Abstract
BACKGROUND AND PURPOSE:
Patients with acute CTO generally have a poor prognosis, despite IV or IA thrombolytic treatment. The goal of this study was to analyze the results of patients with CTO who had IA urokinase treatment with or without initial IV rtPA based on a bridging protocol.
MATERIALS AND METHODS:
Sixteen consecutive patients with acute ischemic stroke due to CTO who had combined IV and IA or a single IA thrombolytic treatment were enrolled. The baseline characteristics and prognosis were described. The patients who did and did not develop a PH shortly after treatment were compared.
RESULTS:
The mean age was 66.4 years, and the median initial NIHSS score was 17. The median dose of IA urokinase was 320,000 U, and recanalization (TICI grade II-III) was achieved in 12 patients (75%). However, 5 patients died and 10 patients had poor prognosis with mRS 5–6 at discharge. Six patients (37.5%) with a PH had a higher NIHSS score 1 day after treatment (26.7 versus 13.6, P = .002), and they had more frequent mortality (66.7% versus 10.0%, P = .018) and worse prognosis (mRS 5–6; 100% versus 40%, P = .016) at discharge than patients without PH.
CONCLUSIONS:
Patients with CTO who received IA urokinase treatment based on a bridging protocol had a poor prognosis. The development of PH might affect this outcome.
CTO, which simultaneously involves the ICA and ipsilateral middle and anterior cerebral arteries, is one of the most fatal causes of acute ischemic stroke. The mortality has been reported to be approximately 50%, irrespective of the type of therapy.1–5 Recently, recanalization has been regarded as one of the most important predictive factors for patients with acute ischemic stroke.5–7 Endovascular treatment is powerful to in its ability recanalize major occluded cerebral arteries. IA urokinase infusion has been a treatment option since the PROACT II trial was published.8 Specifically, IA pro-urokinase infusion in patients with acute ischemic stroke achieved a much higher recanalization rate and better outcomes. The MELT study also showed more frequent excellent outcomes and mRS scores ≤1 in patients who received IA urokinase infusions than in those who were treated conservatively.9 Because the recanalization rate with IV rtPA is very low,6,10 additional IA chemical treatments have also been tried. Bridging thrombolytic treatments have been reported to be feasible and effective in recanalizing occluded cerebral arteries.11–15 Nevertheless, the effects of bridging therapy on patients with CTO and large thrombi have not been reported.
In this study we report our experiences by using IA urokinase infusions for recanalization of patients with CTO. According to our bridging treatment protocol, patients received IA urokinase treatment with or without an initial IV rtPA infusion based on the onset-to-treatment interval. A PH was the focus as a prognostic factor in patients with acute ischemic stroke and CTO.
Materials and Methods
Patient Enrollment
Between February 2006 and March 2009, 16 consecutive patients with acute ischemic stroke due to CTO who received an IA urokinase infusion were enrolled in the study, which was conducted at a university hospital. Those who had received other IA thrombolytics or mechanical thrombolytic treatments other than microwire disruption were not included in this study. CTO was diagnosed by initial CT angiography. All data, which were retrieved from the prospectively collected data base of the stroke registry, were retrospectively reviewed. The indications for IV rtPA (0.9 mg/kg) were applied according to the NINDS rtPA Criteria,16 with the exception of age. In brief, patients were included who had acute stroke syndrome, based on an examination by neurologists, an onset-to-decision interval <3 hours, and no evidence of hemorrhage on the initial nonenhanced CT. The indications for IA thrombolytic treatment were severe neurologic status, major cerebral artery occlusion on CT angiography, and a duration of onset-to-presentation within 5 hours. Informed consent for endovascular treatment was obtained from patients or their legal representatives.
