SUMMARY:
Transcranial Doppler ultrasonography has been used to detect microemboli in the middle cerebral artery during orthopedic surgery. We conducted a comprehensive systematic literature review of transcranial Doppler ultrasonography in orthopedic surgery to evaluate its status in this setting. Fourteen studies were selected for qualitative analysis. The highest number of patients studied was 45; emboli were detected in all studies, occurring in 20%–100% of patients. Most embolic counts were below 10, but some high counts were noted. No study reported all the technical parameters of the transcranial Doppler ultrasonography. All studies assessed neurologic status, and 6 studies evaluated cognitive function postoperatively. No study identified an association between postoperative cognitive function and embolic count. Six studies sought the presence of right-to-left shunts.
Transcranial Doppler uses sonography to study cerebral blood flow velocity and can be used to detect microembolic material in the cerebral circulation. In orthopedic surgery, sonography was originally introduced to detect fat embolism in the venous system after long bone fracture1–3 and subsequently to detect microemboli in the middle cerebral artery.4 In general, microemboli that enter the venous circulation are largely filtered out by the pulmonary circulation but, in some cases, may cross into the systemic circulation by either pulmonary shunts or a right-to-left shunt within the heart.
In addition to fat, it is believed that other solid particles such as thrombus, tissue, and cement may pass into the circulation as microemboli during orthopedic surgery. Gas microemboli have also been noted during orthopedic surgery,5 though the exact source is open to conjecture. Air may enter blood vessels in the surgical field, originate in the intravenous solutions, or arise from agitation during surgical manipulation.
Transcranial Doppler ultrasonography (TCD) has been used in orthopedic surgery to count these microemboli that are detected as high-intensity transient signals in (HITS) the MCA. In addition to counting the number of microemboli in the cerebral circulation, investigators have also sought to identify which specific surgical manipulations lead to microemboli entering the cerebral circulation and thus potentially placing the patient at risk.6,7
Large emboli in the arterial circulation that reach the brain may cause overt neurologic symptoms (ie, stroke). Smaller microemboli may cause more subtle neurologic damage manifesting as postoperative cognitive dysfunction.8 Several studies have sought to identify whether neurologic or cognitive change is related to the number of HITS (microemboli) detected in the MCA.
The use of TCD requires technical expertise and accurate interpretation of the signal. Differences in criteria used for signal identification originally caused confusion and led a Consensus Committee in 1995 to recommend the criteria for identification of HITS (Table 1).9 The Consensus Committee suggested that investigators specify ultrasonic frequency, dynamic range, gain settings, and identification criteria for classifying HITS. A subsequent International Consensus Group recommended that specific parameters of the ultrasonic device be reported (Table 2) in addition to the detection criteria and also stated that automatic emboli detection did not have the required sensitivity and specificity for clinical use.10
Table 1:
Criteria for identification of high-intensity transient signals
| Criteria |
|---|
| 1) Doppler microembolic signal is transient, usually lasting <300 ms |
| 2) The amplitude is usually at least 3 dB higher than the background blood flow |
| 3) A signal is unidirectional within the Doppler velocity spectrum |
| 4) Microembolic signal is accompanied by an audible output |
Table 2:
Recommended parameters to be reported for transcranial Doppler ultrasound
| Parameters |
|---|
| 1) Ultrasound device |
| 2) Transducer type |
| 3) Insonated artery |
| 4) Insonation depth |
| 5) Algorithms for signal-intensity measurement |
| 6) Scale settings |
| 7) Detection threshold |
| 8) Axial extension of sample volume |
| 9) Fast Fourier transformation size (no. of points used) |
| 10) Fast Fourier transformation length (time) |
| 11) Fast Fourier transformation overlap |
| 12) Transmitted ultrasound frequency |
| 13) High-pass filter settings |
| 14) Recording time |
We systematically reviewed studies that used TCD intraoperatively in orthopedic surgery to detect cerebral emboli with the following specific aims:
-
1)
To identify whether the investigators have documented the features of the TCD device and detection criteria (as recommended)
-
2)
To determine the number of emboli released during orthopedic surgery.
We also recorded whether the studies also sought to identify right-to-left shunts as an explanation for the cerebral emboli count or measure neurologic or cognitive change as a result of the cerebral emboli count.
