Abstract
It is important to assess the cerebral arteries near the clip after cerebral aneurysm clipping. Contrast-enhanced computed tomography angiography has side effects of contrast medium and radiation exposure. Time-of-flight magnetic resonance angiography (TOF-MRA) is a fast and non-invasive method, but clip-induced artifact limits the assessment around the clip. Recently, 3 tesla MRA with ultrashort echo time called SILENT MRA (GE Healthcare Life Sciences, UK) has been reported to have the potential to overcome these disadvantages. We herein present consecutive 19 cerebral aneurysm patients treated by clipping and evaluated using SILENT MRA. The 19 patients (15 women and 4 men) underwent TOF-MRA and SILENT MRA during the same scan session. Two neurosurgeons independently assessed the visibility of the mother vessel at the clipping site in TOF-MRA and SILENT MRA. We also investigated the factors related to visibility in SILENT MRA. All patients’ mother vessels were not described in TOF-MRA, and that of 16 patients (84%) were described in SILENT MRA. Overall agreement was 100% in the two neurosurgeons, and the fixed marginal kappa = 1.00 (95% CI: 0.36–1.00). Univariate analysis revealed that larger aneurysm dome and long clip blade length contributed to the visibility of the mother vessel in SILENT MRA. (p = 0.023, 0.007, each). In conclusion, SILENT MRA can be applied for the assessment of the arteries and aneurysm neck remnants near the clip. Using clips with long blade and ligation with its tip would be related to the visibility of the mother vessels in SILENT MRA.
Keywords: cerebral aneurysm, clipping, less invasive, SILENT MRA, ultrashort echo time magnetic resonance angiography (UTE-MRA)
Introduction
Aneurysmal clipping has been widely performed for cases of both unruptured and ruptured cerebral aneurysms. Considering the possibility of the aneurysm neck remnant, de novo aneurysm, and aneurysm regrowth, follow-up imaging in the acute stage, chronic stage, and the outpatient is important.1) Usually, three-dimensional contrast-enhanced computed tomography angiography (3D-CTA) is performed for the evaluation of the clipped cerebral aneurysm and its mother vessel, but it has side effects of contrast medium and radiation exposure.2) Time-of-flight magnetic resonance angiography (TOF-MRA) is a fast and non-invasive method, but clip-induced artifact limits assessment of the artery in the vicinity of the clip.3,4) Recently, MRA with ultrashort echo time (UTE-MRA) has been attracting and reported to have the potential to overcome these disadvantages.3,5,6–14)
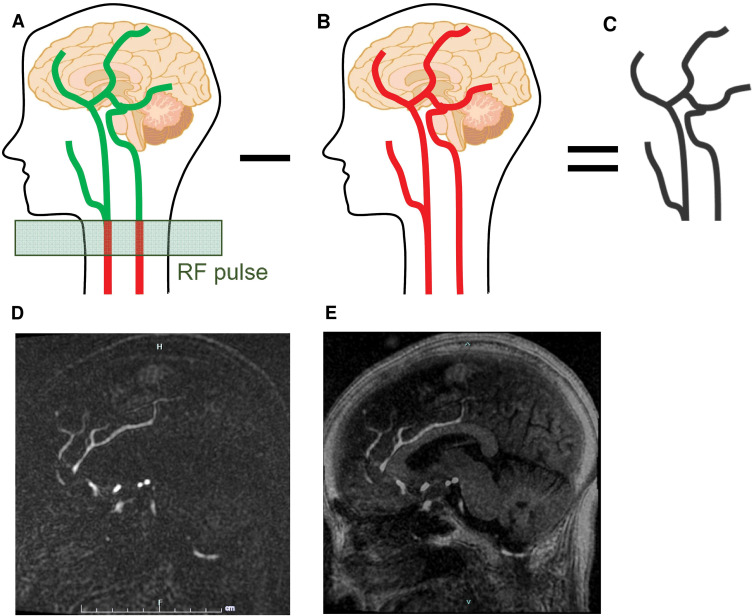
UTE-MRA is a sequence using arterial spin labeling (ASL) technique15) or inversion recovery pulses16) as well as subtraction processing.7,15–17) SILENT MRA15) (GE Healthcare Life Sciences, Buckinghamshire, UK),12,14) one of the UTE-MRA sequences, makes the blood within the carotid arteries “labeled” using a long radiofrequency inversion pulse. Once the blood is labeled, it is allowed to flow into the arteries and to be captured by the MR acquisition (Fig. 1A). The collection of a control dataset follows this (Fig. 1B), and these two datasets are subtracted to eliminate the background (Fig. 1C). Then, we can acquire the depiction of the arteries like cerebral angiography (Fig. 1D). After adding T1-weighted images to the SILENT MRA images, we can obtain detailed images similar to the conventional TOF-MRA (Fig. 1E). Notably, UTE-MRA reduces metal artifact,3,5–7) and its usefulness has been reported for the assessment of the internal flow in the intracranial stent,8,9,18) moyamoya disease,19) and arteriovenous malformation.20,21) However, a few studies reported the usefulness of specific UTE-MRA, such as pointwise encoding time reduction with radial acquisition (PETRA16); Siemens Healthcare, Erlangen, Germany)10,11) and SILENT MRA12,14) for the assessment of arteries and aneurysm neck remnant in the vicinity of a clip.
