Abstract
BACKGROUND AND PURPOSE:
ILS is a rare lesion that has a different management from the more common “acoustic” schwannoma. To date, only 137 cases have been reported. We present a classification scheme based on labyrinthine anatomy to describe and localize these lesions. Treatment and prognosis hinge on the appropriate localization of these tumors; thus, a concise terminology that can be used by both the otolaryngologist and radiology communities is desirable.
MATERIALS AND METHODS:
After approval of the institutional review board, a retrospective study of all patients with the diagnosis of ILS imaged between 1996 and 2010 was performed. Clinical and imaging data were collected. Patients were imaged with thin-section high-resolution T2 and contrast-enhanced MR imaging.
RESULTS:
There were 45 patients with a diagnosis of ILS. Forty-three had complete histories. There were 18 male and 25 female patients with an age range of 21–78 years with a mean age of 53 years. The most common presenting symptom was progressive sensorineural hearing loss. Lesions were characterized on the basis of their location. Intracochlear was most common (14/45) followed by transmodiolar (13/45), intravestibular (7/45), vestibulocochlear (5/45), transmacular (4/45), and transotic (2/45). Sixteen patients underwent surgical resection. The remaining patients were followed clinically and by serial MR imaging.
CONCLUSIONS:
ILS is an uncommon but under-reported tumor. We characterized the MR imaging appearance of these tumors by using high-resolution techniques. In addition, an anatomically based classification system is presented that will help the radiologist accurately describe ILS within the inner ear and help the surgeon determine which tumors are potential surgical candidates.
Schwannoma is a benign neoplasm of the nerve sheath and is the most common neoplasm of the IAC and CPA. “Acoustic” schwannomas most often arise from the vestibular division of the vestibulocochlear nerve. These tumors arise from the perineural Schwann cells surrounding the vestibular and cochlear nerves. This tumor most commonly originates near the vestibular ganglion at the junction of the central and peripheral myelin near the fundus of the IAC at the Schwann cell−glial junction but can be found anywhere along the nerve from the IAC to the terminal ends of the eighth cranial nerve within the vestibule, cochlea, or semicircular canals.1–19
ILSs are defined as tumors arising primarily from within the membranous labyrinth: cochlea, vestibule, or semicircular canals.
Treatment of patients with simple IAC schwannomas involves surgical resection with a goal of preserving hearing and facial nerve function. Classically, these schwannomas are treated surgically by using 1 of 3 approaches (translabyrinthine, middle fossa, or retrosigmoid/suboccipital), depending on the size and location of the mass as well as the level of hearing.1,20–23 When schwannomas involve the inner ear, surgical approaches and prognostic implications are affected. Hearing preservation surgery is not an option when a lesion extends into the labyrinth because removing a tumor from the labyrinth would be expected to result in profound sensorineural hearing loss.1 Thus, proper anatomic localization by the radiologist is essential in the preoperative assessment of these patients.
Primary ILS in the past has been considered a rare lesion in isolation. To date, there have been 137 reported cases of ILS.1–30 However, these tumors are likely much more common than previously thought. Improved imaging allows detection and characterization and stresses the needs for a heightened sense of awareness among radiologists to their presence.
The objectives of our study were to define the radiologic features of ILS and present a classification scheme to describe and localize these lesions. The system uses anatomy as the basic tenet and defines the lesions by their location. Treatment and prognosis hinge on the appropriate localization of these tumors; thus, a concise terminology that can be used by both the otolaryngology and radiology communities is desirable.
Materials and Methods
After approval of the institutional review board, we retrospectively reviewed all patients with the diagnosis of vestibular schwannoma from the University of Utah, imaged between 1996 and 2010. A review of 645 patients with the diagnosis of acoustic neuroma was examined to identify patients with tumors that primarily involve the labyrinth (cochlea, vestibule, and semicircular canals). Patients with known neurofibromatosis type 2 were excluded because the pathophysiology of these patients is different. Additionally, these patients are known to have tumors that often invade the cochlea. Forty-five patients with schwannomas with their major bulk and center within the labyrinth (cochlea, vestibule, and/or semicircular canals) based on MR imaging were included.
All images were reviewed by 2 neuroradiologists (K.L.S., H.R.H.) with Certificates of Added Qualification and a board-certified neuroradiology fellow (A.M.C.) from the University of Utah. Medical records were reviewed, and demographic data were collected, including age, sex, and date of presentation. In addition, clinical data including presenting symptoms, treatment, and length of follow-up were included.