Materials and Methods
Eligibility Criteria
Eligibility criteria for article selection were any publication from 1965 through 2012 that used TCD during orthopedic surgery to detect HITS in the MCA. The publications must have been in the English language and contain surgical reports of intraoperative patient data of microemboli in the cerebral arteries in >1 case (ie, single case reports were excluded, but case series were included).
Study Identification
Electronic searches of MEDLINE and EMBASE were performed with results limited to the English language from 1965 to August 2012 inclusive. Three broad categories were combined to find appropriate articles for review. The categories were Doppler transcranial ultrasonography, orthopedic surgery, and embolism. The specific search strategies used in each data base are listed below.
Full-text articles were retrieved for any citations that were considered potentially relevant. Additional articles were sought by searching bibliographies of previous articles and review articles. Relevant journal and conference proceedings were also hand-searched. Inclusion criteria were English language, prospective, intraoperative, orthopedic surgery, and use of TCD. Exclusion criteria were the use of TCD preoperatively or postoperatively, Doppler used other than transcranially, and nonorthopedic surgery. Reports of single cases were excluded, but case series consisting of multiple cases were included.
MEDLINE (Searched on www.EBSCOhost.com)
A combination of MeSH terms and keywords were used as follows: [{(MH “Ultrasonography, Doppler” OR MH “Blood Flow Velocity” OR “blood flow velocity” OR “Doppler”) AND “transcranial”} OR MH “Ultrasonography, Doppler, Transcranial” OR “high-intensity transient signals”] AND [MH “Orthopedic Procedures +” OR “orthopedic*” OR “orthopedic*” OR “arthroplasty” OR “fracture*” OR MH “Musculoskeletal Diseases+/SU”] AND [MH “Intracranial Embolism” OR MH “Embolism” OR MH “Embolism, Fat+” OR “embol*” or “microembol*” or “micro-embol*”].
Twenty-nine results were selected from the MEDLINE search.
www.EMBASE.com
A combination of EMTREE terms and keywords were used as follows: [(“Doppler echography”/de OR “Doppler” OR “high-intensity transient signals” OR “blood flow velocity” OR “blood flow velocity”/de) AND “transcranial”] AND [“orthopedic surgery”/exp OR “orthopedic*” OR “orthopedic*” OR “arthroplasty” OR “fracture*”] AND [“embolism”/de OR “fat embolism”/de OR “embol*” OR “microembol*” OR “microembolism”].
Thirty results were selected from the EMBASE search.
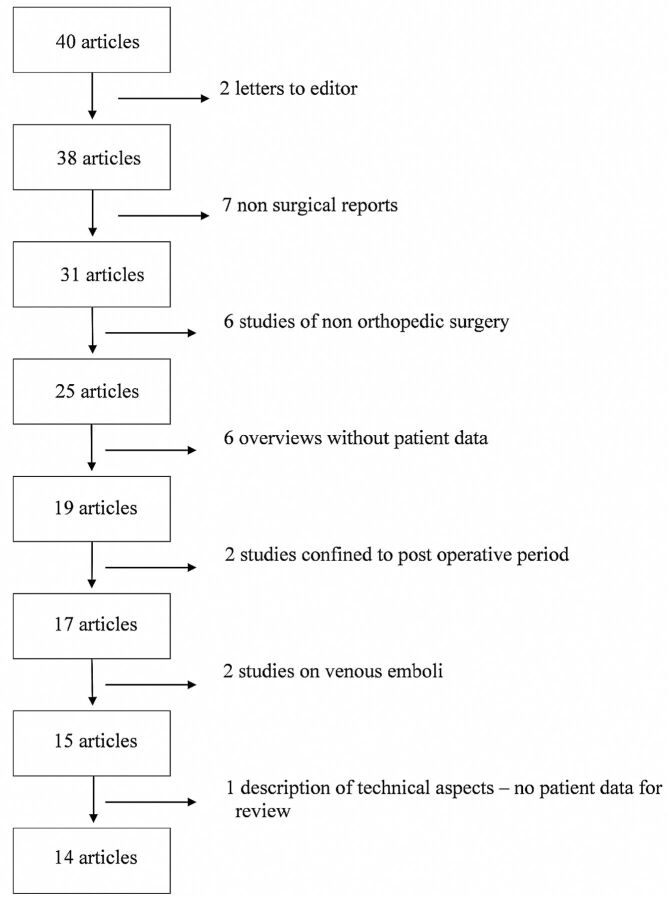
Fifty-nine articles were identified; screening removed 19 duplicates, leaving 40 eligible articles for review by 2 independent researchers. Fourteen articles satisfied the selection criteria (vide supra) for both researchers and were included for qualitative synthesis. Both independent researchers selected these 14 articles, so there was no need for an adjudication process. The reasons for eliminating articles are shown in Fig 1.
