SUMMARY:
Interhypothalamic adhesion is a newly described disease entity, characterized by an abnormal parenchymal band connecting the medial margins of the hypothalami across the third ventricle. Additional anomalies, including cleft palate, gray matter heterotopia, cerebellar hypoplasia, optic atrophy, hippocampal under-rotation, and white matter lesions, may coexist. The purpose of this clinical report is to describe the imaging findings from a series of 13 patients with interhypothalamic adhesions discovered on brain MR imaging.
An interhypothalamic adhesion (IHA) is a horizontally oriented parenchymal band connecting the hypothalami across the third ventricle. Its architecture resembles that of a normal interthalamic adhesion. Although there are no known cases with histologic correlation, it may represent a choristoma. IHA could be considered a form fruste holoprosencephaly because additional midline abnormalities may be present in these patients.1 Indeed, failed hypothalamic separation of varying degrees is a constant feature of the classic holoprosencephaly spectrum.2
After institutional review board approval and waiver, all cases of IHA encountered by the neuroradiology service during a 1-year period from 2 separate academic children's hospitals were retrospectively reviewed. We present MR imaging findings from 13 separate patients with interhypothalamic adhesions.
Materials and Methods
Case Series
Thirteen patients were documented, with a mean age of 4 ± 3 years (range, 3 days to 15 years) and a balanced sex distribution (On-line Table). MR imaging examinations from each patient were analyzed by 2 board-certified neuroradiologists (M.T.W. and G.V.); a consensus was reached in each case. All scans were obtained on either a 1.5 or 3T MR imaging scanner (Signa HDxt; GE Healthcare, Milwaukee, Wisconsin) and included at least the following pulse sequences: sagittal spoiled gradient-recalled-echo (SPGR) T1WI reformatted into axial and coronal planes, axial T2WI, axial T2 FLAIR, axial DWI, and coronal fat-saturated T2WI. In each patient, the signal intensity of the IHA was evaluated in the following planes and sequences: axial (T2WI, T2 FLAIR, and SPGR T1WI), coronal (SPGR T1WI and fat-saturated T2WI), and sagittal (SPGR T1WI). All examinations were of diagnostic quality. No exclusionary criteria prevented accurate evaluation.
The mean interhypothalamic adhesion volume was 29.4 ± 30.1 mL (range, 2.1–187.2 mL), assuming an ellipsoid with 3 orthogonal caliper-based measurements acquired from the sagittal SPGR T1WI and coronal and axial reformats representing the axes (volume = d1 × d2 × d3 × pi/6) where d = diameter. IHA locations involved portions of the expected area of the dorsomedial (n = 10), ventromedial (n = 7), arcuate (n = 6), paraventricular (n = 5), and preoptic (n = 3) nuclei; IHA locations were categorized on the basis of pre-existing literature.3 In all cases, the IHA was isointense to gray matter on all sequences and connected the medial walls of the hypothalami horizontally. Additional brain abnormalities were detected in 12 of 13 patients; midline anomalies were present in 12 of 13 patients. Intravenous gadolinium was administered in 6 patients; none of the interhypothalamic adhesions demonstrated contrast enhancement. Pituitary hypoplasia was present in 1 patient. The pituitary gland and stalk were normal in all remaining patients.
Case 1.
A 9-month-old boy status post cleft palate repair was referred to exclude congenital brain anomalies. Brain MR imaging revealed an interhypothalamic adhesion in addition to olfactory bulb hypoplasia, hippocampal under-rotation, and falx cerebri hypoplasia (Fig 1). An IHA connected the hypothalami in the expected area of the dorsomedial hypothalamic nuclei.
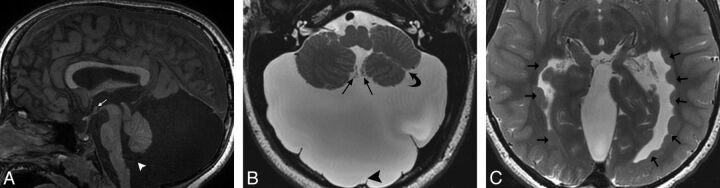
Fig 1.