Protocols
Immediately after the patients in whom symptoms were suspicious for stroke arrived at the emergency department, a CT scan (including nonenhanced and enhanced axial parenchymal images) and CT angiography were obtained. The patients who were diagnosed with acute ischemic stroke were classified according to a bridging thrombolytic protocol through a critical pathway. The CT angiography was reconstructed with maximal intensity projection and a volume-rendering technique. IV rtPA was induced when the infusion was started within 3 hours after symptom onset. Patients without contraindications for endovascular treatment were brought to the angiographic suite and treated with the IA approach as soon as possible if the onset-to-decision interval was <5 hours, irrespective of IV rtPA infusion. Recanalization was determined by the final angiography, which was obtained immediately after the thrombolytic treatment was completed. Immediately after the endovascular procedure was completed, a nonenhanced CT was obtained. MR imaging, including diffusion-weighted imaging, was performed within 1 or 2 days after onset. A follow-up CT scan and CT angiography were performed within 4–7 days after the onset of symptoms. Neurologic scales were checked daily until discharge and every 3 months thereafter.
Intervention Procedures
A guiding catheter (Envoy; Cordis, Miami Lakes, Florida or Shuttle-SL guide sheath; Cook, Bloomington, Indiana) was used to select the ICA. In most cases, after the length of the thrombus was measured with a microcatheter (Excelsior SL-10; Boston Scientific, Natick, Massachusetts) and a microwire (Transend, Boston Scientific), the distal tip of the microwire was shaped like a pigtail, as modified according to a previously reported method.17 The curved loop of the distal tip was repeatedly passed into the thrombus to achieve mechanical thrombectomy. Urokinase (10,000 U/mL for 1 minute) was manually infused at the distal portion of the thrombus first, and subsequently in the middle and proximal portions.
Analysis
Baseline patient data, including vascular risk factors, stroke etiology, laboratory and radiologic results, and initial neurologic severity scales were recorded. The ASPECTS was evaluated on the initial nonenhanced CT.18 The collateral vessels in the Sylvian area and the anterior and posterior communicating arteries were evaluated by maximal intensity projection images of CT angiography, as modified by a previously reported method.19 The ipsilateral collateral vessels were regarded as “decreased” when the arterial attenuation was less than that of the contralateral vessels. Clinical outcomes were measured by the NIHSS score on the day following revascularization therapy, and mRS at discharge and 3 months and 1 year later. Radiologic outcomes were determined by the modified TICI grade,20,21 and ICHs were classified according to the ECASS study.22,23 In brief, each TICI grade was defined as follows: 0, no perfusion; I, minimal perfusion; IIa, partial filling of less than one-half of the occluded arterial distribution; IIb, partial filling of one-half or greater of the occluded arterial distribution; and III, full perfusion.20 Recanalization was defined as TICI grades IIa, IIb, or III for this study. We included TICI grade IIa as recanalization because the leptomeningeal collaterals are associated with a better prognosis, even though the main branch, such as the middle cerebral artery, is not recanalized.2 The classification of hemorrhages was defined as follows: hemorrhagic infarction type I, small petechiae along the margins of the infarcts; hemorrhagic infarction type II, more confluent petechiae within the infarcted area but without a space-occupying effect; PH type I, blood clot <30% of the infarcted area with a mild space-occupying effect; and PH type II, attenuated blood clots ≥30% of the infarct volume with a significant space-occupying effect.23 Finally, to evaluate the association of the development of PH with prognosis, patients were classified into 2 groups according to the presence or absence of PH.
Statistics
To compare the frequency and continuous values, the X2 test and t test were used, respectively. To evaluate the relationship of the degree of ICH with the degree of recanalization, the degrees were reordered as an integer. Thus, the degree of ICH was ordered from 0–4, representing no hemorrhage, hemorrhagic transformation types 1 and 2, and PH types 1 and 2, respectively. The degree of recanalization was also reordered from 0–4, representing TICI grades 0, I, IIa, IIb, and III, respectively. The Spearman correlation coefficient was calculated for correlative analysis. A P value <0.05 was considered statistically significant. Statistical analyses were performed by using a commercially available software package (SPSS, version 12.0 for Windows; SPSS, Chicago, Illinois).