Fig 1.
Reasons for exclusion of articles after selection.
Of the 2 articles by Rodriguez et al in 2001,11,12 the first describes 13 adolescents and the second describes 4 adolescents who appear to be a subset of the first article but have been included because they additionally underwent transesophageal echocardiography (TEE). Similarly, the 12 patients included by Patel et al13 were also described in the subsequent article as part of a larger study7; both articles have been included because cognitive decline was sought in the larger study but not in the former study.
Although the primary search related to the use of TCD to detect microemboli in the cerebral circulation, we also noted whether the studies additionally sought the presence of a patent foramen ovale (PFO) or assessed the neurologic or cognitive changes postoperatively.
Results
We found 14 publications that satisfied the inclusion criteria (Table 3). Ten of these publications additionally investigated the presence of PFO by using echocardiography or TCD after intravenous injection of agitated saline. All 14 publications made some comment on postoperative neurologic status, ranging from the presence or absence of neurologic complications to more subtle cognitive changes.
Table 3:
Studies selected for review
| Study No. | Study Name | Year | No. of Patients Studied |
|---|---|---|---|
| 1 | Forteza et al4 | 1999 | 5 |
| 2 | Sulek et al20 | 1999 | 22 |
| 3 | Edmonds et al17 | 2000 | 23 |
| 4 | Rodriguez et al11 | 2001 | 13 |
| 5 | Rodriguez et al12 | 2001 | 4 |
| 6 | Riding et al15 | 2004 | 41 |
| 7 | Rodriguez et al19 | 2005 | 37 |
| 8 | Kalairajah et al18 | 2006 | 24 |
| 9 | Koch et al8 | 2007 | 24 |
| 10 | Barak et al5 | 2008 | 22 |
| 11 | Gray et al6 | 2008 | 20 |
| 12 | Gray et al22 | 2009 | 20 |
| 13 | Patel et al13 | 2009 | 24 |
| 14 | Patel et al7 | 2010 | 45 |
On-line Tables 1 and 2 contain a description of each study included and whether the 14 specifications of the TCD used to detect HITS as recommended by the Consensus Committee10 were documented. Ideally, each of the 14 points should be made available in accordance with the consensus article. However, as evident from On-line Tables 1 and 3, none of the studies documented all 14 requirements.
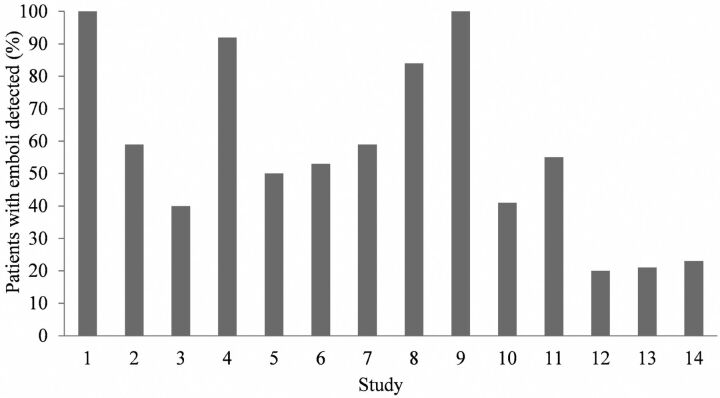
For each study, On-line Table 3 shows the type of surgery, the number of patients, the criteria for emboli detection, the method of counting HITS, and monitoring of either unilateral or bilateral MCAs. Ten studies conformed to the recommended detection criteria, 3 studies raised the threshold amplitude >3 dB higher than background flow, and 1 study reduced the duration to <100 ms. On-line Table 4 lists the emboli count for each study and whether a PFO was sought and neurologic or cognitive change was identified. Emboli were detected in all 14 studies and ranged from 20% to 100% of patients (Fig 2). Most counts were low (<10), but high counts were present in some patients.