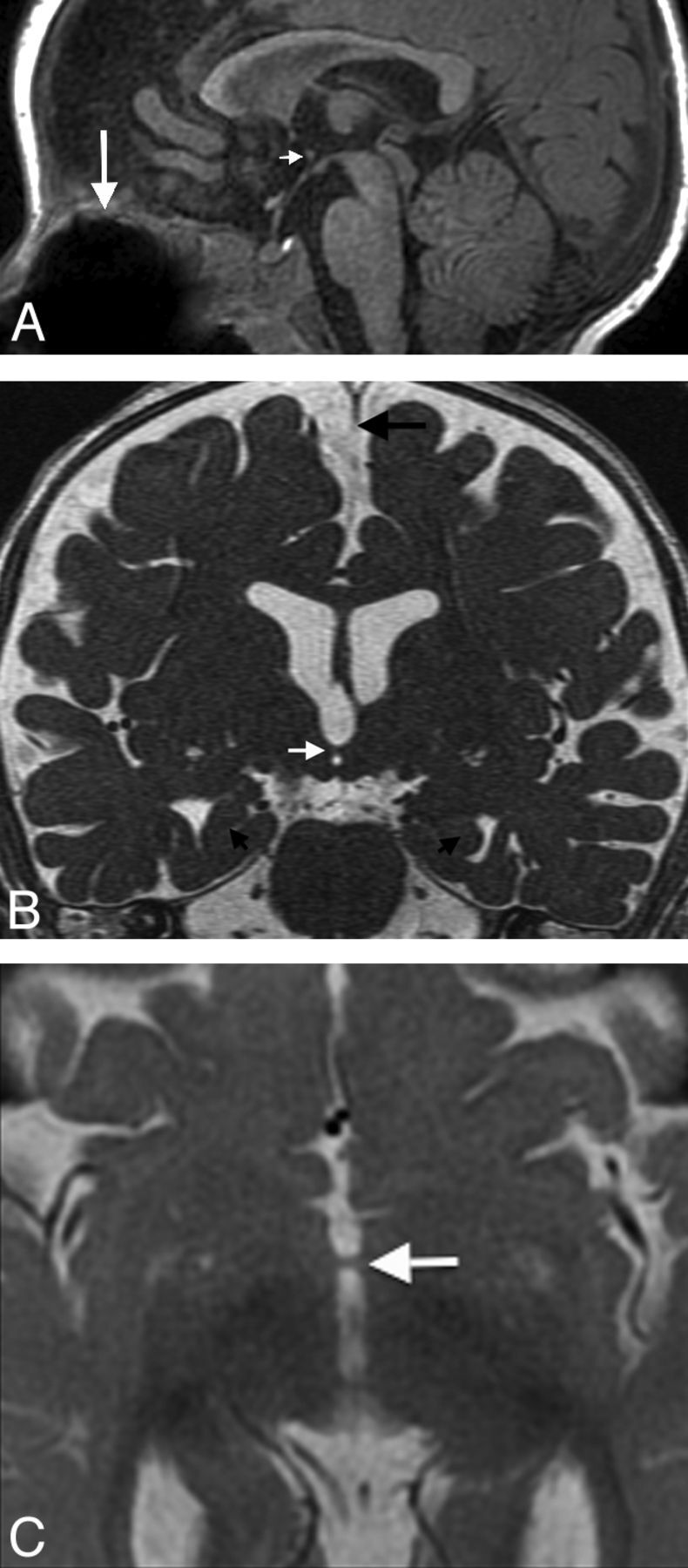
A, Sagittal fast-spoiled gradient recalled brain volume T1WI (TR/TE/TI, 10/4/450 ms) showing a parenchymal band representing an interhypothalamic adhesion in the third ventricle between the anterior commissure and mammillary body (small arrow). The pituitary infundibulum, adenohypophysis, and neurohypophysis are normal. Note metallic susceptibility artifacts in the roof of the oral cavity from prior cleft palate repair (large arrow). B, Coronal FIESTA with phase cycling (TR/TE, 10/4 ms) demonstrates an IHA traversing the third ventricle (white arrow), oblique axes of the hippocampal heads representing under-rotation (small black arrows), and incomplete formation of the falx cerebri (large black arrow). C, Axial T2WI (TR/TE, 5000/85 ms) shows an IHA connecting the medial hypothalamic margins across the third ventricle (arrow).
Case 2.
