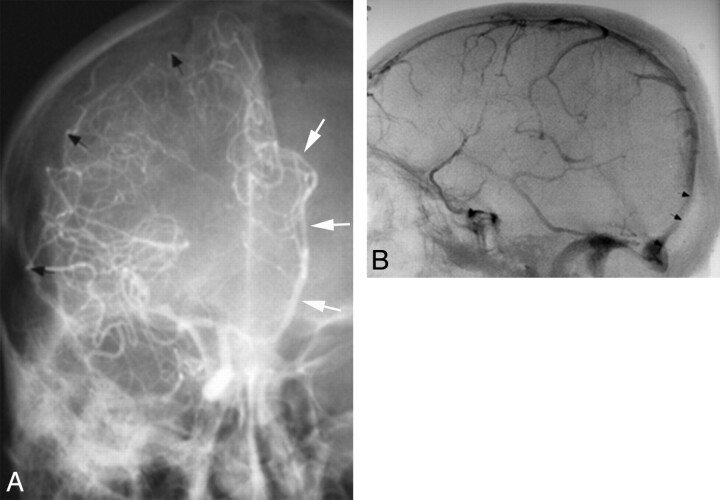
Fig 4.
Before CT and MR imaging, angiography was used to diagnose extra-axial hematomas in patients with acute head trauma. A, Frontal view from a right ICA angiogram shows displacement of right MCA branches away from the inner table of skull (black arrows) by a crescentic collection compatible with a SDH. There is also a distal-type midline shift with the anterior cerebral artery displaced across the midline (white arrows). B, Lateral angiographic image shows anterior displacement of distal superior sagittal sinus and torcular herophili (arrows), compatible with stripping of the dura from inner table of skull due to an EDH.
The Development of Cross-Sectional Imaging: CT and MR Imaging
In 1961, frustrated by the invasiveness, morbidity, and indirect imaging of the brain associated with pneumoencephalography and angiography, American neurologist William Oldendorf described and built from household products a prototype to transmit a beam of x-rays through the head and reconstruct its image.48,54 He obtained a patent for his idea but could not obtain funding for further development. One manufacturer of x-ray equipment commented, “Even if it could be made to work as you suggest, we cannot imagine a significant market for such an expensive apparatus which would do nothing but make a radiographic cross-section of a head.”55 In articles published in 1963 and 1964, physicist Alan Cormack described the mathematic algorithm of tomographic reconstruction without knowledge of Oldendorf's device.56,57 Nearly a decade later, also independently, computer engineer Godfrey Hounsfield working at EMI Laboratories in England began working on a technique to reconstruct the internal structure of a body from a number of x-ray transmission measurements resulting in development of his “backprojection” technique.6,58 Ultimately, Hounsfield and Cormack were awarded the Nobel Prize in Medicine in 1979 for their work and many think that Oldendorf should have shared in this prize.48 The first CT scanners were installed in the United States in 1973. EMI initially estimated a worldwide need for only 25 CT scanners!59
This was a monumental advance in neuroradiology, allowing direct visualization of the internal structure of the brain noninvasively. The ventricles, gray matter, white matter, and skull could be delineated from one another. Even the earliest CT scans were able to depict acute hemorrhage and calcification as areas of increased attenuation with edema, necrosis, and cystic lesions as areas of decreased attenuation. With the use of iodinated contrast material, arteries, veins, meninges, and abnormal vascularity could be visualized.45 Initially by using air and later iso-osmolar nonionic contrast agents, such as metrizamide, CT cisternography could be performed to diagnose lesions in the subarachnoid space.60,61 Results were initially displayed as a numeric printout of Hounsfield values, a cathode ray display of processed information from magnetic tape, or a Polaroid (Polaroid Corporation, Minnetonka, Minnesota) picture of the cathode ray display.48 By today's standards, the images would be unacceptable with a section thickness of 8–13 mm with 3 × 3 mm pixels, while the scanning time of 4 minutes per section with 1.5 minutes for reconstruction time seems interminable.58
Compared with neuroradiologic examinations in the pre-CT era, however, even the earliest CT scans offered savings in time, money, and danger to the patient.13 Diagnoses could be made faster and less invasively with therapy instituted sooner. For example, before CT, the best method for localization of an SDH was carotid angiography, with a mortality rate between 60% and 80% for patients with posttraumatic SDHs.62 A 2011 study on mortality associated with traumatic SDH indicated a mortality rate of between 10% and 20%.63 While clearly the decline in mortality cannot be attributed to improved imaging alone, it certainly plays a role. Within a few years of the introduction of CT, pneumoencephalography was extinct and the use of angiography decreased.35,45