Fig. 1.
Schematic explanation of the SILENT MRA. SILENT MRA makes the blood within the carotid arteries “labeled” using a long RF inversion pulse. Once the blood is labeled, it is allowed to flow into the arteries and to be captured by the MR acquisition (A). The collection of a control dataset follows this (B), and these two datasets are subtracted to eliminate the background (C). One of the original SILENT MRA images (D). After adding T1-weighted images to the SILENT MRA images, we can obtain detailed images (E). MRA: magnetic resonance angiography, RF: radiofrequency.
We routinely use SIGNA Pioneer 3.0T (GE Healthcare Life Sciences) and perform SILENT MRA12,15) for the postoperative assessment of the arteries and aneurysm neck remnant after clipping. We hypothesized that SILENT MRA could be a first-line non- invasive imaging modality for follow-up of clipped aneurysms regardless of aneurysm location or treatment method. However, only our one case report12) and case series with 16 patients14) describe its utility for the assessment of the arteries in the vicinity of a clip. Therefore, we herein report 19 patients who underwent aneurysm clipping and were evaluated using SILENT MRA as a continuation study of our previous case report.12) Furthermore, we also statistically investigated the factors related to visibility in SILENT MRA.
Materials and Methods
Study population
This retrospective study included 19 consecutive patients who underwent surgical clipping for ruptured and unruptured aneurysms and were performed TOF-MRA, SILENT MRA between January 2019 and August 2020 in our hospital. No cases were excluded due to severe motion artifact, and all aneurysms were completely clipped without neck remnants, confirmed intraoperatively. All the patients were treated using the fourth-generation YASARGIL Aneurysm Clip System (B. BRAUN, Melsungen, Germany) made of TiAl6V4 titanium alloy (ISO 5832-2).
The study was approved by our hospital’s research ethics committee, and we gained written informed consent for this study from all of the patients, the legally authorized representative of the patients, or next of kin of the deceased patients. All methods were carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki). All personal patient information was deleted from the database for this study to protect patient privacy.
Imaging Acquisition
TOF-MRA and SILENT MRA on postoperative day 7 were used for this study, and they were acquired during the same scan session. We performed MRA on postoperative day 7 because of the following reasons. (1) Some cases would have angiographic or symptomatic vasospasms on postoperative day 7. We intentionally investigated the visibility of the mother vessels when the vessels would be relatively narrow due to vasospasm and tested the utility of SILENT MRA. (2) Some cases would still have subarachnoid hemorrhage (SAH) on postoperative day 7, affecting the vessels’ visibility, especially in TOF-MRA. Similarly, we investigated the effects of SAH for visibility in the SILENT MRA sequence. We used SIGNA Pioneer 3.0T by a 24-channel head-neck coil. The detailed scan parameters for TOF-MRA: repetition time/echo time, 24/2.8 ms; flip angle, 18 degrees; field of view, 180 × 180 mm; matrix, 182; thickness, 1.0 mm; and acquisition time, 5 minutes 13 seconds. The detailed scan parameters for SILENT-MRA: repetition time/echo time, 751/0.016 ms; flip angle, 5 degrees; field of view, 180 × 180 mm; matrix, 384; thickness, 1.2 mm; and acquisition time, 6 minutes 44 seconds. The images of SILENT MRA were acquired as sagittal images.
3D-CTA before surgery was used to measure the narrowest mother vessel diameter. 3D-CTA examinations were performed using 320-row CT scanner (Aquillion ONE; Canon Medical Systems, Tochigi, Japan). Scan parameters comprised rotation period, 1.0 sec; tube voltage, 100 kV; tube current, 420 mA; slice thickness, 0.5 mm; matrix, 512 × 512; and field of view, 200 mm. Iopamidol was used as a contrast medium.
TOF-MRA image was made with maximum intensity projection (MIP) methods using AW server (GE Healthcare Life Sciences). SILENT-MRA was made with MIP methods and volume rendering (VR) methods using AW server. Both images were used for analysis. 3D-CTA images were made using VR methods using Ziostation2 (Ziosoft, Tokyo, Japan).
Image analysis
The narrowest diameters of the mother vessels within 5 mm of the aneurysm neck were measured using a preoperative 3D-CTA reconstruction image using picture archiving and communication system (SYNAPSE; software version 2.5.3, FUJIFILM Medical Co. Ltd., Tokyo, Japan). In reviewing the images, the window width and level could be modified for evaluation. The distal anterior cerebral artery (ACD) and anterior communicating artery (ACoA) themselves were defined as mother vessels for the ACD and ACoA aneurysms, the posterior communicating (PC) artery for the internal carotid-PC (IC-PC) artery aneurysm, and the M2 portion of the middle cerebral artery (MCA) and its bifurcation for the MCA aneurysm. When the posterior communicating artery was not shown in the 3D-CTA reconstruction image, the internal carotid artery was defined as the mother vessel for the IC-PC aneurysm.