A 2-month-old preterm female, delivered early for intrauterine growth retardation due to maternal lupus and hypertension, underwent a term-equivalent brain MR imaging. It demonstrated an IHA, mild hippocampal under-rotation, and mild cerebral white matter hypoplasia. The IHA was located in the anterior/inferior third ventricle. It was centered in the expected area of the dorsomedial/ventromedial hypothalamic nuclear junction but extended inferiorly to abut the median eminence in the expected area of the arcuate nucleus.
Case 3.
A 4-year-old boy underwent brain MR imaging for headaches and vomiting. Medical history was remarkable for Leber congenital amaurosis, small body habitus, facial dysmorphia, hearing loss, developmental delay, and hypotonia. Findings of a chromosomal microarray were normal. Brain MR imaging depicted an IHA, optic pathway atrophy, and an old frontal lobe white matter injury. The IHA traversed the anterior/inferior third ventricle, partially inseparable from the forniceal columns and spanning to the third ventricular floor. It was centered in the expected area of the dorsomedial and ventromedial hypothalamic nuclei and extended inferiorly to the region of the arcuate nucleus.
Case 4.
A 6-month-old term-gestation boy, with a history of global hypotonia, failure to thrive, cyanosis, and upper respiratory tract infection, presented for brain MR imaging evaluation. A chromosomal microarray showed areas of homozygosity consistent with parental consanguinity. Metabolic work-up demonstrated normal urine organic acid levels, plasma amino acid levels, creatine kinase levels, and lactate/pyruvate ratios. Imaging revealed an IHA in the anterior/inferior third ventricle and under-rotation of the left hippocampus. Centered at the level of the superior margin of the brain stem, the IHA was located anterior to the forniceal columns in the region of the dorsomedial and ventromedial hypothalamic nuclei. A follow-up brain MR imaging at 11 months showed no change apart from normal myelination progression.
Case 5.
A 5-year-old boy with developmental delay, seizures, and presumed Dandy-Walker malformation continuum diagnosed on prenatal sonography underwent brain MR imaging. An IHA was present in the anterior/inferior third ventricle, partially integrated into the forniceal columns. It was centered in the expected area of the dorsomedial and ventromedial hypothalamic nuclei and extended inferiorly to the region of the arcuate nucleus. Additional abnormalities included cerebellar hypoplasia/dysplasia, brain stem hypoplasia, Blake pouch cyst, falx cerebri deficiency, malformations of occipital development, hippocampal dysgenesis, and periventricular nodular heterotopia (Fig 2). A follow-up brain MR imaging performed at 9 years of age showed no change.
Fig 2.
A, Sagittal SPGR T1WI (TR/TE/IR, 8/3/450 ms) demonstrating a nodular structure isointense to gray matter consistent with an IHA (white arrow) located at the level of the upper midbrain in the anterior/inferior third ventricle, spanning to the third ventricular floor. A Blake pouch cyst is present, enlarging the posterior fossa and communicating with the fourth ventricle via a widened foramen of Magendie (white arrowhead) and without accompanying vermian malrotation. Note also generalized vermian and brain stem hypoplasia. B, Axial T2 fast-spoiled gradient recalled image (TR/TE, 3049/99 ms) shows a large retrocerebellar cystic lesion markedly enlarging the posterior fossa, associated with an incomplete falx cerebelli (arrowhead). Choroid plexus is seen extending from the foramen of Magendie along its anteromedial walls (straight arrows), consistent with a Blake pouch cyst. The cerebellum is dysplastic with irregular foliation and a left cerebellar hemispheric cleft (curved arrow). C, Axial T2 fast-spoiled gradient recalled scan (TR/TE, 3049/99 ms) depicts periventricular nodular heterotopia along the lateral ventricular atria and occipital and temporal horns (arrows).
Case 6.
An 8-month-old girl presented with lethargy and seizures after an upper respiratory tract infection. She was found to have hyponatremia, presumed secondary to polydipsia. MR imaging demonstrated an IHA and hippocampal under-rotation. The IHA was located midway between the anterior commissure and tuber cinereum, just posterior to the lamina terminalis. It was centered at the expected junction of the paraventricular and dorsomedial hypothalamic nuclei.
Case 7.