Fig 2.
The percentage of patients with emboli detected in each of the 14 studies listed in Table 3.
On-line Table 4 also lists whether a right-to-left shunt was sought. The passage of microemboli from the venous into the arterial circulation can be confirmed by 2 methods either before or after surgery. Air microbubbles in an intravenous injection of agitated saline may pass into the arterial circulation via either cardiac or pulmonary communications and can be detected by TCD in the MCA as HITS. The early appearance of microemboli (eg, within the first 6 heart beats after injection14) suggests a cardiac communication (eg, functional or anatomic PFO) rather than a pulmonary communication (arteriovenous shunt). In the 2 studies by Patel et al,7,13 the diagnostic time window for microemboli was 25 seconds. Riding et al15 used a time window of 12 cardiac cycles, which is still arbitrary and open to challenge. Koch et al8 used contrast agents with microbubbles, which do not survive pulmonary passage.16
The second method involves the use of echocardiography (either TEE or transthoracic echocardiography) to identify right-to-left intracardiac shunts. This is also achieved by an intravenous injection of agitated saline. Echocardiography is then used to track these bubbles as they pass into the left atrium or ventricle as opposed to detecting them in the MCA. Unlike the detection of a right-to-left shunt by using TCD, which may not clearly distinguish cardiac from pulmonary communications, TEE definitively identifies cardiac communications. On-line Table 4 suggests that when TEE identifies a PFO, emboli counts may be high, but pulmonary communications also account for the appearance of HITS. No definitive conclusions can be drawn from these studies on the size and number of microemboli originating from cardiac or pulmonary communications.
Finally, On-line Table 4 lists whether the presence of neurologic or cognitive change was sought and whether any such change was related to the number of emboli.
The 14 articles spanned the 13 years from 1999 to 2010. Five studies were on hip replacements, 3 on knee replacements, 2 on both operations, 2 on fractured long bones, and 2 on scoliosis corrections in adolescents. Among the adult studies, the number of patients in each study ranged from 5 to 45. Two of the articles5,17 did not conform to the consensus criteria for detection of microemboli.9 Despite the use of automatic HITS counting, 13 articles reviewed the HITS count off-line. One article neither conformed to the consensus criteria nor stated how the emboli count was undertaken.18
Echocardiography was used to detect PFO in 6 studies (transthoracic echocardiography in 1 study, transthoracic echocardiography and TEE together in 1 study, and TEE in the remaining 4 studies). TCD was used to detect arteriovenous shunts in 3 studies and both TEE and TCD in 1 study.19 In general, higher counts were found in the presence of arteriovenous shunts.
All studies commented on the presence or absence of neurologic changes postoperatively. This appears to be assessed as part of routine follow-up, though 1 study specifically undertook neurologic examinations.20 Six studies measured cognitive function, and 1 study measured quality of life postoperatively as a proxy for cognition.13 No study found an association between neurologic or cognitive change and embolic count.
Discussion
Articles that described the use of TCD to detect microemboli during orthopedic surgery were sought and reviewed for the accuracy of reporting and the number of microemboli counted. Additionally, we recorded whether the detection of right-to-left shunts was sought and whether any neurologic or postoperative cognitive changes were noted. Fourteen articles were included for qualitative synthesis, and the largest number of patients in a single study was 45. The data for this important topic are thus limited, and this review highlights the need for larger and better reported studies in this area.
There are limitations on the quality of the reports. Few investigators explicitly stated all the features of the sonographic device used, particularly the 14 parameters recommended by the consensus statement.10 Settings that were consistently included were the insonated artery (MCA), insonation depth (40–60 mm), and sonographic frequency (2 MHz). The specific device, sample volume, threshold, and recording time were all inconsistent and poorly reported.