Patients were imaged on a 1.5T magnet. Forty-two patients were imaged with high-resolution FSE T2-weighted imaging. Thirty-eight patients were scanned with phased array surface coils. Thin-section T2-weighted images were obtained by using either a 2D acquisition (4000 ms/102 ms/6 [TR/TE/NEX] with 2-mm section thickness and a 1-mm intersection gap; echo-train length, 32) or a 3D acquisition (4000 ms/130 ms/1 [TR/TE/NEX] with an 0.8-mm section thickness and a 0-mm intersection gap and an echo time of 64). Four patients were scanned with the head coil by using a 3D coronal acquisition (750 ms/115 ms/1 [TR/TE/NEX] with a 1.0-mm section thickness and a 0-mm intersection gap and an FOV of 180). The axial imaging of these 3 patients was performed by using a 3D-constructive interference in steady state acquisition (6.8 ms/3.4 ms/1/80° [TR/TE/NEX/flip angle] with a section thickness of 0.8 mm). If there was replacement of the normal high signal intensity in the labyrinth of the IAC on T2-weighted imaging, the patient underwent additional MR imaging, including pre- and postcontrast sequences with gadolinium to look for an enhancing mass. The parameters included T1-weighted imaging (800 ms/72 ms/2 [TR/TE/NEX]; 3-mm sections with a 0-mm intersection gap). Three of the patients had contrast-enhanced imaging only.
“Intracochlear” ILS is defined as a tumor confined to the turns in the cochlea. “Intravestibular” ILS is defined as a tumor confined to the vestibule with or without extension into the semicircular canals. “Vestibulocochlear” tumors are those that fill the cochlea and vestibule without extension into the middle ear or IAC. “Transmodiolar” ILS is defined as a tumor that extends through the modiolus from the cochlea into the IAC via the cochlear nerve canal. “Transmacular” ILS is defined as a tumor that extends through the macula cribrosa from the vestibule into the IAC. Finally the term “transotic” is used when the tumor extends through the labyrinth into the IAC and middle ear.
Results
Clinical Data
There were 45 patients with a diagnosis of ILS. Forty-three patients had complete histories available. There were 18 male and 25 female patients with an age range of 21–78 years and a mean age of 53 years. Twenty-three tumors were on the right and 22 were on the left.
The most common presenting symptom was hearing loss with all patients (45/45) being affected. The range of symptom duration was 1–252 months with an average of 40 months. Most hearing loss in documented cases was progressive (27/45) with a smaller number presenting with sudden hearing loss (14/45) and 4 patients, with fluctuating hearing loss.
The second most common symptom was tinnitus with 23 patients affected, followed by imbalance (16 patients) and vertigo (10 patients). Aural fullness was seen in 1 patient (Table 1).
Table 1:
Presenting symptoms and location of tumor
| Symptom | No. of Patients | Locationa (No.) |
|---|---|---|
| Hearing loss | 45 | Intracochlear (14) |
| Transmodiolar (13) | ||
| Intravestibular (7) | ||
| Transmacular (4) | ||
| Vestibulocochlear (5) | ||
| Transotic (2) | ||
| Tinnitus | 23 | Intracochlear (8) |
| Transmodiolar (10) | ||
| Intravestibular (4) | ||
| Transmacular (1) | ||
| Vestibulocochlear (0) | ||
| Transotic (0) | ||
| Imbalance | 16 | Intracochlear (6) |
| Transmodiolar (4) | ||
| Intravestibular (3) | ||
| Transmacular (2) | ||
| Vestibulocochlear (0) | ||
| Transotic (1) | ||
| Vertigo | 10 | Intracochlear (3) |
| Transmodiolar (3) | ||
| Intravestibular (1) | ||
| Transmacular (2) | ||
| Vestibulocochlear (0) | ||
| Transotic (1) | ||
| Aural fullness | 1 | Intracochlear (0) |
| Transmodiolar (1) | ||
| Intravestibular (0) | ||
| Transmacular (0) | ||
| Vestibulocochlear (0) | ||
| Transotic (0) |
Patients often presented with multiple symptoms.
Imaging Data
MR imaging was performed in all 45 patients. Forty-two patients underwent high-resolution thin-section T2-weighted scanning. Forty-five patients underwent pre- and postcontrast enhanced T1-weighted imaging.