A 7-year-old boy was imaged for emesis and headaches. Brain MR imaging showed an interhypothalamic adhesion centered in the anterior/inferior third ventricle (Fig 3A). The IHA was partially integrated into the forniceal columns and mammillary bodies, centered at the upper margin of the mesencephalon but spanning to the tuber cinereum. It was centered in the area of the dorsomedial and ventromedial hypothalamic nuclei and extended inferiorly to the arcuate nucleus. Additional abnormalities included cerebral white matter hypoplasia, remote bifrontal white matter injury, and hippocampal dysgenesis and under-rotation. An unusually deep sulcus was present, extending through the left anterior precuneus and posterior cingulate gyrus into the margin of the callosal splenium, associated with focal thinning of the splenium (Fig 3B, -C).
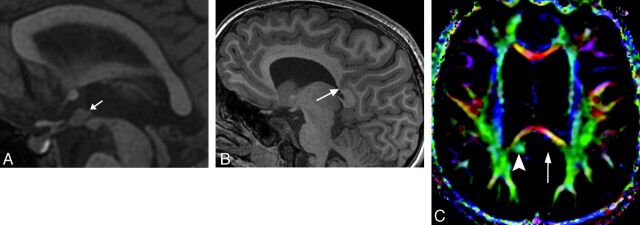
Fig 3.
A, Sagittal SPGR T1WI (TR/TE/IR, 8/3/450 ms) demonstrating a nodular structure isointense to gray matter consistent with IHA (arrow) located at the level of the upper midbrain in the inferior third ventricle, extending to the tuber cinereum. B, Sagittal SPGR T1WI (TR/TE/IR, 8/3/450 ms) demonstrates an unusual deep parietal sulcus extending through the cingulate gyrus and distorting the callosal splenium architecture (arrow). C, Axial directionally encoded color map of DTI data (15 directions of encoding; TR/TE, 10,000/82 ms) shows absence of the normal green hue of the left cingulum isthmus (long arrow). Note the normal right cingulum isthmus (arrowhead).
Case 8.
A 14-year-old girl was referred to brain MR imaging for delirium. An IHA was centered across the anterior third ventricle at the level of the superior midbrain margin, abutting the lamina terminalis. The IHA spanned portions of the area where one would expect to locate the preoptic, paraventricular, and dorsomedial hypothalamic nuclei. The brain was otherwise normal.
Case 9.
A 4-month-old girl underwent brain MR imaging for infantile spasms. She had no other significant medical history. An IHA was present in the anterior third ventricle, centrally located among the lamina terminalis, forniceal columns, optic chiasm, and anterior commissure in the region of the paraventricular nucleus. Parieto-occipital white matter hypoplasia and hippocampal under-rotation were also evident.
Case 10.
A 26-month-old girl with a history of Chiari II malformation status post myelomeningocele repair presented for MR imaging to exclude hydrocephalus. The patient was also status post cranioplasty for unicoronal synostosis. An IHA was seen in the anterior third ventricle midway between the median eminence and anterior commissure (Fig 4A) in the approximate location of the paraventricular nucleus. Parenchymal stigmata of Chiari II malformation were present, including a small posterior fossa, tectal beaking, and mild corpus callosum dysgenesis (Fig 4B). In addition, partial rhombencephalosynapsis (Figs 4A, -B) and moderate ventriculomegaly were present. The hippocampi were under-rotated.
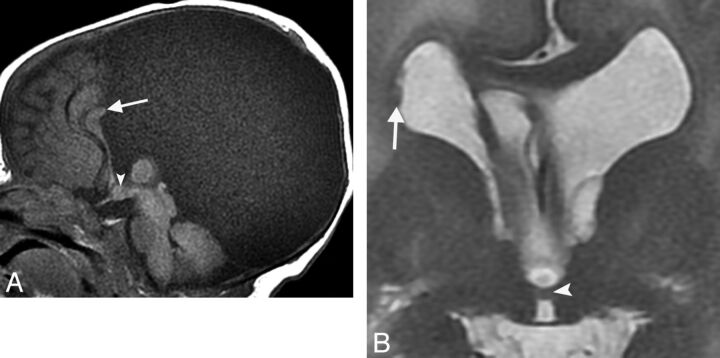
Fig 4.