Once data have been recorded, it is common practice to use an independent observer to count the HITS. The criteria for classification of HITS should be documented to allow comparisons of studies. The Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium addressed this concern in 1995 and recommended the criteria for classification.21 The results in On-line Tables 1 and 2 show that all studies stated the criteria for emboli detection, though 4 articles adjusted the threshold or duration from the recommended criteria. Additionally, while 12 of 14 articles included the guidelines used to count microemboli, the studies by Edmonds et al17 and Kalairajah et al18 did not specify how microemboli were counted. The validity of the results of these articles is of concern because the reliability of the TCD detection remains operator-dependent. Without independent analysis and strict classification guidelines, recorded data run the risk of including TCD signals which, in fact, are not real microemboli. Rodriguez et al11 noted that diathermy created interference that could easily be misinterpreted as HITS (especially by automatic detection), but this was not mentioned by other authors.
This review of the literature confirms that microemboli are detectable in the MCA during orthopedic surgery for the specific procedures identified. The embolic count may be absent but is more commonly present in small numbers (<10) or high numbers (>100), the highest reported being 550 for the unifrequency probe.6 A multifrequency probe detected 464 solid and 479 gas emboli in 1 patient,5 but counting was automatic with off-line verification by the same operator. The count distribution appears bimodal, and there is evidence that the high counts are associated with PFO when this has been sought by TEE (On-line Table 4). There is no apparent relationship between the type of surgery and the embolic count. Patient factors obviously play a role in addition to surgical factors. Rather than a specific surgical operation being implicated, any operation involving bone and intramedullary instrumentation is most likely to lead to embolic events.22
Of the 6 studies that tested for cognitive change, 1 did not explicitly describe the cognitive tests used, simply referring to a mental test score without describing the tests.18 The other 5 studies used a battery of neuropsychological tests at various time intervals before and after surgery. The definition of cognitive change is controversial.23,24 One study calculated the incidence of cognitive change by using the 20% rule (a decrease in cognitive score of 20% in 20% of the tests)25 and showed that the number of emboli was not different in patients with and without cognitive change.8 Three studies used group analyses to calculate the mean change in cognitive scores and showed that there was no difference in cognitive change between patients with and without detectable HITS.6,7,22 Only 1 study converted a cognitive score to a standardized score by using normative means and SDs19 but still found no association between the incidence of postoperative cognitive dysfunction and HITS count. Despite the use of highly differing methods of analysis, no study has found any association between HITS and neurologic or cognitive change.
This lack of association between embolic count and neuropsychological change brings into question the concept that microemboli released in orthopedic surgery may lead to cognitive changes. Even the patient with the highest embolic count (>500) was discharged with no detectable cognitive dysfunction.6
The early concern regarding microembolism to the brain during cardiac surgery being a cause of postoperative cognitive dysfunction26,27 has not been confirmed in more recent studies28 and systematic reviews.29,30 Liu et al31 reported a median embolic count of 430 (range, 155–2088) in patients undergoing cardiac surgery with cardiopulmonary bypass against a median embolic count of 2 (range, 0–66) in off-pump surgery but could show no difference in cognitive outcome. While the number of microemboli seen during cardiac surgery is much greater than that during orthopedic surgery, these are more likely to be predominantly gaseous. Cardiac surgical postoperative cognitive dysfunction has more recently been considered to be multifactorial.32,33
Several studies have used MR imaging and diffusion-weighted imaging after cardiac surgery to identify the relationship of new cerebral lesions to quantitative microemboli counts.34,35 None of the studies in this review used MR imaging or diffusion-weighted imaging to explore the incidence of cerebral lesions in relation to microemboli count.
Conclusions
Studies using TCD to detect cerebral microemboli in orthopedic surgery have used small numbers of patients. Many of these studies have not disclosed the full details of the TCD machine specifications, though most have used the recommended detection criteria. TCD often detects microemboli in the MCA during orthopedic surgery, in which the counts are frequently low but may be high in approximately 20% cases. High counts appear to be associated with right-to-left shunts when verified by agitated saline detected in the MCA by using TCD or when visualized by using TEE or transthoracic echocardiography. There is no evidence that these microemboli are associated with cognitive change after surgery. A study with high patient numbers, accurate reporting of TCD machine specifications, and criteria for emboli detection, together with comprehensive cognitive testing and analysis, is required to definitively confirm these conclusions.