The underlying basis for MR imaging, NMR, was developed in 1946 and initially used in analytic chemistry.64,65 The first study of NMR signal intensity from a living animal was published in 1968.66 Three years later Raymond Damadian demonstrated tumors in mice having T1 and T2 relaxation times different from normal tissue.67 In 1973, Paul Lauterbur published his work on creating NMR images by applying backprojection to the data.7 Two years later, the use of selective excitation methods for spatial localization of data was described.68 The first images of live humans, consisting of a finger and the chest, were published in 1977, and research on brain imaging began to be published in 1980.69–71 The first commercial MR imaging scanner was introduced in 1980, and the first superconducting magnet was put in clinical use in 1981.9,48 This technique continued to be referred to as NMR imaging until the early 1980s when it was changed to MR imaging to allay public fears over the term “nuclear.”72 The major imaging-equipment manufacturers entered the field in the mid-1980s, introducing 1.5T magnets.9,48 Gadolinium contrast agents were first suggested in the early 1980s, with FDA approval of gadolinium chelates in 1988.73
Even though the spatial resolution on the earliest images of the brain was poor, the contrast resolution was superior to that of CT.45 Imaging could be performed in multiple planes with varying pulse sequences that took advantage of different types of tissue contrast.48 Use of gadolinium contrast agents allowed detection of small lesions, such as vestibular schwannomas and pituitary microadenomas, which were not visible on CT or noncontrast MR imaging examinations.73 As MR imaging has matured, it has replaced CT as the primary technique for imaging the brain in most circumstances because it more clearly depicts normal and abnormal structures, often permitting a specific histologic diagnosis. CT is primarily used in emergency settings for rapidly imaging acutely ill or injured patients, patients in whom hemorrhage or calcified/ossified lesions are suspected, and patients with contraindications to MR imaging.45
Advances in CT and MR Imaging
CT and MR imaging have continued to evolve during the past 20 years. Advances in computer technology have resulted in improved spatial resolution and faster imaging in CT. Additionally, development of multidetector CT scanners in the late 1990s has resulted in the development of new CT applications.74 Submillimeter section thickness and 3D imaging with nearly isotropic voxels has become a reality. Scanning time has been significantly reduced, permitting dynamic scanning. In neuroradiology, these advances have been applied in the development of CTA and CT perfusion.75,76 The 1990s saw the introduction of more rapid MR imaging techniques such as fast spin-echo and gradient-echo sequences. Spatial resolution improved and 3D imaging with MR imaging became possible.48 MRA, FLAIR, DWI, DTI, MR spectroscopy, fMRI, and perfusion MR imaging were all introduced.77–82 More recently, 3T scanners have been introduced into routine clinical work.83 With perfusion imaging, DWI, DTI, MR spectroscopy, and fMRI techniques, brain imaging has become not only morphologic but physiologic. These techniques have brought dramatic changes to the diagnosis and subsequent treatment of patients with a variety of brain lesions compared with the days of ventriculography, pneumoencephalography, and angiography.
Conclusions
Without looking back at history, particularly for those trained in the era of cross-sectional imaging, it is difficult to appreciate the impact advances in neuroimaging have had on patient care. We have progressed from looking at gross changes on skull radiographs caused by generally advanced disease, to painful invasive tests, which, for the most part, only indirectly imaged brain lesions, to cross-sectional imaging, which not only shows anatomy and pathology in exquisite details but also gives physiologic information. It is impossible to know (but exciting to contemplate) what developments will take place in neuroradiology in the next 100 years. Hopefully, progress will continue to lead to less invasive, safer, faster, and more specific diagnostic techniques, resulting in even earlier diagnosis and treatment with a continuing positive impact on patient outcome.