A, Sagittal SPGR T1WI (TR/TE/IR, 11/5/500 ms) demonstrating a small nodular structure representing an IHA located in the midanterior third ventricle in the expected area of the paraventricular nucleus (arrowhead). Parenchymal stigmata of a Chiari II malformation include a small posterior fossa, tectal beaking (curved arrow), and mild corpus callosum dysgenesis. In addition, partial rhombencephalosynapsis is present with loss of the normal architecture of the posterior vermis (straight arrow). B, Coronal fat-saturated T2WI (TR/TE, 3931/100 ms) shows continuous transverse cerebellar hemispheric folia and fissures extending across the midline, representing absence of the posterior vermis and partial rhombencephalosynapsis.
Case 11.
A 3-day-old term boy was referred for evaluation of a prenatally diagnosed intracranial cyst. Brain MR imaging demonstrated an IHA connecting the expected areas of the dorsomedial, ventromedial, and arcuate nuclei (Fig 5A). A large midline meningeal cyst, septum pellucidum fenestration, and corpus callosum dysgenesis were present (Fig 5A). Periventricular nodular heterotopia (Fig 5B), a thickened thalamic mass intermedia, under-rotation and dysgenesis of the hippocampi, and hydrocephalus were also evident.
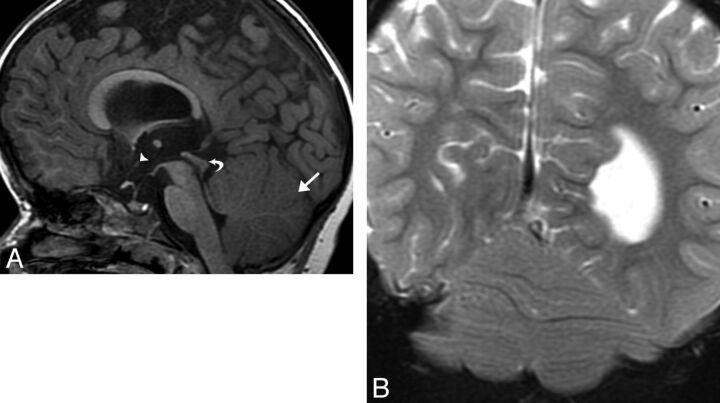
Fig 5.
A, Sagittal T1WI (TR/TE, 400/9 ms) depicting a nodular structure occupying the anterior/inferior third ventricle, consistent with an IHA (arrowhead). A large interhemispheric meningeal cyst is associated with corpus callosal dysgenesis (arrow). B, Coronal T2WI (TR/TE, 2217/103 ms) shows a horizontal parenchymal band adjoining the medial thalami, representing an IHA (arrowhead), and periventricular nodular heterotopia (arrow).
Case 12.
A 3-year-old girl presented for evaluation of developmental delay and encephalopathy. Brain MR imaging revealed a tiny IHA connecting the region of the preoptic and paraventricular hypothalamic nuclei. With the exception of a 10-mm complicated pineal cyst, the remainder of the brain was normal for age.
Case 13.
A 15-year-old boy was referred for evaluation of anosmia. His medical history was positive for congenital digital hypoplasia. Brain MR imaging showed an IHA encompassing nearly the entire medial surface area of the hypothalamus. The bases of the mammillary bodies were apposed. The corpus callosum was mildly thickened, and the pituitary gland was hypoplastic. Olfactory bulbs and tracts were aplastic. A subependymal nodular heterotopian was present in the right parietal lobe.
Discussion
An interhypothalamic adhesion is a newly described entity characterized by an abnormal band of tissue adjoining the medial hypothalami to one another across the third ventricle.1 The appearance of the interhypothalamic adhesion is quite characteristic, always visible in the sagittal plane as an additional small ovoid structure between the fornices and lamina terminalis, centered near the level of the midbrain cranial margin (Fig 6). Anatomically, this location corresponds to the anterior and tuberalis regions of the periventricular hypothalamus. Although there are no known cases with histologic correlation, interhypothalamic adhesion may represent a choristoma or a focal area of failed hypothalamic separation.