On T2-weighted images, these lesions appear as focal filling defects with replacement of the normal high-signal-intensity fluid (42/42). On postgadolinium imaging, they appear as focal homogeneously enhancing masses (45/45). The contrast enhancement in the patients who had both T2 and enhanced imaging corresponded to the T2 abnormality. The intracochlear schwannoma occurs within the turns of the cochlea (Fig 1). The intravestibular tumor occurs within the vestibule with or without extension into the semicircular canals (Fig 2). The vestibulocochlear tumor occurs within the vestibule and cochlea without extension into the IAC or middle ear (Fig 3). The transmacular tumor occurs within the vestibule, with the major portion within the vestibule with additional extension through the macula cribrosa into the IAC (Fig 4). The transmodiolar tumor occurs within the cochlea, with the major portion within the cochlea and extension through the modiolus into the IAC (Fig 5). The transotic tumor appears as a tumor that extends through the labyrinth into the IAC and middle ear (Fig 6). Tumors in our series ranged from 2 to 55 mm. The site of tumor was characterized on the basis of location as detailed in Table 2. Intracochlear was the most common location (14/45), followed by transmodiolar (13/45), intravestibular (7/45), vestibulocochlear (5/45), transmacular (4/45), and transotic (2/45).
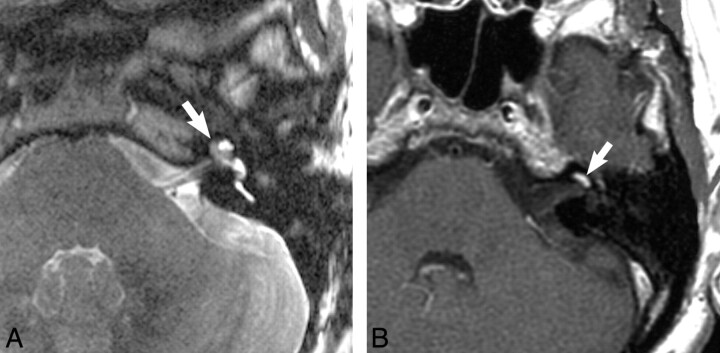
Fig 1.
Intracochlear schwannoma. A, Axial, high-resolution FSE T2-weighted MR image (4000 ms/102 ms/6 [TR/TE/NEX]) at the level of the cochlea reveals a hypointense filling defect within the cochlea (arrow), replacing the normal hyperintense CSF signal intensity, representing the intracochlear schwannoma. B, Axial enhanced T1-weighted MR image (800 ms/72 ms/2 [TR/TE/NEX]) at the level of the cochlea reveals a homogeneously enhancing mass in the basal turn of the cochlea (arrow), representing the intracochlear schwannoma.
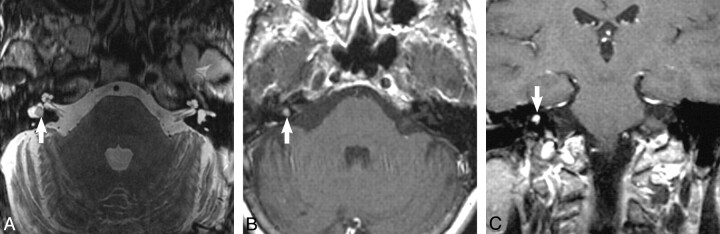
Fig 2.
Intravestibular schwannoma. A, Axial high-resolution FSE T2-weighted MR image (4000 ms/102 ms/6 [TR/TE/NEX]) at the level of the vestibule reveals a hypointense filling defect within the vestibule (arrow) replacing the normal hyperintense CSF signal intensity, representing the intravestibular schwannoma. B, Axial enhanced T1-weighted MR image (800 ms/72 ms/2 [TR/TE/NEX]) at the level of the vestibule reveals a homogeneously enhancing mass (arrow) confined to the vestibule, which corresponds to the T2 abnormality. C, Coronal enhanced T1-weighted MR image (800 ms/72 ms/2 [TR/TE/NEX]) at the level of the vestibule reveals a homogeneously enhancing mass (arrow) confined to the vestibule, representing the intravestibular schwannoma.
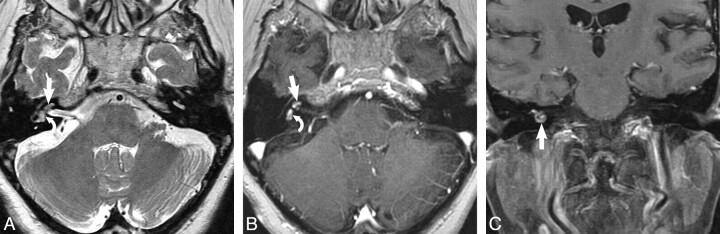
Fig 3.