Results
The demographics, risk factors, and clinical information are listed in On-line Table 1. The 16 patients who were enrolled consisted of 10 men and 6 women. The mean age was 66.4 years (range, 52–85 years). The median initial NIHSS score was 17 (range, 7–25). Eight, 7, and 1 patient(s) had right, left, and bilateral ICA involvement, respectively. Cardioembolism and atherosclerosis were the etiologies underlying occlusion in 12 (75%) and 4 patients (25.0%), respectively. The vascular risk factors included hypertension in 8 (50.0%), diabetes in 3 (18.8%), atrial fibrillation in 12 (75.0%), valvular heart disease in 4 (25.0%), smoking in 3 (20.0%), metabolic syndrome in 6 (37.5%), and previous stroke in 3 patients (18.8%). Four (25%) and 2 patients (12.5%) had taken any type of antiplatelet medications and anticoagulants, respectively. IV rtPA was administered to 8 patients (50%). The median dose of urokinase was 320,000 U (40,000–700,000U).
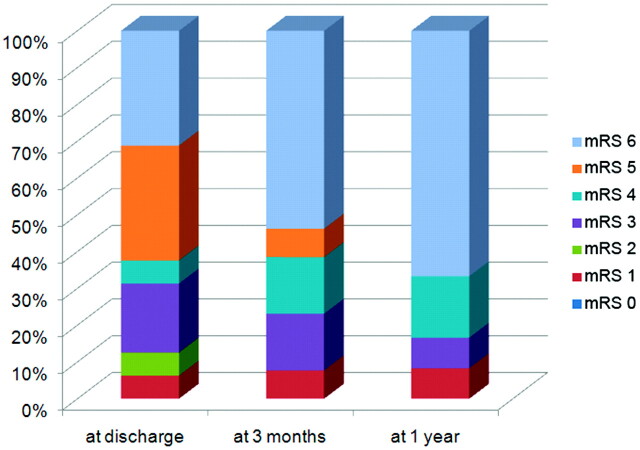
Recanalization was achieved in 12 patients (75%). Six patients each had TICI grades IIa and IIb; however, there was no complete recanalization. Overall, 10 patients (62.5%) had postintervention ICH; hemorrhagic transformation type 1, hemorrhagic transformation type 2, PH type 1, and PH type 2 occurred in 2 (12.5%), 2 (12.5%), 5 (31.3%), and 1 patient(s) (6.3%), respectively. A subarachnoid hemorrhage occurred in 1 patient (6.3%). Craniectomies with or without lobectomies were performed in 3 patients (18.8%). The prognosis was not favorable; mortality rate was 31.3% at discharge, 53.8% at 3 months, and 66.7% at 1 year after onset (Fig 1). The rate of patients with mRS 4–5 was 37.5% at discharge, 23.1% at 3 months, and 16.7% at 1 year after onset.
Fig 1.
Functional outcomes as judged by mRS after IA urokinase with or without prior IV rtPA treatment for CTO.
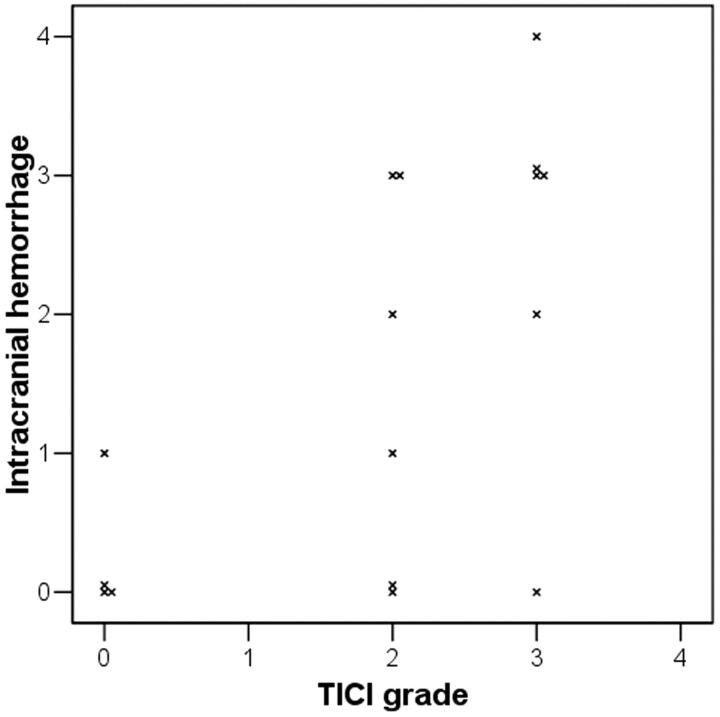