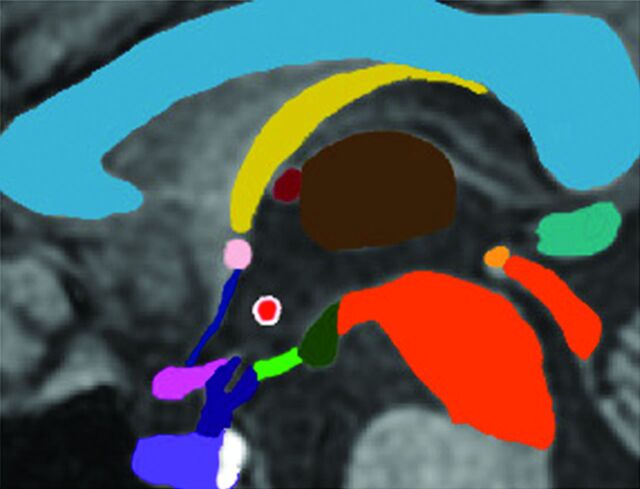
Fig 6.
Midline sagittal graphic depicting the hypothalamus and neighboring structures. With the exception of the mammillary bodies (dark green) and tuber cinereum (bright green), the hypothalami are not visible at midline. The location of the IHA (bright red/white margin) is typical, centered in the anterior/inferior third ventricle. Choroid plexus is present in the anterior/superior third ventricle (dark red). The corpus callosum (light blue), fornix (yellow), thalamus (brown), midbrain (dark orange), posterior commissure (bright orange), pineal gland (teal), lamina terminalis (dark blue), anterior commissure (light pink), optic chiasm (dark pink), pituitary stalk (dark purple), adenohypophysis (light purple), and neurohypophysis (white) are represented.
The hypothalamus borders much of the anteroinferior third ventricle (Fig 6). The normal anatomic boundaries of the hypothalamus are the hypothalamic sulcus (superior), roof of the suprasellar cistern (inferior), an imaginary line between the intraventricular foramina and mid-optic chiasm (rostral), and mammillary body/midbrain tegmentum junction (caudal).4 Various nuclei and white matter tracts are the foundation of this functionally diverse and critical organ. Although current imaging techniques are incapable of defining the precise boundaries of the hypothalamic zones and nuclei, Lemaire et al3 have devised a scheme for segmentation based on universally visible anatomic landmarks.
The interhypothalamic adhesion is a band of tissue that structurally connects, at the very least, the periventricular hypothalamic zones and, potentially, portions of the hypothalamic nuclei. In most patients (10/13), the IHA traversed the expected area of the dorsomedial and/or ventromedial hypothalamic nuclei. The arcuate or paraventricular nuclei appeared involved in 6 patients. The IHA involved the preoptic nucleus area in 3 patients. One patient with Kallmann syndrome (case 13) had pituitary hypoplasia. None of the other patients in this cohort had a documented history of endocrinopathy, hypotension, thermoregulatory abnormality, obesity, or abnormal fear response; the pituitary gland was structurally normal in all other patients.
Holoprosencephaly represents a spectrum of failed prosencephalon separation. The range of classic holoprosencephaly includes 3 subtypes in decreasing order of severity: alobar, semilobar, and lobar.5–7 The mildest form of classic holoprosencephaly manifests with partial forebrain and diencephalic union. The hypothalamus is incompletely separated in the mildest of forms of classic holoprosencephaly. A retrospective study by Simon et al2 demonstrated hypothalamic union in all patients with holoprosencephaly in their cohort. An isolated interhypothalamic adhesion could represent form fruste holoprosencephaly, and as such, its presence should prompt a search for additional midline anomalies.1 Additional manifestations of holoprosencephaly may include ocular anomalies, cleft lip, cleft palate, absent septum pellucidum, falx cerebri hypoplasia, under-rotation of the hippocampi, and/or corpus callosum dysgenesis.8–11 Hippocampal abnormalities were present in 9 of 13 patients, and hypoplasia of the falx cerebri was present in 2 patients. Symmetric hypoplasia of the optic bulbs was present in 2 patients.
Rhombencephalosynapsis is a midline cerebellar anomaly that can coexist with holoprosencephaly12; partial rhombencephalosynapsis associated with Chiari II malformation was present in 1 patient. Additional midline anomalies outside the typical holoprosencephaly disease spectrum were also encountered in this case series. Some of these midline abnormalities included corpus callosum dysgenesis, cysts (Blake pouch, pineal, meningeal), Kallmann syndrome, brain stem hypoplasia, and malformations of cerebral and cerebellar development.