ABBREVIATIONS:
- CPA
cerebellopontine angle
- EDH
epidural hematoma
- LP
lumbar puncture
- NMR
nuclear magnetic resonance
- SDH
subdural hematoma
References
- 1. Church A. Cerebellar tumor: recognized clinically, demonstrated by x-ray—proved at autopsy. Am J Med Science 1899;177:125–30 [Google Scholar]
- 2. Pfahler GE. Cerebral skiagraphy: transactions of the American Roentgen Ray Society. In: Proceedings of the 5th Annual Meeting of the American Roentgen Ray Society, September 9, 10, 12, 13. St. Louis, Missouri. 1904;4:175–83 [Google Scholar]
- 3. Bull JW. The history of neuroradiology. Proc Roy Soc Med 1970;63:637–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dandy WE. Ventriculography following the injection of air into the cerebral ventricles. Ann Surg 1918;68:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dandy WE. Röntgenography of brain after the injection of air into the spinal canal. Ann Surg 1919;70:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hounsfield GN. Computerized transverse axial scanning (tomography). Part 1. Description of system. Br J Radiol 1973;46:1016–22 [DOI] [PubMed] [Google Scholar]
- 7. Lauterbur PC. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature 1973;242:190–91 [PubMed] [Google Scholar]
- 8. Schüller A. Röentgen-diagnostik der erkrankugen des kopfas. Vienna: Holder; 1912 [Google Scholar]
- 9. Taveras JM. Neuroradiology: past, present and future. Radiology 1990;175:593–602 [DOI] [PubMed] [Google Scholar]
- 10. Alper MG. Three pioneers in the early history of neuroradiology: the Snyder lecture. Doc Ophthalmol 1999;98:29–49 [DOI] [PubMed] [Google Scholar]
- 11. Schindler E. Arthur Schüller: pioneer of neuroradiology. AJNR Am J Neuroradiol 1997;18:1297–302 [PMC free article] [PubMed] [Google Scholar]
- 12. Heuer GJ, Dandy WE. Roentgenography in the localization of brain tumor, based on a series of one hundred consecutive cases. Bull Johns Hopkins Hosp 1916;27:311–22 [Google Scholar]
- 13. Bull J. The changing face of neuroradiology over nearly 40 years. Neuroradiology 1975;9:111–15 [DOI] [PubMed] [Google Scholar]
- 14. Wolpert SM. On the radiologic diagnosis of cerebral aneurysm with plain films and angiography: a historical survey. AJNR Am J Neuroradiol 1995;16:181–84 [PMC free article] [PubMed] [Google Scholar]
- 15. Bingel A. Encephalographie eine method zur röntgenographischen darstellung des gehims. Chen darstellung des gehirns. Fortschr Rontgenstr Nuklearrned Erganzungsband 1921;28:205–17 [Google Scholar]
- 16. Müller J, Hermes M, Piepgras U. Adolf Bingel, the second inventor of lumbar pneumoencephalography. AJNR Am J Neuroradiol 1995;16:487–90 [PMC free article] [PubMed] [Google Scholar]
- 17. Doby T. Cerebral angiography and Egas Moniz. AJR Am J Roentgenol 1992;159:364. [DOI] [PubMed] [Google Scholar]
- 18. Wolpert SM. Neuroradiology classics. AJNR Am J Neuroradiol 1999;20:1752–53 [PMC free article] [PubMed] [Google Scholar]
- 19. Moniz E. L'encephalographie arterielle, son importance dans la localization des tumeurs cerebrales. Rev Neurol (Paris) 1927;2:72–90 [Google Scholar]
- 20. Lindgren E, Greitz T. The Stockholm school of neuroradiology. AJNR Am J Neuroradiol 1995;16:351–60 [PMC free article] [PubMed] [Google Scholar]