Vestibulocochlear schwannoma. A, Axial high-resolution FSE T2-weighted MR image (4000 ms/102 ms/6 [TR/TE/NEX]) at the level of the cochlea and vestibule reveals a hypointense filling defect within the cochlea (arrow) and vestibule (curved arrow), replacing the normal hyperintense CSF signal intensity, representing the vestibulocochlear schwannoma. B, Axial enhanced T1-weighted MR image (800 ms/72 ms/2 [TR/TE/NEX]) at the level of the cochlea and vestibule reveals a homogeneously enhancing mass in the cochlea (arrow) and vestibule (curved arrow), which corresponds to the T2 abnormality, representing the vestibulocochlear schwannoma. C, Coronal enhanced T1-weighted MR image (800 ms/72 ms/2 [TR/TE/NEX]) at the level of the cochlea and vestibule reveals a homogeneously enhancing mass nearly filling the cochlea (arrow), representing the vestibulocochlear schwannoma.
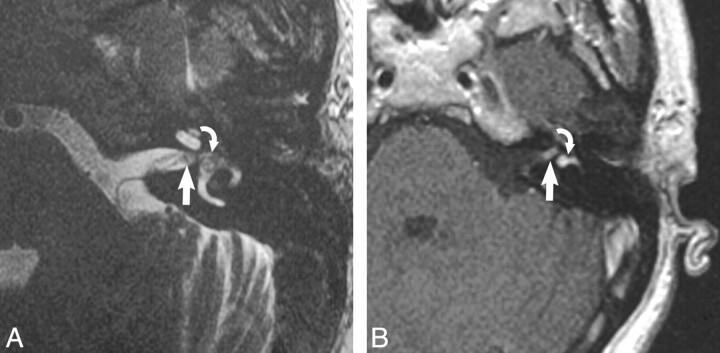
Fig 4.
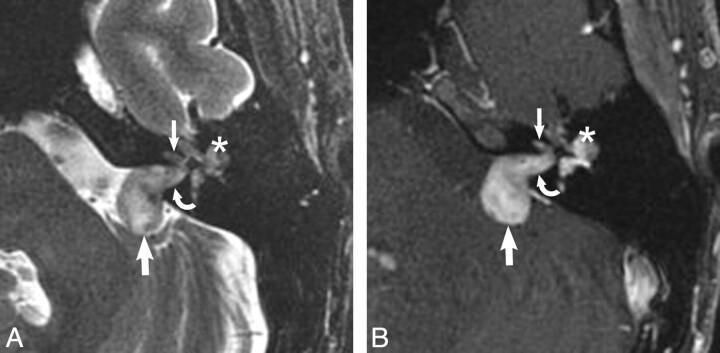
Transmacular schwannoma. A, Axial high-resolution FSE T2-weighted MR image (4000 ms/102 ms/6 [TR/TE/NEX]) at the level of the cochlea and vestibule reveals a hypointense filling defect within the IAC (arrow) and vestibule (curved arrow), replacing the normal hyperintense CSF signal intensity, representing the transmacular schwannoma. B, Axial enhanced T1-weighted MR image (800 ms/72 ms /2 [TR/TE/NEX]) at the level of the IAC reveals a homogeneously enhancing mass in the vestibule (curved arrow) with extension through the macular cribrosa into the IAC fundus (arrow), representing the transmacular schwannoma.
Fig 5.
Transmodiolar schwannoma. A, Axial high-resolution FSE T2-weighted MR image (4000 ms/102 ms/6 [TR/TE/NEX]) at the level of the cochlea reveals a hypointense filling defect within the cochlea (arrow), replacing the normal hyperintense CSF signal intensity, representing the transmodiolar schwannoma. Note the extension through the modiolus and cochlear nerve canal (curved arrow) into the IAC. B, Axial enhanced T1-weighted MR image (800 ms/72 ms/2 [TR/TE/NEX]) at the level of the cochlea reveals a homogeneously enhancing mass within the cochlea (arrow) with extension through the modiolus and cochlear nerve canal to involve the IAC fundus (curved arrow). C, Coronal enhanced T1-weighted MR image (800 ms/72 ms/2 [TR/TE/NEX]) at the level of the cochlea reveals a homogeneously enhancing mass in the turns of the cochlea (arrow), representing the transmodiolar schwannoma.
Fig 6.
