Abstract
Background
Recent advances in mosquito eradication and antimalarial treatments have reduced the malaria burden only modestly. An effective malaria vaccine remains a high priority, but its development has several challenges. Among many potential candidates, the RTS,S/AS01 vaccine (MosquirixTM) remains the leading candidate.
Objective and Method
This review aims to understand the advances in the RTS,S/AS01 vaccine, and future comments regarding the vaccine’s effectiveness in malaria eradication. Literature review for the past five decades was performed searching PubMed, EMBASE Ovid, and Cochrane Library, with using the following search items: (“malaria” OR “WHO’s malaria” OR “Plasmodium falciparum” OR “RTS,S” OR “RTS,S/AS01” OR “RTS,S/AS02” OR “pre-erythrocytic malaria” OR “circumsporozoite” OR “Mosquirix”) AND (“vaccine” OR “vaccination”).
Results
RTS,S/AS01, a recombinant pre-erythrocytic vaccine containing Plasmodium falciparum surface-protein (circumsporozoite) antigen, is safe, well-tolerated, and immunogenic in children. Three doses, along with a booster, have a modest efficacy of about 36% in children (age 5–17 months) and about 26% in infants (age 6–12 weeks) against clinical malaria during a 48-month follow-up. However, the efficacy varies among population subgroups and with the parasite strain, it reduces without a booster and offers protection for a limited duration. Because of its potential cost-effectiveness and positive public health effect, the vaccine is being investigated in a pilot program for mortality benefits and broader deployment.
Conclusion
The RTS,S/AS01 vaccine prevents malaria; however, it should be considered another addition to the malaria-control program and not as an eradication tool because of its relatively low to modest efficacy.
Keywords: malaria, vaccine, pre-erythrocytic, RTS‚S, RTS‚S/AS01, WHO, circumsporozoite
Introduction
Malaria remains the most important human parasitic infection worldwide and is caused by protozoal infection of the genus Plasmodium. It is a vector-borne disease transmitted through the bites of infected female mosquitoes of the Anopheles family. The disease affects nearly half of the world’s population, mainly the low-middle income countries (LMIC) of Africa and South-east Asia. Malaria causes more than 40 thousand deaths each year, with the most significant risk among children under five years.1 Five Plasmodium species – P. falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi (previously known as monkey parasite), have been documented to infect humans, with the first two being the most important.2,3
A recent expansion of treatment and prevention efforts, including novel antimalarial drugs and insecticides, has reduced malaria’s global burden, albeit only modestly.2 Besides, the increasing resistance of the parasite to antimalarial or insecticidal agents and drug-induced toxic effects with chemoprophylaxis lend urgency to the quest for new tools for malaria control.2 World Health Organization (WHO) has identified vaccine as an cost-effective measure to eliminate malaria in the world’s endemic areas.1,4–6 Thus, in the last decade, notably, the focus has shifted to accelerate the efforts towards an effective vaccine, and one candidate has emerged to reach a large phase-III trial while other potential candidates are showing encouraging results.4–10
Challenges in Vaccine Development
Natural immunity against malaria develops in the residents of endemic areas but only after prolonged exposure and is usually incomplete. Therefore, malaria vaccines, which induce antibodies that are qualitatively or quantitatively superior to those occurring naturally, are the need of the hour.11,12 A safe and effective vaccine enabling the world to eradicate malaria would significantly advance public health. However, the hope for an effective and widely available vaccine has been tempered by several challenges.5,10,13,14
The first and major barrier is the genetic and pathophysiologic complexity of the malaria parasite.5,6,10 Plasmodium genome is much larger and more complex than bacterial or viral genomes. Moreover, the parasite has a complicated life cycle, including an asexual phase (schizogony) in man and a sexual phase (gametogony) in the mosquito.2 Therefore, different immune system arms are required for different stages of the parasite, depending on its extracellular or intracellular location and distinct immunogenic properties. The protective antibodies against sporozoites (sexual forms transmitted by the mosquito in man) fail to recognize merozoites (asexual erythrocytic stages that cause clinical malaria). Thus, if one sporozoite escapes the vaccine-induced antibodies, 10–40 thousand merozoites will cause the disease a week later. This poses a considerable challenge in designing an ideal malaria vaccine.5,6,10 Second, because malaria disproportionately affects LMIC lacking the robust health infrastructure, the vaccine manufacturers have little incentive for malaria vaccines and continued targeting vaccines for industrialized world markets.15,16 Third, the stringent regulation imposed by national vaccine licensing authorities increases the cost of clinical development pathways for vaccine licensure quite heavily. Therefore, a newly produced vaccine has to cost more than usual for the industry to regain its investments if not supported by non-government organizations and made available at subsidized rates through public-private partnerships.15,16