- 21. Lysholm E. Apparatus and technique for roentgen examination of the skull. Stockholm: P.A. Norstedt & Söner; 1931 [Google Scholar]
- 22. Lysholm E, Ebenius B, Sahlstedt H. Das Ventrikulogramm. I Teil. Röntgentechnik. Stockholm: P.A. Norstedt & Söner; 1935 [Google Scholar]
- 23. Lysholm E, Ebenius B, Lindblom K, et al. Das Ventrikulogramm. II Teil. Dritter und vierter Ventrikel. Stockholm: P.A. Norstedt & Söner; 1937 [Google Scholar]
- 24. Lysholm E, Ebenius B, Sahlstedt H. Das Ventrikulogramm. III Teil. Die Seitenventrikeln. Stockholm: P.A. Norstedt & Söner; 1935 [Google Scholar]
- 25. Kieffer SA. Cornelius G. Dyke and the Neurological Institute of New York: the foundations of American neuroradiology. AJNR Am J Neuroradiol 1997;18:801–09 [PMC free article] [PubMed] [Google Scholar]
- 26. Davidoff LM, Dyke CG. An improved method of encephalography. Bull Neurol Inst N Y 1932;2:75–94 [Google Scholar]
- 27. Davidoff LM. Cornelius Gysbert Dyke: pioneer neuroradiologist. Bull N Y Acad Med 1969;45:665–80 [PMC free article] [PubMed] [Google Scholar]
- 28. Davidoff LM, Dyke CG. The Normal Encephalogram. Philadelphia: Lea; 1937 [Google Scholar]
- 29. Amplatz K. An improved chair for pneumoencephalography and autotomography. AJR Am J Roentgenol 1963;90:184–88 [PubMed] [Google Scholar]
- 30. Potts DG, Taveras JM. A new somersaulting chair for pneumoencephalography. AJR Am J Roentgenol 1964;91:1144–49 [PubMed] [Google Scholar]
- 31. Suqueira EB, Bucy PC, Cannon AH. Positive contrast ventriculography, cisternography and myelography. AJR Am J Roentgenol 1968;104:132–38 [DOI] [PubMed] [Google Scholar]
- 32. Wilson M, Snodgrass SR. Positive contrast ventriculography. Radiology 1959;72:810–15 [DOI] [PubMed] [Google Scholar]
- 33. Campbell RL, Campbell JA, Heimburger RF, et al. Ventriculography and myelography with absorbable radiopaque medium. Radiology 1964;82:286–89 [DOI] [PubMed] [Google Scholar]
- 34. Martin CM. Myelography with sodium diatrizoate (Hypaque). Calif Med 1971;115:57–59 [PMC free article] [PubMed] [Google Scholar]
- 35. Huckman MS. Neuroradiology without benefit of computers: a memoir. AJNR Am J Neuroradiol 2010;31:783–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lindgren E. Some aspects on the technique of encephalography. Acta Radiol 1949;31:161–77 [DOI] [PubMed] [Google Scholar]
- 37. Taveras JM, Wood EH. Diagnostic Neuroradiology. Baltimore: The Williams and Wilkins Company; 1964:1.248–1.262 [Google Scholar]
- 38. Loman J, Myerson A. Visualization of the cerebral vessels by direct intracarotid injection of thorium dioxide (Thoratrast). AJR Am J Roentgenol 1936;35:188–93 [Google Scholar]
- 39. Schechter MM, de Gutierrez-Mahoney CG. The evolution of vertebral angiography. Neuroradiology 1973;5:157–64 [DOI] [PubMed] [Google Scholar]
- 40. Seldinger SI. Catheter replacement of the needle in percutaneous arteriography a new technique. Acta Radiol 1953;39:368–76 [DOI] [PubMed] [Google Scholar]
- 41. Greitz T. The history of Swedish neuroradiology. Acta Radiol 1996;37:455–71 [DOI] [PubMed] [Google Scholar]
- 42. Rosenbaum AR, Eldevik OP, Mani JR, et al. In re: Amundsen P—Cerebral angiography via the femoral artery with particular reference to cerebrovascular disease. Acta Neurol Scand 1967; Suppl. 31:115. AJNR Am J Neuroradiol 2001;22:585–89 [PMC free article] [PubMed] [Google Scholar]