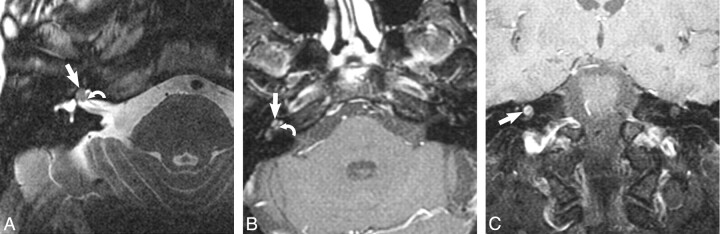
Transotic schwannoma. A, Axial high-resolution FSE T2-weighted MR image (4000 ms/102 ms/6 [TR/TE/NEX]) at the level of the IAC reveals a hypointense filling defect within the IAC (curved arrow), replacing the normal hyperintense CSF signal intensity, representing the very rare transotic schwannoma. There is involvement of the CPA (large arrow), labyrinth (small arrow), and middle ear cavity (asterisk). B, Axial enhanced T1-weighted MR image (800 ms/72 ms/2 [TR/TE/NEX]) at the level of the IAC reveals an avidly enhancing mass within the CPA (large arrow) and IAC (curved arrow), representing the transotic schwannoma. Note the involvement of the labyrinth (small arrow) and middle ear cavity (asterisk).
Table 2:
Classification of ILS
| Class (No.) | Definition |
|---|---|
| Intracochlear (14) | Tumor confined to the cochlea |
| Transmodiolar (13) | Tumor centered in the cochlea with extension through the modiolus into the IAC |
| Intravestibular (7) | Tumor centered in vestibule (± semicircular canal involvement) |
| Transmacular (4) | Tumor centered in the vestibule with extension into the IAC via the macula cribrosa |
| Vestibulocochlear (5) | Tumor within the vestibule and cochlea |
| Transotic (2) | Tumor within the labyrinth with extension into the IAC and middle ear |
Sixteen patients underwent surgical intervention. All had benign ILS found at surgery. Ten patients underwent surgery for increasing size; 3, for intractable vertigo; 2, for extension into the CPA; and 1 tumor was removed during cochlear implant surgery. The approach was transotic in 7, transcochlear in 5, and translabyrinthine in 3, and the final surgery was for cochlear implant. The remaining patients were followed clinically and with imaging with the presumed diagnosis of ILS based on characteristic imaging features and stability. Surgery is only indicated for patients with intractable vertigo, extension of tumor to the CPA, or evidence of tumor growth.
Follow-up ranged from 0 to 168 months. Annual MR imaging was performed as the standard of care with biannual follow-up in patients whose first postdiagnosis scan was unchanged. To date, 2 patients had small interval growth in their tumors and continue to be observed. One additional patient was recommended for surgical resection on the basis of increasing tumor size and progressive symptoms. The remaining patients are stable or have been lost to follow-up.
Discussion
ILS is an under-reported cause of sensorineural hearing loss. This slow-growing tumor is often diagnosed many years after the initial report of mild unilateral sensorineural hearing loss. Once diagnosed, it frequently does not undergo surgery because the operation inevitably results in complete hearing loss in the affected ear. Instead, surgery is reserved for those patients with intractable vertigo or evidence of tumor leaving the inner ear to enter the IAC (transmacular, transmodiolar, or transotic lesions). The proposed anatomy-based nomenclature is meant to help the radiologist accurately describe the ILS within the inner ear and indicate which tumors are potential surgical candidates.
Higher field magnets with refined MR imaging sequences have increased the sensitivity of the detection of ILS. However, previous reports suggesting that this is a very rare lesion are also the consequence of lack of awareness of the radiologist as to the existence of ILS. On the basis of this series and others, it is now possible to suggest that this tumor occurs a good deal more frequently than previously realized.1–30 The prevalence in a recent series of 52 cases of ILS was 10%, much larger than previously reported.30 Our series was collected in a large academic center with a large otologic referral area, which may reflect a selection bias. To make this diagnosis however, the radiologist will have to inspect not just the CPA and IAC but also the inner ear on all examinations completed for sensorineural hearing loss. The key to its detection lies in the appropriate MR imaging protocol as well as a heightened sense of awareness of the interpreting radiologist. Given the advances in high-resolution MR imaging, these lesions may be detected at very small sizes, including tumors measuring 2–3 mm.