The amount of SAH around the clipping site was assessed in a fluid-attenuated inversion recovery sequence and classified into three groups: no SAH (no hematoma), small SAH (hematoma thickness <10 mm), and large SAH (10 mm ≤ hematoma thickness).
Other variables
In addition to the previous radiological variables regarding MRA or 3D-CTA, we also investigated the age, sex, Hunt and Kosnik grade, surgical approach (bifrontal or pterional), location of the aneurysm, dome and neck size (mm), the direction of the clip against the mother vessel (parallel or perpendicular), clip blade length (mm), its shape (straight, curved, bayonet, fenestrated), and the number of the clips. The presence of angiographic or symptomatic vasospasm when the SILENT MRA was performed was also investigated. Two neurosurgeons, who did not know the patients’ information, independently assessed the visibility of the mother vessel at the clipping site in TOF-MRA and SILENT MRA referring to the preoperative CTA and intraoperative findings.
Statistical analysis
To assess the association between the visibility of the mother vessels in SILENT MRA and the variables, we used the Mann–Whitney U test or Fisher’s exact test. Clip blade length of the single clip was used for statistical analysis, and those of double clips were excluded because the metal artifacts would be large in the double clips and the length of each clips varied. Interrater agreement for the visibility in TOF-MRA of SILENT MRA was evaluated by Fleiss’s fixed marginal multi-rater kappa using Online Kappa Calculator.22,23) The results were interpreted as poor (<0.40), intermediate to good (0.40–0.75), or excellent (>0.75). Continuous variables are summarized as median (range). A two-tailed p <0.05 was considered statistically significant. We conducted this calculation using SPSS software version 24.0.0. (IBM, Armonk, New York, USA).
Results
Clinical characteristics
Clinical characteristics of the 19 patients (15 women and 4 men) treated by clipping are summarized in Table 1. The median (range) age was 71 (46–84). Six patients had bifrontal approaches and 13 pterional ones. Five patients had aneurysms at the ACoA, 2 patients at the ACD, 10 at the MCA, and 2 at the IC-PC. The median dome size was 5.46 (1.30–12.00) mm, and the median neck length was 2.75 (1.38–4.88) mm. The median narrowest mother vessel diameter was 1.40 (0.49–2.03) mm. In all, 11 clips were applied parallel to the mother vessel, and 8 clips perpendicular to the mother vessel. In total, 14 patients had a single clip, and 5 had double clips. The median blade length was 9.0 (3.0–13.7) mm. Angiographic vasospasms were observed in four patients, and symptomatic ones in two patients. SAH amount was moderate in three patients and massive in the other three patients. All patients’ mother vessels were not described in TOF-MRA, and that of 16 patients (84%) were described in SILENT MRA in both MIP methods and VR methods. Overall agreement was 100% in the two neurosurgeons, and the fixed marginal kappa = 1.00 (95% CI: 0.36–1.00) (Table 1).
Table 1. Patients characteristics.
| Case No. | Age | Sex | HK Gr. | Approach | Location of an. | Dome (mm) | Neck (mm) | Narrowest mother vessel diameter (mm) | Direction of the clip | Clip | Clip blade length (mm) | Clip shape | SAH amount | Angiographic vasospasm on POD 7 | Symptomatic vasospasm on POD 7 | Mother vessel visibility in SILENT MRA (MIP) | Mother vessel visibility in SILENT MRA (VR) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | W | 2 | F | Lt. ACD | 12.00 | 4.70 | 0.67 | Parallel | FT722T, FT760T | 6.6, 11.0 | Curved, straight | - | - | - | + | + | |
| 2 | 67 | W | 3 | F | Lt. ACD | 5.00 | 1.76 | 0.80 | Perpendicular | FT602T, FT750T | 7.8, 9.0 | Fenestrated & curved, straight | - | - | - | + | + | |
| 3 | 46 | M | 2 | F | ACoA | 4.46 | 1.79 | 1.82 | Perpendicular | FT720T | 7.0 | Straight | ++ | - | - | - | - | |
| 4 | 49 | W | 2 | F | ACoA | 2.40 | 2.44 | 1.61 | Perpendicular | FT700T | 3.0 | Straight | - | - | - | - | - | |
| 5 | 71 | W | 2 | F | ACoA | 5.75 | 4.77 | 1.00 | Perpendicular | FT752T | 8.3 | Curved | + | - | - | + | + | |
| 6 | 76 | W | 2 | F | ACoA | 3.58 | 2.04 | 1.14 | Perpendicular | FT728D | 7.0 | Bayonet | - | - | - | + | + | |