The severity of postintervention ICH was associated with the recanalization grade (Figure 2). When the ICH and TICI grades were reordered by severity, there was a correlation and statistical significance (Spearman coefficient R = 0.595, P = .015). There were significantly more patients having a poor prognosis (defined as a mRS 5 or 6) in the group with PH than in the group without (On-line Table 2). Age (71.0 versus 63.6 years, P = .241) and initial NIHSS score (19.0 versus 14.8, P = .064) tended to be higher in patients with PH than in patients without PH but were not statistically significant. However, prior IV rtPA infusion was significantly more frequent in those with PH than without PH (83.3% versus 30.0%, P = .039). With respect to short-term prognosis, the NIHSS score 1 day after treatment (26.7 versus 13.6, P = .002) was higher, and mortality (66.7% versus 10.0%, P = .018) and a mRS score of 5–6 (100% versus 40%, P = .016) at discharge were more frequent in patients with PH than in patients without PH. The significant difference of mortality and mRS 5–6 was also shown at 3 months and at 1 year. Representative radiologic figures for patients with or without PH are shown in Fig 3. Patients with and without prior IV rtPA were grouped and compared; however, there was no significant difference in baseline characteristics and outcomes (data not shown).
Fig 2.
Relationship of the degree of intracranial hemorrhage with that of recanalization after intra-arterial urokinase infusion. Both degrees are shown to have positive correlation (Spearman correlation coefficient R = 0.595, P = .015).
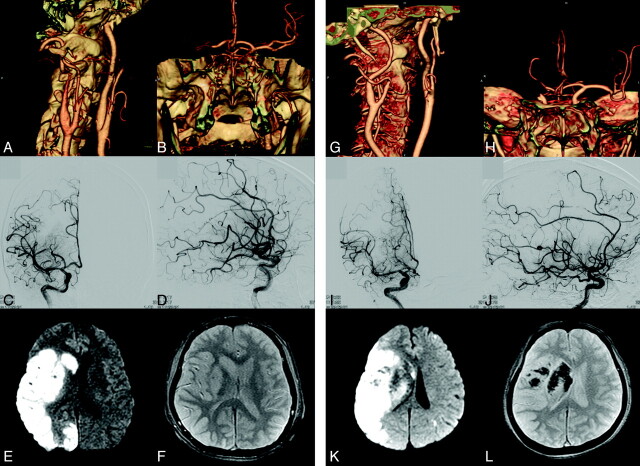
Fig 3.
Illustrative figures for patients without PH (A-F) and with PH (G-L), respectively. Initial NIHSS scores of patients 7 and 15, who had right major hemispheric syndrome and CTO on CTA (A-B and G-H), were 16 and 18, respectively. They had taken IV rtPA and IA vrokinase 400,000U and 300,000U, respectively. Their CTO was recanalized with TICI IIb (C-D and I-J), but a large area with diffusion restriction was shown (E and K). Whereas patient 7, whose gradient-echo MR imaging revealed no hemorrhage (F), had relatively good outcome with mRS 2 at discharge, patient 15, whose gradient-echo MR imaging showed PH type 1 (L), had poor outcome with mRS 5 at discharge.