Supplementary Material
ABBREVIATIONS:
- HITS
high-intensity transient signals
- PFO
patent foramen ovale
- TCD
transcranial Doppler ultrasonography
- TEE
transesophageal echocardiography
Footnotes
Disclosures: Peter F. Choong—UNRELATED: Consultancy: Depuy, Zimmer, Comments: for being part of a surgeon consultation team involved in design of surgical instruments (Depuy), Grants/Grants Pending: National Health and Medical Research Council,* Australian Research Council,* Australian Orthopaedic Association Research Foundation,* Comments: peer-reviewed grant projects, Royalties: Zimmer, Comments: royalties for being part of design team of tumor endoprosthesis. *Money paid to the institution.
REFERENCES
- 1. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br 1970;52:732–37 [PubMed] [Google Scholar]
- 2. Kelly GL, Dodi G, Eiseman B. Ultrasound detection of fat emboli. Surg Forum 1972;23:459–61 [PubMed] [Google Scholar]
- 3. Herndon JH, Bechtol CO, Crickenberger DP. Use of ultrasound to detect fat emboli during total hip replacement. Acta Orthop Scand 1975;46:108–18 [DOI] [PubMed] [Google Scholar]
- 4. Forteza AM, Koch S, Romano JG, et al. Transcranial Doppler detection of fat emboli. Stroke 1999;30:2687–91 [DOI] [PubMed] [Google Scholar]
- 5. Barak M, Kabha M, Norman D, et al. Cerebral microemboli during hip fracture fixation: a prospective study. Anesthe Analg 2008;107:221–25 [DOI] [PubMed] [Google Scholar]
- 6. Gray AC, Torrens L, Howie CR, et al. Cognitive function and cerebral emboli after primary hip arthroplasty. Hip Int 2008;18:40–45 [DOI] [PubMed] [Google Scholar]
- 7. Patel RV, Stygall J, Harrington J, et al. Cerebral microembolization during primary total hip arthroplasty and neuropsychologic outcome: a pilot study. Clin Orthop Relat Res 2010;468:1621–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koch S, Forteza A, Lavernia C, et al. Cerebral fat microembolism and cognitive decline after hip and knee replacement. Stroke 2007;38:1079–81 [DOI] [PubMed] [Google Scholar]
- 9. Basic identification criteria of Doppler microembolic signals: Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium. Stroke 1995;26:1123. [PubMed] [Google Scholar]
- 10. Ringelstein EB, Droste DW, Babikian VL, et al. Consensus on microembolus detection by TCD: International Consensus Group on Microembolus Detection. Stroke 1998;29:725–29 [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez RA, Letts M, Jarvis J, et al. Cerebral microembolization during pediatric scoliosis surgery: a transcranial Doppler study. J Pediatr Orthop 2001;21:532–36 [PubMed] [Google Scholar]
- 12. Rodriguez RA, Sinclair B, Weatherdon D, et al. Patent foramen ovale and brain microembolization during scoliosis surgery in adolescents. Spine (Phila Pa 1976) 2001;26:1719–21 [DOI] [PubMed] [Google Scholar]
- 13. Patel R, Stygall J, Harrington J, et al. Intra-operative cerebral microembolisation during primary hybrid total hip arthroplasty compared with primary hip resurfacing. Acta Orthop Belg 2009;75:671–77 [PubMed] [Google Scholar]
- 14. Klötzsch C, Janssen G, Berlit P. Transesophageal echocardiography and contrast-TCD in the detection of a patent foramen ovale: experiences with 111 patients. Neurology 1994;44:1603–06 [DOI] [PubMed] [Google Scholar]
- 15. Riding G, Daly K, Hutchinson S, et al. Paradoxical cerebral embolization: an explanation for fat embolism syndrome. J Bone Joint Surg Br 2004;86:95–98 [PubMed] [Google Scholar]
- 16. Droste DW, Lakemeier S, Wichter T, et al. Optimizing the technique of contrast transcranial Doppler ultrasound in the detection of right-to-left shunts. Stroke 2002;33:2211–16 [DOI] [PubMed] [Google Scholar]
- 17. Edmonds CR, Barbut DM, Hager D, et al. Intraoperative cerebral arterial embolization during total hip arthroplasty. Anesthesiology 2000;93:315–18 [DOI] [PubMed] [Google Scholar]