| 7 | 81 | W | 2 | P (left) | ACoA | 4.00 | 3.00 | 1.89 | Parallel | FT752T | 8.3 | Curved | - | + | + | + | + | |
| 8 | 70 | W | 1 | P | Rt. IC-PC | 3.50 | 3.50 | 0.80 | Parallel | FT728D, FT700T | 7.0, 3.0 | Bayonet, straight | - | - | - | + | + | |
| 9 | 76 | W | 2 | P | Lt. IC-PC | 7.00 | 2.87 | 1.48 | Parallel | FT752T | 8.3 | Curved | + | + | - | + | + | |
| 10 | 51 | W | 3 | P | Lt. MCA | 6.48 | 3.43 | 1.70 | Parallel | FT750T | 9.0 | Straight | ++ | - | - | + | + | |
| 11 | 65 | M | 2 | P | Lt. MCA | 3.10 | 2.47 | 0.49 | Parallel | FT762T | 10.2 | Curved | - | + | + | + | + | |
| 12 | 69 | W | 3 | P | Lt. MCA | 6.94 | 4.84 | 0.71 | Parallel | FT782T | 13.7 | Curved | - | - | - | + | + | |
| 13 | 70 | W | unrup. | P | Lt. MCA | 6.69 | 2.75 | 1.03 | Perpendicular | FT740T, FT750T | 7.0, 9.0 | Straight, straight | - | - | - | + | + | |
| 14 | 71 | W | unrup. | P | Lt. MCA | 9.52 | 1.38 | 0.80 | Parallel | FT750T | 9.0 | Straight | - | - | - | + | + | |
| 15 | 75 | W | 2 | P | Lt. MCA | 1.30 | 1.52 | 1.40 | Perpendicular | FT722T | 6.6 | Curved | + | - | - | - | - | |
| 16 | 75 | W | unrup. | P | Rt. MCA | 5.46 | 2.00 | 2.03 | Parallel | FT750T | 9.0 | Straight | - | - | - | + | + | |
| 17 | 82 | M | unrup. | P | Rt. MCA | 5.91 | 4.88 | 1.50 | Parallel | FT750T, FT760T | 9.0, 11.0 | Straight, straight | - | - | - | + | + | |
| 18 | 83 | W | 4 | P | Rt. MCA | 6.64 | 3.44 | 2.00 | Parallel | FT782T | 13.7 | Curved | ++ | + | - | + | + | |
| 19 | 84 | M | unrup. | P | Rt. MCA | 5.20 | 2.10 | 1.60 | Perpendicular | FT782T | 13.7 | Curved | - | - | - | + | + | |
| Median (range) | 71 (46-84) | 15 Women/ 4 Men | 5 unruptured, 14 ruptured | 6 bifrontal, 13 pterional | 5 ACoA, 2 ACD, 10 MCA, 2 IC-PC |
5.46 (1.30-12.00) | 2.75 (1.38-4.88) | 1.40 (0.49-2.03) | 11 parallel, 8 perpendicular | 14 single, 5 double | 9.0 (3.0-13.7) | 3 moderate, 3 massive | 4 angiographic vasospasm | 2 symptomatic vasospasm | 16 visible (84%) | 16 visible (84%) |
ACoA: anterior communicating artery, ACD: distal anterior cerebral artery, F: bifrontal approach, HK Gr.: Hunt and Kosnik grade, IC-PC: internal carotid-posterior communicating artery, MCA: middle cerebral artery, MIP: maximum intensity projection, P: pterional approach, POD: postoperative day, SAH: subarachnoid hemorrhage, unrup.: unruptured aneurysm, VR: volume rendering.
Relationship between visibility in SILENT MRA and variables
We performed the Mann–Whitney U test and Fisher’s exact test. Large aneurysm dome size and long clip blade length contributed to the visibility of the mother vessel in SILENT MRA (p = 0.023, 0.007. each) (Table 2).
Table 2. Patient characteristics and difference between visible and invisible groups.
| Variables (median, range) | Visible (n = 16) | Invisible (n = 3) | p value† |
|---|---|---|---|
| Age (years) | 71 (51-84) | 49 (46-75) | 0.109 |
| Female: Male | 13:3 | 2:1 | 0.530 |
| Ruptured: Unruptured | 11:5 | 3:0 | 0.281 |
| Approach | 0.222 | ||
| bifrontal | 4 | 2 | |
| pterional | 12 | 1 | |
| Laterality of aneurysm | Uncalculated | ||
| left | 8 | 1 | |
| right | 5 | 0 | |
| center | 3 | 2 | |
| Location of aneurysm | Uncalculated | ||
| ACoA | 3 | 2 | |
| ACD | 2 | 0 | |
| MCA | 9 | 1 | |
| IC-PC | 2 | 0 | |
| Dome (mm) | 5.83 (3.10-12.0) | 2.4 (1.30-4.46) | 0.023* |
| Neck (mm) | 2.93 (1.38-4.88) | 1.79 (1.52-2.44) | 0.085 |
| Narrowest mother vessel diameter (mm) | 1.08 (0.49-2.03) | 1.61 (1.40-1.82) | 0.303 |
| Direction of the clip to the mother vessel | 0.058 | ||
| perpendicular | 5 | 3 | |
| parallel | 11 | 0 | |
| Clip blade length (mm); single clip only (n = 14) | 9.0 (7.0-13.7) | 6.6 (3.0-7.0) | 0.007* |
| Shape | Uncalculated | ||
| straight | 3 | 2 | |
| curved | 7 | 1 | |
| others or multiple | 6 | 0 | |
| Number of clips | 0.530 | ||
| single | 11 | 3 | |
| double | 5 | 0 | |
| Presence of angiographic vasospasm on POD 7 | 4 | 0 | Uncalculated |
| Presence of symptomatic vasospasm on POD 7 | 2 | 0 | Uncalculated |
| SAH | 0.222 | ||
| none | 12 | 1 | |
| present | 4 | 2 |
*p <0.05 by Mann-Whitney U test, †Mann-Whitney U test or Fisher’s exact test was performed. ACoA: anterior communicating artery, ACD: distal anterior cerebral artery, IC-PC: internal carotid-posterior communicating artery, MCA: middle cerebral artery, POD: postoperative day, SAH: subarachnoid hemorrhage.