Discussion
Since IV rtPA was developed and approved in the mid-1990s, more patients with acute ischemic stroke have survived and been relieved from grave morbidity compared with the natural course of acute ischemic stroke.16 Nonetheless, the so-called CTO is still associated with a poor prognosis and high mortality rate.1–5 CTO must be associated with a large clot burden because large-caliber arteries, such as the ICA and its 2 major branches (the anterior and middle cerebral arteries), are concomitantly occluded. Thus, the recanalization rate is too low and the prognosis is not good despite IV rtPA infusion.1,6,10 IA thrombolytic infusion also has been tried in severe cases, but the prognosis was not favorable.1,5 In this retrospective study, a bridging protocol, based on IA urokinase treatment with or without prior IV rtPA infusion, revealed that the prognosis of patients who had acute CTO was still poor. Our data showed that a poor prognosis existed especially when the patients with acute CTO had PH after the intervention. This result is consistent with a previous report in that all patients who received IV or IA chemical therapy for recanalization of CTO died when PH was found on CT after treatment.1 With respect to mechanisms, PH might be affected by reperfusion injury and chemical toxicity because all patients with PH had recanalization after intervention.
Most CTOs cause a large hemispheric infarction shortly after the onset of symptoms, and the brain tissue might be vulnerable to reperfusion injury following recanalization. The breakdown of the blood-brain barrier among reperfusion injury mechanisms can be an important factor to induce intracranial hematomas. In an animal study, increased blood-brain barrier disruption was associated with 3-hour reperfusion following a 3-hour occlusion model rather than the 6-hour permanent occlusion model.24 The researchers used the rat suture model for middle cerebral artery occlusion, which is very similar to CTO in that most collaterals from the circle of Willis are blocked. It has been reported in both animal and clinical studies that the blood-brain barrier disruption is associated with ICH development after thrombolytic infusion in patients with acute ischemic stroke.25,26
With respect to high rates of postinterventional ICHs, the probability of chemical injury should be considered. First, prior IV rtPA infusion to the intervention was associated with the occurrence of PH in the current study. Since IV rtPA treatment was approved following the NINDS rtPA Study in the mid-1990s, a dose of 0.9 mg/kg has been used.16 In the late 1990s, the PROACT II study showed the feasibility and efficacy when IA pro-urokinase infusion was applied to patients with moderate-to-severe acute ischemic stroke within 6 hours after the onset of symptoms.8 These 2 important treatments have been combined for patients with major cerebral artery occlusion since that time. Whereas previous reports have suggested combined IV rtPA (0.9 mg/kg) and an additional IA thrombolytic infusion was safe in patients with acute ischemic stroke,14,15 our data, which included only CTO among acute ischemic stroke subtypes, were not consistent with these previous findings, even though a relatively small amount of urokinase was used in our bridging protocol. The rtPA dose of 0.9 mg/kg for IV infusion might be too much for the combined treatment. Alteplase and urokinase have a similar mechanism of action that involves the conversion of plasminogen to plasmin. Indirectly calculated, more than 0.9 mg/kg of rtPA was infused when both treatments were applied together. This is comparable to the results of the first ECASS trial, which used a 1.1 mg/kg rtPA IV infusion and showed high frequency (19.8%) of PH.23 On the other hand, the frequency of symptomatic ICHs was relatively low when a 0.6 mg/kg IV rtPA infusion was used with the subsequent IA rtPA infusion.11,12 There was no PH or symptomatic ICH in the combined treatment group of the EMS bridging trial.11 The IMS I trial showed only 6.3% of symptomatic ICH, a rate which was comparable to 6.6% of ICH in the 0.9 mg/kg IV rtPA infusion of the NINDS rtPA study.12,16
Selection of the most appropriate drug among thrombolytics seems to be another important factor for additional IA thrombolytic treatment. Despite a recent study that showed a full dose of IV rtPA followed by an IA thrombolytic infusion was relatively safe and achieved a high recanalization rate and a favorable outcome, urokinase appears to be relatively harmful compared with 3 other types of thrombolytic drugs such as reteplase, alteplase, and urokinase.15 Of 6 patients with additional IA urokinase infusions, 2 had symptomatic ICH and 4 died. Reteplase and alteplase appear to be relatively safe. In the previous PROACT II and MELT studies, by using pro-urokinase and urokinase, the rates of symptomatic ICH (10% and 9%, respectively) were relatively high without prior IV rtPA infusion.8,9 Against this background, selection of a new-generation thrombolytic other than urokinase as a boosting thrombolytic drug should be considered.