Symptoms of ILS can be varied. Previous reports have proposed that the location on imaging may predict the symptoms.10,19–21 In earlier reports, it was suggested that tumors confined to the vestibule more commonly cause vertigo or symptoms similar to Meniere disease.12,19,23 However, in our series, tumor location had no reproducible bearing on symptoms. Sensorineural hearing loss in our series was universal, seen in all 45 patients, and most patients' symptoms were progressive. Other symptoms included tinnitus, vertigo, imbalance, and aural fullness.
The imaging identification of ILS has increased during the past 20 years with imaging descriptions evolving along with MR imaging technology. In 1990, the first cases of ILS were globally described on enhanced MR imaging as homogeneous enhancement in the labyrinth.10,27 Following this, several reports used enhanced MR imaging as a primary diagnostic tool for ILS.12,14,16,17,22,23,26 More advanced imaging and characterization with high-resolution T2 and gradient recalled-echo sequences appeared in the literature in the early 2000s.1,21,28–31 Some reports have suggested that the lesions can be invisible on T2 and even postgadolinium T1-weighted images.10,17,26 This insensitivity may have been due to section thickness and less optimized imaging sequences at that time. In all of our cases, these lesions were visualized on both sequences. However, smaller lesions may even still be missed today, and the evolution of imaging will undoubtedly continue to increase detection. High-resolution imaging with improved awareness of the radiologist will undoubtedly lead to increased detection. The key to diagnosis is the appropriate imaging parameters. On thin-section high-resolution T2 images, ILS appears as a replacement of the normal high signal intensity within the membranous labyrinth. After the administration of intravenous contrast, there is uniform enhancement.
There are other lesions that can mimic ILS on contrast-enhanced MR imaging, including labyrinthitis (typically viral in etiology), labyrinthitis ossificans, hemorrhage, or lipoma. Labyrinthitis most commonly shows diffuse enhancement. In the rare focal enhancement case, labyrinthitis can be differentiated by performing a high-resolution thin-section T2-weighted study. In the case of labyrinthitis, no soft-tissue mass or filling defect in the labyrinth as in ILS will be seen.10,32 Labyrinthitis ossificans, which can enhance during its fibro-osseous phase and will have T2 signal intensity within the inner ear, can most often be distinguished both by history and a temporal bone CT scan. The patient will have a history of previous meningitis or suppurative otomastoiditis. On a thin-section temporal bone CT scan, there will be bony encroachment on the membranous labyrinth.32–35 None of our patients with presumed ILS had a history of prior meningitis or otomastoiditis. Although it is possible to confuse the early imaging of labyrinthine ossificans with ILS, the clinical setting helps separate these entities. Hemorrhage and lipoma, though rare, can easily be differentiated on precontrast T1-weighted imaging, appearing as high signal intensity without enhancement.24,32
With improved spatial resolution, characterization will naturally follow. The proposed classification system is simple with straightforward anatomy as its foundation. ILSs are intracochlear, intravestibular, vestibulocochlear, transmodiolar, transmacular, and transotic. Within this classification schema, our series included 14 intracochlear, 13 transmodiolar, 7 intravestibular, 5 vestibulocochlear, 4 transmacular, and 2 transotic tumors. Cochlear ILS is the dominant location in our series, with 27 tumors either in the intracochlear or transmodiolar locations. In other reported series, intracochlear and transmodiolar tumors were reported in 88 cases; intravestibular and transmacular, in 40 cases; vestibulocochlear, in 5 cases; and transotic, in 4 cases.1,23,29,30 A similar classification scheme was offered on many of the same patients.1 An additional 17 cases were added to our original dataset since the initial presentation. The tympanolabyrinthine class was excluded from our description due to the fact that there were no observed cases fitting the description and there was redundancy with the transotic subtype.
The treatment of ILS depends on the location, symptoms, and the behavior of the tumor with time. Hearing-preservation surgery is not possible in the removal of ILS; thus, management is usually observation with serial MR imaging, to avoid excessive morbidity. Surgery is only indicated for intractable vertigo or evidence of tumor growth leaving the membranous labyrinth into the IAC or middle ear. In cases where surgery is performed due to symptoms, localization aids in presurgical planning and decreases morbidity. Radiation therapy is discussed with patients and is a viable option in the poor operative candidate who exhibits growth. In our study with an average follow-up of all 45 patients for 56 months, 7 patients showed growth and underwent surgery. Most ILSs in our study did not significantly grow, making follow-up with MR imaging the preferred approach to tumor management.
The main limitation of our study is that only 16 of 45 patients underwent surgical resection. The remaining 29 patients are presumed schwannomas on the basis of clinical evaluation, characteristic imaging features, and stability on follow-up MR studies. There was 1 patient with no follow-up imaging. The remaining 28 patients had follow-up imaging data ranging from 12 to 108 months, with an average follow-up of 58.2 months.