Representative cases as visible in SILENT MRA
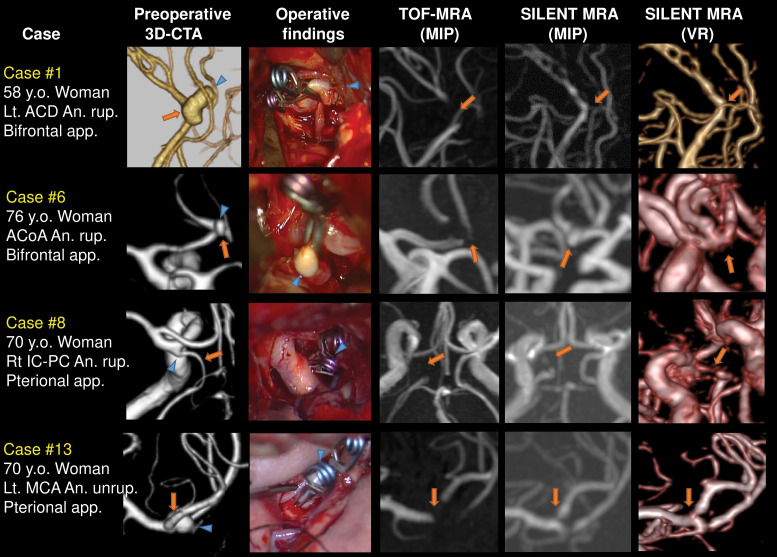
We herein describe the representative cases of clipped aneurysms at each location. Case 1: A ruptured 12.00 mm left ACD aneurysm was clipped using two clips parallel to the distal part of the anterior cerebral artery. Case 6: A ruptured 3.58 mm ACoA aneurysm was clipped using 7.0 mm bayonet clip perpendicularly to the ACoA. Case 8: A ruptured 3.50 mm right IC-PC aneurysm was clipped using two clips along the narrow posterior communicating artery, and the posterior communicating artery was revealed in SILENT MRA. Case 13: An unruptured 6.69 mm left MCA aneurysm was clipped using two clips perpendicularly to the bifurcation. TOF-MRA did not describe the mother vessels, but SILENT MRA described them. Arrows indicate the mother vessels, and arrowheads indicate the aneurysms (Fig. 2).
Fig. 2.
Representative cases, whose mother vessels were VISIBLE in SILENT MRA. Case 1: A ruptured 12.00 mm left ACD aneurysm was clipped using two clips parallel to the distal part of the anterior cerebral artery. Case 6: A ruptured 3.58mm ACoA aneurysm was clipped using 8.3 mm curved clip perpendicularly to the ACoA. Case 8: A ruptured 3.50 mm right IC-PC aneurysm was clipped using two clips parallel along the narrow posterior communicating artery, and it was revealed in SILENT MRA. Case 13: An unruptured 6.69 mm left MCA aneurysm was clipped using two clips perpendicularly to the bifurcation. Arrows indicate the mother vessels, and arrowheads indicate the aneurysms. ACD: anterior cerebral artery, ACoA: anterior communicating artery, IC-PC: internal carotid posterior communicating, MCA: middle cerebral artery, MRA: magnetic resonance angiography.
Cases as invisible in SILENT MRA
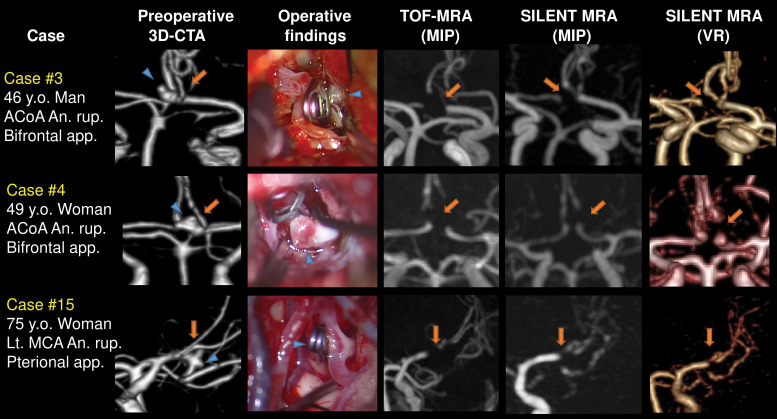
We herein describe the three cases whose mother vessels were not revealed in SILENT MRA. Case 3: A ruptured 4.46 mm ACoA aneurysm was clipped using 7.0 mm straight clip perpendicularly to the ACoA. Case 4: A ruptured 2.40 mm ACoA aneurysm was clipped using 3.0 mm short straight clip perpendicularly to the ACoA. Their mother vessels (ACoA) were not described in both TOF-MRA and SILENT MRA. Case 15: A ruptured 1.30 mm left MCA aneurysm was clipped using 6.6 mm curved clip perpendicular to the bifurcation. Both TOF-MRA and SILENT MRA did not reveal the origins of the M2 portions. Arrows indicate the mother vessels, and arrowheads indicate the aneurysms (Fig. 3).