Endovascular neurointervention is an emerging field for the treatment of acute ischemic stroke. Several methods, which include mechanical aspiration, mechanical clot fragmentation, as well as the use of thrombolytics, have been tried for acute ischemic stroke.27–32 Mechanical recanalization therapies, such as intracranial self-expandable stent deployment, clot capture with a Merci device (Concentric Medical, Mountain View, California) and forceful clot suction with a Penumbra system (Penumbra, Alameda, California) can be alternatives to chemical therapy for the treatment of acute CTO. Those methods effectively recanalize the occluded major cerebral arteries and achieve a relatively high rate of good outcomes for patients with severe initial neurologic symptoms.27–32 Stent-assisted chemical treatment is also feasible, and the use of a lower dosage of chemicals is expected.33
Our data were strengthened by a relatively homogeneous study cohort with IA thrombolytics without other IA mechanical or combined recanalization techniques. However, group comparison might possess low statistical power and independent predictors, for the occurrence of PH could not be achieved due to the small number of patients. Factors such as age, NIHSS score on arrival, and decreased collaterals could have confounded our results and would be better controlled in a larger cohort study with multivariate analysis. For getting more consistent results, analysis with data gathered from various centers will be needed. Moreover, whether mechanical revascularization techniques rather than IA thrombolytics are more effective in patients with acute CTO should be studied.
Conclusions
Cerebral infarction resulting from CTO caused poor prognosis despite IA urokinase treatment. The prognosis was associated with the development of parenchymal hematoma. Newer mechanical recanalization methods are warranted for CTO treatment because a lower rate of hemorrhagic complication is expected.
Supplementary Material
ABBREVIATIONS
- ASPECTS
Alberta Stroke Program Early CT Score
- CTO
carotid terminus occlusion
- ECASS
European Cooperative Acute Stroke Study
- IA
intra-arterial
- EMS
Emergency Management of Stroke
- ICH
intracranial hemorrhage
- IMS
Interventional Management of Stroke
- MELI
MCA-Embolism Local Fibrinolytic Intervention Trial
- mRS
modified Rankin Scale
- PH
parenchymal hematoma
- PROACT
Prolyse in Acute Cerebral Thromboembolism
- TICI
thrombolysis in cerebral ischemia
Footnotes
This study was supported by a grant of the Korea Healthcare technology R & D Project, Ministry of Health & Welfare, Republic of Korea (A100463-1001-0000100).
References
- 1. Jansen O, von Kummer R, Forsting M, et al. Thrombolytic therapy in acute occlusion of the intracranial internal carotid artery bifurcation. AJNR Am J Neuroradiol 1995;16:1977–86 [PMC free article] [PubMed] [Google Scholar]
- 2. Urbach H, Ries F, Ostertun B, et al. Local intra-arterial fibrinolysis in thromboembolic “T” occlusions of the internal carotid artery. Neuroradiology 1997;39:105–10 [DOI] [PubMed] [Google Scholar]
- 3. Arnold M, Nedeltchev K, Mattle HP, et al. Intra-arterial thrombolysis in 24 consecutive patients with internal carotid artery T occlusions. J Neurol Neurosurg Psychiatry 2003;74:739–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wunderlich MT, Stolz E, Seidel G, et al. Conservative medical treatment and intravenous thrombolysis in acute stroke from carotid T occlusion. Cerebrovasc Dis 2005;20:355–61 [DOI] [PubMed] [Google Scholar]
- 5. Eckert B, Kucinski T, Neumaier-Probst E, et al. Local intra-arterial fibrinolysis in acute hemispheric stroke: effect of occlusion type and fibrinolytic agent on recanalization success and neurological outcome. Cerebrovasc Dis 2003;15:258–63 [DOI] [PubMed] [Google Scholar]
- 6. Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 2010;41:2254–58 [DOI] [PubMed] [Google Scholar]
- 7. Nogueira RG, Yoo AJ, Buonanno FS, et al. Endovascular approaches to acute stroke, part 2: a comprehensive review of studies and trials. AJNR Am J Neuroradiol 2009;30:859–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. JAMA 1999;282:2003–11 [DOI] [PubMed] [Google Scholar]