Conclusions
ILS is a diagnosis that has been previously under-reported. This series of 45 cases collected over 14 years supports the contention that ILS is more common than previously thought. Careful interrogation of the inner ear structures for the presence of filling defects (high-resolution T2 MR imaging) or focal enhancement (T1 enhanced MR imaging) will yield more frequent identification of this tumor. In this article, we characterize the MR imaging appearance of these lesions by using high-resolution techniques. In addition, we presented an anatomically based classification system, which will help the radiologist accurately describe ILS within the inner ear and help the surgeon determine which tumors are potential surgical candidates.
ABBREVIATIONS:
- CPA
cerebellopontine angle
- FSE
fast spin-echo
- IAC
internal auditory canal
- ILS
intralabyrinthine schwannomas
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, May 21–27, 2005; Toronto, Ontario, Canada.
Disclosures: H. Ric Harnsberger, Ownership Interest: CEO, Amirsys (unrelated to article). Karen L. Salzman, Consultant: Amirsys; Ownership Interest: Amirsys. Clough Shelton, Research Support: Cochlear.
References
- 1. Kennedy RJ, Shelton C, Salzman KL, et al. Intralabyrinthine schwannomas: diagnosis, management, and a new classification system. Otol Neurotol 2004;25:160–67 [DOI] [PubMed] [Google Scholar]
- 2. Karlan MS, Besek M, Potter GB. Intracochlear neurilemmoma. Arch Otolaryngol 1972;96:573–75 [PubMed] [Google Scholar]
- 3. Wannamaker HH. Acoustic neuroma primary arising in the vestibule. Laryngoscope 1972;82:1040–44 [DOI] [PubMed] [Google Scholar]
- 4. Hoshino T, Ishii D. Intralabyrinthine neurilemmoma: a histopathological report. ORL J Otorhinolaryngol Relat Spec 1972;34:117–23 [DOI] [PubMed] [Google Scholar]
- 5. DeLozier HL, Gacek RR, Dana ST. Intralabyrinthine schwannoma. Ann Otol Rhinol Laryngol 1979;88(2 pt 1):187–91 [DOI] [PubMed] [Google Scholar]
- 6. Miyamoto RT, Isenberg SF, Culp WM, et al. Isolated intralabyrinthine schwannoma. Am J Otol 1980;1:215–17 [PubMed] [Google Scholar]
- 7. Vernick DM, Graham MD, McClatchey KD. Intralabyrinthine schwannoma. Laryngoscope 1984;94:1241–43. [DOI] [PubMed] [Google Scholar]
- 8. Huang TS. Primary intralabyrinthine schwannoma. Ann Otol Rhinol Laryngol 1986;95(2 pt 1):190–92 [DOI] [PubMed] [Google Scholar]
- 9. Sataloff RT, Roberts BR, Feldman M. Intralabyrinthine schwannoma. Am J Otol 1988;9:323–26 [PubMed] [Google Scholar]
- 10. Mafee MF, Lachnauer CS, Kumar A, et al. CT and MR imaging of intralabyrinthine schwannoma: report of two cases and review of the literature. Radiology 1990;174:395–400 [DOI] [PubMed] [Google Scholar]
- 11. Birzgalis AR, Ramsden RT, Curley JW. Intralabyrinthine schwannoma. J Laryngol Otol 1991;105:659–61 [DOI] [PubMed] [Google Scholar]
- 12. Doyle KJ, Brackmann DE. Intralabyrinthine schwannomas. Otolaryngol Head Neck Surg 1994;110:517–23 [DOI] [PubMed] [Google Scholar]
- 13. Ozluoglu L, Jenkins HA. Intralabyrinthine schwannoma. Arch Otolaryngol Head Neck Surg 1994;120:1404–06 [DOI] [PubMed] [Google Scholar]
- 14. Saeed SR, Birzgalis AR, Ramsden RT. Intralabyrinthine schwannoma shown by magnetic resonance imaging. Neuroradiology 1994;36:63–64 [DOI] [PubMed] [Google Scholar]
- 15. Donnelly MJ, Daly CA, Briggs JS. MR imaging features of an intracochlear acoustic schwannoma. J Laryngol Otol 1994;108:111–14 [DOI] [PubMed] [Google Scholar]