Fig. 3.
Three cases whose mother vessels were INVISIBLE in SILENT MRA. Case 3: A ruptured 4.46 mm ACoA aneurysm was clipped using 7.0 mm straight clip perpendicularly to the ACoA. Case 4: A ruptured 2.40 mm ACoA aneurysm was clipped using 3.0 mm short straight clip perpendicularly to the ACoA. Their mother vessels (ACoA) were not described in SILENT MRA. Case 15: A ruptured 1.30 mm left MCA aneurysm was clipped using 6.6 mm curved clip perpendicular to the bifurcation. SILENT MRA did not reveal the origins of the M2 portions. Arrows indicate the mother vessels, and arrowheads indicate the aneurysms. ACOA: anterior communicating artery, MCA: middle cerebral artery, MRA: magnetic resonance angiography.
Discussion
We previously published the case report on the utility of SILENT MRA in the same way as this study.12) As a continuation study, we herein report 19 patients who underwent aneurysm clipping and were evaluated using SILENT MRA, and this report contains a relatively large number of clipped cases compared to previous reports.14) The mother vessels were revealed in 16 of the 19 patients (84%). Also, in the univariate analysis, aneurysm dome size and clip blade length were larger in the visible group compared to the invisible group in SILENT MRA. This statistical analysis is a novel point compared to our previous case report.12)
SILENT MRA and other modality
SILENT MRA can minimize the phase dispersion of the labeled blood flow signal, and decrease the metal artifact derived from clips. Therefore, SILENT MRA can describe cerebral arteries like cerebral angiography, leading to the flow visualization in the vicinity of the clip.12,14) Also, SILENT MRA can improve visualization of turbulent flow by minimizing flow-related signal dropout,24) and the SILENT MRA detect blood flow independently on the flow direction because of the ASL preparation pulse.8) Therefore, the visualization of the aneurysm remnant10) or tortuous artery like IC-PC in SILENT MRA can be better than that in TOF-MRA. Also, SILENT MRA can suppress background compared to TOF-MRA, leading to increased better quality image even in the presence of T1 hyperintense lesions such as intracranial hemorrhage, hematoma, Rathke’s cleft cyst, or paranasal sinus retention cyst.24)
The disadvantages of SILENT MRA were as follows: the total required time is longer in SILENT MRA than that in TOF-MRA because SILENT MRA needs ASL preparation7) and subtraction processing.17) As another problem, the signal in UTE-MRA is weaker than that in TOF-MRA, especially in the distal part from the neck where blood was labeled.3) Furthermore, ASL may not always be performed if there is severe neck carotid artery stenosis or metal around the neck, such as an intubation tube cuff.12) Also, the cost and the availability of the 3 tesla magnetic resonance imaging (MRI) are problems.17) Furthermore, in this study, all patients were treated using the fourth-generation YASARGIL Aneurysm Clip System made of TiAl6V4 titanium alloy (ISO 5832-2). We could not investigate metal artifacts from other clips made of other materials. Therefore, a detailed study must be done, although we should pay attention to the torque, translational force, and the metal temperature in the 3 tesla magnetic fields.25,26)
Mother vessel visibility in SILENT MRA
Compared to the Gönner’s first study3) on the assessment of the clipped artery with UTE-MRA with a TE of 2.4 ms, SILENT MRA with a much shorter TE of 0.016 ms can obtain better quality images and smaller metal artifact of clips. Besides, 3 tesla MRI improves image quality compared to 1.5 tesla MRI,10,12) and SIGNA Pioneer 3.0T can obtain with a broad range in a shorter time compared to old MRI types of equipment. This difference would be because of the advances in MRI quality and performance, especially in the technology of much shorter TE.
Kyeong14) reported the utility of SILENT MRA in 126 patients treated by 60 stent-assisted coil embolization, 46 simple coiling, 4 simple stenting, and 16 clipping. They discussed the image quality of SILENT MRA using 5-point assessment scale. The overall 126 image quality scores of TOF-MRA and SILENT MRA were 4.04 ± 0.22 and 4.64 ± 0.48, respectively (p <0.001), so SILENT MRA was superior to TOF-MRA in the image quality. In the subgroup analysis of the 16 clipped patients, the image quality scores of TOF-MRA and SILENT MRA were 1.4 ± 1.0 and 3.7 ± 0.9, respectively (p <0.001). This trend that SILENT MRA is superior to TOF-MRA to evaluate mother vessels was the same as our present study. However, their 5-point image quality scores of the clipped patients were smaller than those of the patients treated by simple coiling (4.5 ± 0.8) and simple stenting (3.8 ± 1.2). Therefore, delineation of the mother vessel after clipping in SILENT MRA seemed inferior to those after coiling or simple stenting. There would be reasons why SILENT MRA sometimes does not describe the clipped arteries.