- 43. Hinck VC, Judkins MP, Paxton HD. Simplified selective femorocerebral angiography. Radiology 1967;89:1048–52 [DOI] [PubMed] [Google Scholar]
- 44. Leeds NE, Kieffer SA. Evolution of diagnostic neuroradiology from 1904 to 1999. Radiology 2000;217:309–18 [DOI] [PubMed] [Google Scholar]
- 45. Amundson P, Dugstad G, Noyes W. Cerebral angiography via the femoral artery with reference to cerebrovascular disease. Acta Neurol Scand 1967;22:115. [DOI] [PubMed] [Google Scholar]
- 46. Ziedes Des Plantes BG. Application of the roentgenograpic subtraction method in neuroradiology. Acta Radiol 1963;1:961–66 [PubMed] [Google Scholar]
- 47. Leeds NE, Isard HJ, Goldberg, et al. Serial magnification cerebral angiography. Radiology 1968;90:1171–75 [DOI] [PubMed] [Google Scholar]
- 48. Kim PE, Zee CS. Imaging of the cerebrum. Neurosurgery 2007;61(1 suppl):123–46 [DOI] [PubMed] [Google Scholar]
- 49. Wolf GL, Arenson RL, Cross AP. A prospective trial of ionic vs nonionic contrast agents in routine clinical practice: comparison of adverse effects. AJR Am J Roentgenol 1989;152:939–44 [DOI] [PubMed] [Google Scholar]
- 50. Osborn AG. Diagnostic Cerebral Angiography. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 1999:421–45 [Google Scholar]
- 51. Aaron JO, Taveras JM. The angiographic localization of supratentorial masses. In: Taveras JM, Ferrucci JT. eds. Radiology Diagnosis-Imaging and Intervention. Vol 3. Philadelphia: Lippincott; 1989:1–9 [Google Scholar]
- 52. Elias DA, Weinberg PE. Vascular stains in intracranial tumors. In: Taveras JM, Ferrucci JT. eds. Radiology Diagnosis-Imaging and Intervention. Vol 3. Philadelphia: Lippincott; 1989:1–15 [Google Scholar]
- 53. Leeds NE, Goldberg HI. Abnormal vascular patterns in benign intracranial lesions: pseudotumors of the brain. AJR Am J Roentgenol 1973;118:576–85 [DOI] [PubMed] [Google Scholar]
- 54. Oldendorf WH. Isolated flying spot detection of radiodensity discontinuities: displaying the internal structural patterns of a complex object. Ire Trans Biomed Electron 1961;8:68–72 [DOI] [PubMed] [Google Scholar]
- 55. Oldendorf WH. The Quest for an Image of the Brain. New York: Raven Press; 1980 [Google Scholar]
- 56. Cormack AM. Representation of a function by its line integrals, with some radiological applications. J Appl Physics 1963;34:2722–27 [Google Scholar]
- 57. Cormack AM. Representation of a function by its line integrals, with some radiological applications. II. J Appl Physics 1964;35:2908–13 [Google Scholar]
- 58. Ambrose J. Computerized transverse axial scanning (tomography). Part 2. Clinical application. Br J Radiol 1973;46:1023–47 [DOI] [PubMed] [Google Scholar]
- 59. The Beatles: CT scanning. Beatle Tracks Band. http://blog.beatletracksband.com/2008/03/10/the-beatles-great-scientic-contribution.aspx. Accessed September 14, 2011
- 60. Kricheff II, Pinto RS, Bergeron RT, et al. Air-CT cisternography and canalography for small acoustic neuromas. AJNR Am J Neuroradiol 1980;1:57–63 [PMC free article] [PubMed] [Google Scholar]
- 61. Zimmerman RA. Past, present and future of pediatric neuroradiology. Childs Nerv Syst 1999;15:624–34 [DOI] [PubMed] [Google Scholar]
- 62. Javid M. Current concepts head injuries. N Engl J Med 1974;291:890–92 [DOI] [PubMed] [Google Scholar]