- 9. Ogawa A, Mori E, Minematsu K, et al. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: the middle cerebral artery embolism local fibrinolytic intervention trial (MELT) Japan. Stroke 2007;38:2633–39 [DOI] [PubMed] [Google Scholar]
- 10. Alexandrov AV. Current and future recanalization strategies for acute ischemic stroke. J Intern Med 2010;267:209–19 [DOI] [PubMed] [Google Scholar]
- 11. Lewandowski CA, Frankel M, Tomsick TA, et al. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke 1999;30:2598–605 [DOI] [PubMed] [Google Scholar]
- 12. IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 2004;35:904–11 [DOI] [PubMed] [Google Scholar]
- 13. IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke 2007;38:2127–35 [DOI] [PubMed] [Google Scholar]
- 14. Lee KY, Kim DI, Kim SH, et al. Sequential combination of intravenous recombinant tissue plasminogen activator and intra-arterial urokinase in acute ischemic stroke. AJNR Am J Neuroradiol 2004;25:1470–75 [PMC free article] [PubMed] [Google Scholar]
- 15. Shaltoni HM, Albright KC, Gonzales NR, et al. Is intra-arterial thrombolysis safe after full-dose intravenous recombinant tissue plasminogen activator for acute ischemic stroke? Stroke 2006;38:80–84 [DOI] [PubMed] [Google Scholar]
- 16. The NINDS rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–87 [DOI] [PubMed] [Google Scholar]
- 17. Sorimachi T, Fujii Y, Tsuchiya N, et al. Recanalization by mechanical embolus disruption during intra-arterial thrombolysis in the carotid territory. AJNR Am J Neuroradiol 2004;25:1391–402 [PMC free article] [PubMed] [Google Scholar]
- 18. Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001;22:1534–42 [PMC free article] [PubMed] [Google Scholar]
- 19. Maas MB, Lev MH, Ay H, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke 2009;40:3001–05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol 2008;29:582–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higashida R, Furlan A, Roberts H, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol 2003;14:S493–94 [DOI] [PubMed] [Google Scholar]
- 22. Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol 1993;14:3–13 [PMC free article] [PubMed] [Google Scholar]
- 23. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–25 [PubMed] [Google Scholar]
- 24. Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke 1994;25:1658–64 [DOI] [PubMed] [Google Scholar]
- 25. Dijkhuizen RM, Asahi M, Wu O, et al. Rapid breakdown of microvascular barriers and subsequent hemorrhagic transformation after delayed recombinant tissue plasminogen activator treatment in a rat embolic stroke model. Stroke 2002;33:2100–04 [DOI] [PubMed] [Google Scholar]
- 26. Kastrup A, Groschel K, Ringer TM, et al. Early disruption of the blood-brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke 2008;39:2385–87 [DOI] [PubMed] [Google Scholar]
- 27. Levy EI, Siddiqui AH, Crumlish A, et al. First Food and Drug Administration-approved prospective trial of primary intracranial stenting for acute stroke: SARIS (stent-assisted recanalization in acute ischemic stroke). Stroke 2009;40:3552–56 [DOI] [PubMed] [Google Scholar]
- 28. Brekenfeld C, Schroth G, Mattle HP, et al. Stent placement in acute cerebral artery occlusion: use of a self-expandable intracranial stent for acute stroke treatment. Stroke 2009;40:847–52 [DOI] [PubMed] [Google Scholar]
- 29. Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205–12 [DOI] [PubMed] [Google Scholar]
- 30. Bose A, Henkes H, Alfke K, et al. The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol 2008;29:1409–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Penumbra Pivotal Stroke Trial Investigators. The Penumbra Pivotal Stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 2009;40:2761–68 [DOI] [PubMed] [Google Scholar]
- 32. Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38 [DOI] [PubMed] [Google Scholar]
- 33. Suh SH, Kim BM, Roh HG, et al. Self-expanding stent for recanalization of acute embolic or dissecting intracranial artery occlusion. AJNR Am J Neuroradiol 2009;31:459–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.