Takubo11) reported the clip artifact characteristics in the 3 tesla UTE-MRA with PETRA16) (MAGNETOM Skyra; Siemens Healthcare). They used a homemade phantom (width 90 mm, depth 90 mm, and height 50 mm) containing a mixture of 0.125 mmol/L gadopentetate dimeglumine to mimic white matter and agarose solution. In their study, the clip head artifacts were larger than that of the clip blade, and they varied depending on the direction to the static magnetic field. Considering these clip-induced artifact characteristics, the distance between the clip head and the mother vessels would be related to the capability of vessel delineation.
Our results showed that aneurysm dome size and clip blade length were larger in the visible group compared to the invisible group in SILENT MRA. In Fig. 2, the visible cases, we used clips with long blades and ligated the aneurysm neck using relatively tips of the clips. The clip heads were distant from the mother vessels. While in Fig. 3, the invisible cases, we used clips with relatively short blades and ligated the aneurysm neck with whole blades, so the clip heads were close to the neck and mother vessels. On comparing Figs. 2 and 3, we hypothesized that the closer the clip head is to the mother vessel, the poorer the mother vessel delineation. Aneurysm dome size was also related to the visibility, and its neck was almost statistically significant (p = 0.085, Table 2) in the univariate analysis. We think that our results were acquired from the univariate analysis, so the aneurysm dome size and neck length would be confounders of the clip blade length. Considering clinical application, while a detailed study is necessary, aneurysm clipping intentionally using a long clip with ligation by the blade tip might allow for follow-up with SILENT MRA without the need for 3D-CTA.
Limitations
First, the sample size was small, and we performed only univariate analysis using the Mann–Whitney U test and Fisher’s exact test, and the number of the patients in the invisible group was very small as 3. We should continue to study what kind of aneurysm, clip shape, and surgical procedure, like how to apply the clip, can be applied to SILENT MRA for the vessel evaluation. Second, we did not perform 3D-CTA nor cerebral angiography after operations, so we could not compare the images of SILENT MRA to them. We need postoperative 3D-CTA or cerebral angiography, which would be helpful to reveal the difference between the visible and invisible groups. Third, SILENT MRA images with MIP and VR techniques can differ depending on the radiology technician, leading to over and underestimation. Fourth, SILENT MRA needs longer acquisition time and subtraction procedure, so we would take unuseful images from patients who cannot keep resting during the MRA acquisition.
Conclusions
SILENT MRA can be applied for the assessment of arteries and aneurysm neck remnant in the vicinity of a clip, and the mother vessels were revealed in 16 of the 19 patients (84%). Using clips with long blade and ligation with its tip would be related to the visibility of the mother vessels in SILENT MRA.
Ethics
This study was approved by our hospital ethics committee. The patients in our report provided written consent forms.
Acknowledgments
We are thankful for the medical staff to support our work and data acquisition.
Conflicts of Interest Disclosure
The authors report no conflicts of interest concerning the materials or methods used in this study, or the findings presented in this paper. No sources of financial or material support were received. This article and content of this study were not published or presented previously.
References
- 1).The Japan Stroke Society: [Japanese Guidelines for the Management of Stroke 2015] . Tokyo, Kyowa Kikaku, 2015. (Japanese) [Google Scholar]
- 2).van Loon JJ, Yousry TA, Fink U, Seelos KC, Reulen HJ, Steiger HJ: Postoperative spiral computed tomography and magnetic resonance angiography after aneurysm clipping with titanium clips. Neurosurgery 41: 851–856; discussion 856–857, 1997 [DOI] [PubMed] [Google Scholar]
- 3).Gönner F, Lövblad KO, Heid O, et al. : Magnetic resonance angiography with ultrashort echo times reduces the artefact of aneurysm clips. Neuroradiology 44: 755–758, 2002 [DOI] [PubMed] [Google Scholar]
- 4).Becker RL, Norfray JF, Teitelbaum GP, et al. : MR imaging in patients with intracranial aneurysm clips. AJNR Am J Neuroradiol 9: 885–889, 1988 [PMC free article] [PubMed] [Google Scholar]
- 5).Koch KM, Hargreaves BA, Pauly KB, Chen W, Gold GE, King KF: Magnetic resonance imaging near metal implants. J Magn Reson Imaging 32: 773–787, 2010 [DOI] [PubMed] [Google Scholar]