- 16. Mafee MF. MR imaging of intralabyrinthine schwannoma, labyrinthitis, and other labyrinthine pathology. Otolaryngol Clin North Am 1995;28:407–30 [PubMed] [Google Scholar]
- 17. Zbar RI, Megerian CA, Khan A, et al. Invisible culprit: intralabyrinthine schwannomas that do not appear on enhanced magnetic resonance imaging. Ann Otol Rhinol Laryngol 1997;106:739–42 [DOI] [PubMed] [Google Scholar]
- 18. Roland PS, Gilmore J. Intracochlear schwannoma. Otolaryngol Head Neck Surg 1998;119:681–84 [DOI] [PubMed] [Google Scholar]
- 19. Green JD, Jr, McKenzie JD. Diagnosis and management of intralabyrinthine schwannomas. Laryngoscope 1999;109:1626–31 [DOI] [PubMed] [Google Scholar]
- 20. Jackson LE, Hoffmann KK, Rosenberg SI. Intralabyrinthine schwannoma: subtle differentiating symptomatology. Otolaryngol Head Neck Surg 2003;129:439–40 [DOI] [PubMed] [Google Scholar]
- 21. Somers T, Casselman J, de Ceukaer G, et al. Prognostic value of magnetic resonance imaging findings in hearing preservation surgery for vestibular schwannoma. Otol Neurotol 2001;22:87–94 [DOI] [PubMed] [Google Scholar]
- 22. Fitzgerald DC, Grundfast KM, Hecht DA, et al. Intralabyrinthine schwannomas. Am J Otol 1999;20:381–85 [PubMed] [Google Scholar]
- 23. Neff BA, Wilcox TO, Jr, Sataloff RT. Intralabyrinthine schwannomas. Otol Neurotol 2003;24:299–307 [DOI] [PubMed] [Google Scholar]
- 24. Hegarty JL, Patel S, Fischbein N, et al. The value of enhanced magnetic resonance imaging in the evaluation of endocochlear disease. Laryngoscope 2002;112:8–17 [DOI] [PubMed] [Google Scholar]
- 25. Forton GE. Intralabyrinthine schwannomas. Am J Otol 2000;21:456. [DOI] [PubMed] [Google Scholar]
- 26. Montague ML, Kishore A, Hadley DM, et al. MR findings in intralabyrinthine schwannomas. Clin Radiol 2002;27:355–58 [DOI] [PubMed] [Google Scholar]
- 27. Brogan M, Chakeres D. Gd-DTPA-enhanced MR imaging of cochlear schwannoma. AJNR Am J Neuroradiol 1990;11:407–08 [PMC free article] [PubMed] [Google Scholar]
- 28. Deux JF, Marsot-Dupuch K, Ouayoun M, et al. Slow-growing labyrinthine masses: contribution of MRI to diagnosis, follow-up and treatment. Neuroradiology 1998;40:684–89 [DOI] [PubMed] [Google Scholar]
- 29. Grayeli AB, Fond C, Kalamarides M, et al. Diagnosis and management of intracochlear schwannomas. Otol Neurotol 2007;28:951–57 [DOI] [PubMed] [Google Scholar]
- 30. Tieleman A, Casselman JW, Somers T, et al. Imaging of intralabyrinthine schwannomas: a retrospective study of 52 cases with emphasis on lesion growth. AJNR Am J Neuroradiol 2008;29:898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hermans R, Van der Goten A, De Foer B, et al. MRI screening for acoustic neuroma without gadolinium: value of 3DFT-CISS sequence. Neuroradiology 1997;39:593–98 [DOI] [PubMed] [Google Scholar]
- 32. Harnsberger R, Glastonbury CM, Michel MA, et al. Diagnostic Imaging: Head and Neck. 2nd ed. Baltimore: Lippincott, Williams, & Wilkins; 2010 [Google Scholar]
- 33. Swartz JD, Mandell DM, Faerber EN, et al. Labyrinthine ossification: etiologies and findings. Radiology 1985;157:395–98 [DOI] [PubMed] [Google Scholar]
- 34. Becker TS, Eisenberg LS, Luxford WM, et al. Labyrinthine ossificans secondary to childhood bacterial meningitis: implications for cochlear implant surgery. AJNR Am J Neuroradiol 1984;5:739–41 [PMC free article] [PubMed] [Google Scholar]
- 35. Hoffman RA, Brookler KH, Bergeron RT. Radiologic diagnosis of labyrinthitis ossificans. Ann Otol Rhinol Laryngol 1979;88(2 pt 1):253–57 [DOI] [PubMed] [Google Scholar]