- 63. Kalanithi P, Schubert RD, Shivanand PL, et al. Hospital costs, incidence, and inhospital mortality rates of traumatic subdural hematoma in the United States. J Neurosurg 2011;115:1013–18. Epub 2011 Aug 5 [DOI] [PubMed] [Google Scholar]
- 64. Bloch F, Hanson WW, Packard M. Nuclear induction. Phys Rev 1946;69:127 [Google Scholar]
- 65. Purcell EM, Torrey HC, Pound RV. Resonance absorption by nuclear magnetic moments in a solid. Phys Rev 1946;69:37–38 [Google Scholar]
- 66. Jackson JA, Langham WH. Whole-body NMR spectrometer. Rev Sci Instrum 1968;39:510–13 [DOI] [PubMed] [Google Scholar]
- 67. Damadian R. Tumor detection by nuclear magnetic resonance. Science 1971;171:1151–53 [DOI] [PubMed] [Google Scholar]
- 68. Kumar A, Welti D, Ernst RR. NMR Fourier zeugmatography. J Magn Res 1975;18:69–83 [DOI] [PubMed] [Google Scholar]
- 69. Goldsmith M, Damadian R, Stanford M, et al. NMR in cancer. XVIII. A superconductive NMR magnet for a human sample. Physiol Chem Phys 1977;9:105–08 [PubMed] [Google Scholar]
- 70. Mansfield P, Maudsley AA. Medical imaging by NMR. Br J Radiol 1977;50:188–94 [DOI] [PubMed] [Google Scholar]
- 71. Holland GN, Moore WS, Hawkes RC. Nuclear magnetic resonance tomography of the brain. J Comput Assist Tomogr 1980;4:1–3 [DOI] [PubMed] [Google Scholar]
- 72. Young IR. Significant events in the development of MRI. J Magn Reson 2004;20:183–86 [DOI] [PubMed] [Google Scholar]
- 73. Runge VM, Carollo BR, Wolf CR, et al. Gd DTPA: a review of clinical indications in central nervous system magnetic resonance imaging. Radiographics 1989;9:929–58 [DOI] [PubMed] [Google Scholar]
- 74. Hu H, He HD, Foley WD, et al. Four multidetector-row helical CT: image quality and volume coverage speed. Radiology 2000;215:55–62 [DOI] [PubMed] [Google Scholar]
- 75. Rubin GD, Shiau MC, Leung AN, et al. Aorta and iliac arteries: single versus multi detector-row helical CT angiography. Radiology 2000;215:670–76 [DOI] [PubMed] [Google Scholar]
- 76. Koenig M, Klotz E, Luka B, et al. Perfusion CT of the brain: diagnostic approach for early detection of ischemic stroke. Radiology 1998;209:85–93 [DOI] [PubMed] [Google Scholar]
- 77. Masaryk TJ, Modic MT, Ross JS, et al. Intracranial circulation: preliminary clinical experience with 3-dimensional volume MR angiography. Radiology 1989;171:793–99 [DOI] [PubMed] [Google Scholar]
- 78. De Coene B, Hajnal JV, Gatehouse P, et al. MR of the brain using fluid-attenuated inversion recovery (FLAIR) pulse sequences. AJNR Am J Neuroradiol 1992;13:1555–64 [PMC free article] [PubMed] [Google Scholar]
- 79. Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996;199:391–401 [DOI] [PubMed] [Google Scholar]
- 80. Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001;13:534–46 [DOI] [PubMed] [Google Scholar]
- 81. Sauter R, Loeffler W, Bruhn H, et al. The human brain: localized H-1 MR spectroscopy at 1.0 T. Radiology 1990;176:221–24 [DOI] [PubMed] [Google Scholar]
- 82. Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A 1992;89:5951–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lu H, Nagae-Poetscher LM, Golay X, et al. Routine clinical brain MRI sequences for use at 3.0 Tesla. J Magn Reson Imaging 2005;22:13–22 [DOI] [PubMed] [Google Scholar]