- 18. Kalairajah Y, Cossey AJ, Verrall GM, et al. Are systemic emboli reduced in computer-assisted knee surgery? A prospective, randomised, clinical trial. J Bone Joint Surg Br 2006;88:198–202 [DOI] [PubMed] [Google Scholar]
- 19. Rodriguez RA, Tellier A, Grabowski J, et al. Cognitive dysfunction after total knee arthroplasty: effects of intraoperative cerebral embolization and postoperative complications. J Arthroplasty 2005;20:763–71 [DOI] [PubMed] [Google Scholar]
- 20. Sulek CA, Davies LK, Enneking FK, et al. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology 1999;91:672. [DOI] [PubMed] [Google Scholar]
- 21. Ringelstein EB. Skepticism toward carotid ultrasonography: a virtue, an attitude, or fanaticism? Stroke 1995;26:1743–46 [DOI] [PubMed] [Google Scholar]
- 22. Gray AC, Torrens L, Christie J, et al. Cerebral emboli and cognitive function after intramedullary fracture fixation. Injury 2009;40:742–45 [DOI] [PubMed] [Google Scholar]
- 23. Rasmussen LS, Larsen K, Houx P, et al. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand 2001;45:275–89 [DOI] [PubMed] [Google Scholar]
- 24. Lewis M, Maruff P, Silbert B. Statistical and conceptual issues in defining post-operative cognitive dysfunction. Neurosci Biobehav Rev 2004;28:433–40 [DOI] [PubMed] [Google Scholar]
- 25. Lewis MS, Maruff P, Silbert BS, et al. The sensitivity and specificity of three common statistical rules for the classification of post-operative cognitive dysfunction following coronary artery bypass graft surgery. Acta Anaesthesiol Scand 2006;50:50–57 [DOI] [PubMed] [Google Scholar]
- 26. Fearn SJ, Pole R, Wesnes K, et al. Cerebral injury during cardiopulmonary bypass: emboli impair memory. J Thoracic Cardiovasc Surg 2001;121:1150–60 [DOI] [PubMed] [Google Scholar]
- 27. Braekken SK, Reinvang I, Russell D, et al. Association between intraoperative cerebral microembolic signals and postoperative neuropsychological deficit: comparison between patients with cardiac valve replacement and patients with coronary artery bypass grafting. J Neurol Neurosurg Psychiatry 1998;65:573–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stroobant N, Van Nooten G, Van Belleghem Y, et al. Relation between neurocognitive impairment, embolic load, and cerebrovascular reactivity following on- and off-pump coronary artery bypass grafting. Chest 2005;127:1967–76 [DOI] [PubMed] [Google Scholar]
- 29. Martin KK, Wigginton JB, Babikian VL, et al. Intraoperative cerebral high-intensity transient signals and postoperative cognitive function: a systematic review. Am J Surg 2009;197:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kruis RW, Vlasveld FA, Van Dijk D. The (un)importance of cerebral microemboli. Semin Cardiothorac Vasc Anesth 2010;14:111–18 [DOI] [PubMed] [Google Scholar]
- 31. Liu YH, Wang DX, Li LH, et al. The effects of cardiopulmonary bypass on the number of cerebral microemboli and the incidence of cognitive dysfunction after coronary artery bypass graft surgery. Anesth Analg 2009;109:1013–22 [DOI] [PubMed] [Google Scholar]
- 32. Selnes OA, McKhann GM. Neurocognitive complications after coronary artery bypass surgery. Ann Neurol 2005;57:615–21 [DOI] [PubMed] [Google Scholar]
- 33. Grocott HP, Homi HM, Puskas F. Cognitive dysfunction after cardiac surgery: revisiting etiology. Semin Cardiothorac Vasc Anesth 2005;9:123–29 [DOI] [PubMed] [Google Scholar]
- 34. Restrepo L, Wityk RJ, Grega MA, et al. Diffusion- and perfusion-weighted magnetic resonance imaging of the brain before and after coronary artery bypass grafting surgery. Stroke 2002;33:2909–15 [DOI] [PubMed] [Google Scholar]
- 35. Knipp SC, Matatko N, Wilhelm H, et al. Evaluation of brain injury after coronary artery bypass grafting: a prospective study using neuropsychological assessment and diffusion-weighted magnetic resonance imaging. Eur J Cardiothorac Surg 2004;25:791–800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.