Care should be taken to distinguish normal regional anatomy from an IHA. Choroid plexus occupying the anterior/superior third ventricle can mimic an IHA; however, its appearance is characteristic (Fig 6). Coronal images can exclude a hypothalamic connection. A small third ventricle with apposing lateral walls could conceivably be mistaken for IHA; thin-section coronal and/or axial images are useful for making the distinction. Furthermore, small interhypothalamic adhesions could be easily missed without thin-section images.
The differential diagnosis for IHA includes hypothalamic hamartoma and glioma. Both hamartoma and IHA are isointense to gray matter. However, a hamartoma is sessile or pedunculated in morphology, often extending into the third ventricle but not connecting the medial hypothalami to one another. Gliomas are of different signal intensity than gray matter and may enhance after contrast material administration. If this diagnosis is in question, gadolinium should be administered and postcontrast images should be acquired for clarification.
Conclusions
Interhypothalamic adhesions are parenchymal bands of tissue connecting the medial hypothalami across the third ventricle. Accompanying structural abnormalities range from mild hippocampal under-rotation to severe malformations of brain development. Midline anomalies are coexistent in most patients, supporting a type of form fruste holoprosencephaly. Therefore, midline structures should be scrutinized carefully on discovery of an interhypothalamic adhesion.
Supplementary Material
ABBREVIATIONS:
- IHA
interhypothalamic adhesion
- SPGR
spoiled gradient-recalled-echo
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology and the Foundation of the ASNR Symposium, May 17-22, 2014; Montreal, Quebec, Canada.
REFERENCES
- 1. Whitehead MT, Angel JD. Interhypothalamic adhesion in a 9 month-old male with cleft palate. Case Rep Radiol 2013;2013:197415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simon EM, Hevner R, Pinter JD, et al. Assessment of the deep gray nuclei in holoprosencephaly. AJNR Am J Neuroradiol 2000;21:1955–61 [PMC free article] [PubMed] [Google Scholar]
- 3. Lemaire J, Nezzar H, Sakka L, et al. Maps of the adult human hypothalamus. Surg Neurol Int 2013;4(suppl 3):S156–6323682342 [Google Scholar]
- 4. Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. 4th ed. Berlin: Springer-Verlag; 2008:289–90 [Google Scholar]
- 5. Tortori-Donati P, Rossi A, Biancheri R. Brain malformations. In: Tortori-Donati P, Rossi A, eds. Pediatric Neuroradiology: Brain, Head, Neck and Spine. Berlin: Springer-Verlag; 2009:86–94 [Google Scholar]
- 6. Swaiman K, Ashwal S, Ferriero DM, et al. Swaiman's Pediatric Neurology: Principles and Practice. 5th ed. China: Elsevier Saunders; 2012:151–84 [Google Scholar]
- 7. Dias M, Partington M. Normal and abnormal embryology of the brain. In: Winn HR, ed. Youmans Neurological Surgery. 6th ed. Philadelphia: Elsevier Saunders; 2011:1883–97 [Google Scholar]
- 8. Cohen MM Jr. Holoprosencephaly: clinical, anatomic, and molecular dimensions. Birth Defects Res A Clin Mol Teratol 2006;76:658–73 [DOI] [PubMed] [Google Scholar]
- 9. Gawrych E, Janiszewska-Olszowska J, Walecka A, et al. Lobar holoprosencephaly with a median cleft: case report. Cleft Palate Craniofac J 2009;46:549–54 [DOI] [PubMed] [Google Scholar]
- 10. Huang J, Wah IY, Pooh RK, et al. Molecular genetics in fetal neurology. Semin Fetal Neonatal Med 2012;17:341–46 [DOI] [PubMed] [Google Scholar]
- 11. Sato N, Hatakeyama S, Shimizu N, et al. MR evaluation of the hippocampus in patients with congenital malformations of the brain. AJNR Am J Neuroradiol 2001;22:389–93 [PMC free article] [PubMed] [Google Scholar]
- 12. Ishak GE, Dempsey JC, Shaw DW, et al. Rhombencephalosynapsis: a hindbrain malformation associated with incomplete separation of midbrain and forebrain, hydrocephalus and a broad spectrum of severity. Brain 2012;135(pt 5):1370–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.