- 6).Ryu KH, Baek HJ, Moon JI, et al. : Usefulness of noncontrast-enhanced silent magnetic resonance angiography (MRA) for treated intracranial aneurysm follow-up in comparison with time-of-flight MRA. Neurosurgery 87: 220–228, 2020 [DOI] [PubMed] [Google Scholar]
- 7).Schmalbrock P, Yuan C, Chakeres DW, Kohli J, Pelc NJ: Volume MR angiography: methods to achieve very short echo times. Radiology 175: 861–865, 1990 [DOI] [PubMed] [Google Scholar]
- 8).Robson MD, Gatehouse PD, Bydder M, Bydder GM: Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. J Comput Assist Tomogr 27: 825–846, 2003 [DOI] [PubMed] [Google Scholar]
- 9).Irie R, Suzuki M, Yamamoto M, et al. : Assessing blood flow in an intracranial stent: a feasibility study of mr angiography using a silent scan after stent-assisted coil embolization for anterior circulation aneurysms. AJNR Am J Neuroradiol 36: 967–970, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Takano N, Suzuki M, Irie R, et al. : Usefulness of non-contrast-enhanced MR angiography using a silent scan for follow-up after Y-configuration stent-assisted coil embolization for basilar tip aneurysms. AJNR Am J Neuroradiol 38: 577–581, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Katsuki M, Kakizawa Y, Yamamoto Y, Nishikawa A, Wada N, Uchiyama T: Magnetic resonance angiography with ultrashort echo time evaluates cerebral aneurysm with clip. Surg Neurol Int 11: 65, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Takubo S, Kawasaki K, Nagatari T, Matsumoto M, Kageyama T: [Clinical usefulness of ultra-short TE MRA for follow-up imaging after cerebral aneurysm clipping]. Jpn. J. Radiol. Technol 76: 177–184, 2020. (Japanese) [DOI] [PubMed] [Google Scholar]
- 13).Katsuki M, Narita N, Ozaki D, Sato Y, Iwata S, Tominaga T: Three tesla magnetic resonance angiography with ultrashort echo time describes the arteries near the cerebral aneurysm with clip and the peripheral cerebral arteries. Surg Neurol Int 11: 224, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Tanoue S, Uchiyama Y, Hirohata M, et al. : Follow-up non-contrast MRA after treatment of intracranial aneurysms using microcoils with prominent metallic artifact: a comparative study of TOF-MRA and Silent MRA. Jpn J Radiol 38: 853–859, 2020. doi:10.1007/s11604-020-00981-x [DOI] [PubMed] [Google Scholar]
- 15).GE Healthcare: SIGNATM Pioneer 3.0T MRI . http://www3.gehealthcare.co.jp/ja-jp/products_and_service/imaging/magnetic_resonance_imaging/signa_pioneer (Accessed on 2020 May 22)
- 16).Grodzki DM, Jakob PM, Heismann B: Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med 67: 510–518, 2012 [DOI] [PubMed] [Google Scholar]
- 17).Kiriki M, Jomoto W, Ikeda T, Kotoura N: Imaging parameter optimization of 3D phase contrast-MRA to reduce susceptibility-artifact and radiofrequency- shielding around the intracranial stent (Japanese). Jpn J Radiol Technol 74: 1293–1301, 2018 [DOI] [PubMed] [Google Scholar]
- 18).Oishi H, Fujii T, Suzuki M, et al. : Usefulness of silent MR angiography for intracranial aneurysms treated with a flow-diverter device. AJNR Am J Neuroradiol 40: 808–814, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Tomura N, Kokubun M, Horiuchi K, Watanabe Z: Comparison of TOF-MRA and silent scan-MRA in depicting cerebral arteries in patients with Moyamoya disease. Acta Radiol 60: 1321–1328, 2019 [DOI] [PubMed] [Google Scholar]
- 20).Tomura N, Saginoya T, Kokubun M, Horiuchi K, Watanabe Z: Comparison of time-of-flight-magnetic resonance angiography from silent scan magnetic resonance angiography in depiction of arteriovenous malformation of the brain. J Comput Assist Tomogr 43: 943–947, 2019 [DOI] [PubMed] [Google Scholar]
- 21).Arai N, Akiyama T, Fujiwara K, et al. : Silent MRA: arterial spin labeling magnetic resonant angiography with ultra-short time echo assessing cerebral arteriovenous malformation. Neuroradiology 62: 455–461, 2020 [DOI] [PubMed] [Google Scholar]
- 22).Fleiss JL: Measuring nominal scale agreement among many raters. Psychol Bull 76: 378—382, 1971 [Google Scholar]
- 23).Randolph JJ: Online kappa calculator [computer software]. http://justus.randolph.name/kappa
- 24).Holdsworth SJ, MacPherson SJ, Yeom KW, Wintermark M, Zaharchuk G: Clinical evaluation of silent T1-weighted MRI and silent MR angiography of the brain. Am J Roentgenol 210: 404–411, 2018 [DOI] [PubMed] [Google Scholar]
- 25).Kakizawa Y, Seguchi T, Horiuchi T, Hongo K: Cerebral aneurysm clips in the 3-tesla magnetic field. Laboratory investigation. J Neurosurg 113: 859–869, 2010 [DOI] [PubMed] [Google Scholar]
- 26).Watanabe A, Seguchi T, Koyama J, et al. : Investigation of radiofrequency-induced temperature elevation of aneurysm clips in a 3.0-tesla magnetic resonance environment. Neurosurgery 61: 1062–1065; discussion 1065–1066, 2007 [DOI] [PubMed] [Google Scholar]