Types of Malaria Vaccines
Parasite vaccines generally face the challenge of generating immunity with an immunogen that represents only a tiny fraction (<1%) of the composition of the organism. Although most malaria antigens, selected as vaccine candidates, are usual targets of natural immunity, there is substantial stage specificity of the parasite’s antigen expression. A candidate vaccine for one stage of the life cycle is unlikely to impact on another stage. Most malaria vaccines mainly target one of the three phases.5,6,10,16
Pre-Erythrocytic Vaccines
The pre-erythrocytic vaccines target sporozoites, the sexual forms that are transmitted by the mosquito in man. They produce antibody-mediated inhibition of sporozoite infection of the liver by blocking their entry into the hepatocytes, thereby stopping the progression of the hepatic (pre-erythrocytic) stage. This stage is a preparatory phase of Plasmodium’s life cycle. The parasite infects only a small number of hepatocytes initially and takes about one week to complete this phase, allowing enough time for a vaccine to act. Besides, the infected hepatocytes, unlike the infected erythrocytes counterparts, express parasite antigens that can induce T-cells to target and kill these cells, thus preventing merozoites’ release into the blood.5,6,16–21 For these reasons, vaccines directed against the pre-erythrocytic stage are considered more effective than other stage vaccines. Among many pre-erythrocytic vaccine candidates under development, the RTS,S/AS01 vaccine (brand name MosquirixTM) is the leading candidate being the first candidate to pass a Phase III trial and enter a pilot program.5,10,22–25
Whole sporozoite vaccines have also been investigated using radiation, chemically or genetically attenuated strains of P. falciparum sporozoites delivered by mosquito bites. After entering into the liver, they partially develop in the hepatocytes and induce a broad immune response, including CD4 T cells, CD8 T cells, and antibodies without causing disease.26–30
Erythrocytic Vaccines
These vaccines act when the merozoites are released from the liver (after completion of the pre-erythrocytic stage) and enter the blood to infect erythrocytes. They are also referred to as blood-stage vaccines. Their goal is to stop the invasion of red cells and prevent the parasite’s asexual reproduction. They produce the antibodies that target the merozoite surface proteins, thereby halting red cells’ invasion. Besides, blood-stage vaccines may also target the variant surface antigens of the erythrocyte membrane.31–33
Transmission Blocking Vaccines
They prevent the transmission of infection from patients to mosquitoes and halt the spread of the disease. At the end of the erythrocytic stage, some of the merozoites differentiate into sexual stages. These are fed upon by mosquitoes and complete the sexual cycle in the gut. These vaccines incorporate surface antigens of sexual forms of the parasite (gametes and zygote) and generate antibodies that prevent sexual reproduction of the parasite by blocking either the fertilization of the gametes or the development of the zygote into sporozoites.34–36 Recently, a vaccine against mosquito saliva targeting the transmission of multiple mosquito-borne pathogens was investigated and found to be safe and immunogenic.37
RTS,S/AS01 Vaccine
A sporozoite vaccine against P. falciparum malaria, developed in 1987 after animal studies, showed that antibodies against circumsporozoite (CS) protein protect against active infection with P. falciparum.11 The vaccine is not effective against other types of malaria, including P. vivax.
Structure
It is a monovalent recombinant protein vaccine that targets a fragment of the CS protein. The CS protein (a 412 amino acid protein) is the principal antigen on the sporozoite’s surface and is also present early in hepatic infection. The identification of P. falciparum CS protein as the major component of the sporozoite coat led to the cloning and sequencing of this gene. RTS,S/AS01 contains a CS protein sequence fused to hepatitis B virus surface antigen that acts as a carrier matrix for CS antigen central repeat region and an immunogenic adjuvant AS01. The “R” stands for the central repeat region of the CS protein; the “T” stands for the T-cell epitope of the CS antigen; and first “S” for “Surface” portion which when co-expressed on yeast cells, displays both CS protein and S at their surfaces, while the next “S” stands for the hepatitis B surface antigen (a carrier matrix). The RTS fusion protein and free S protein assemble in RTS,S particles. AS01 includes the immune-enhancers monophosphoryl lipid A and QS21. Monophosphoryl lipid A consists of a chemically detoxified form of the parent lipopolysaccharide from the gram-negative bacterium Salmonella minnesota. QS21 is a natural saponin molecule purified from the bark of the South American tree, Quillaja saponaria.5,6,18–21,38
Immunogenicity
RTS,S/AS01 vaccine is formed by tandem repeat region of the CS protein, mainly copies of the four amino acid sequence NANP combine with adjuvant-AS01. It induces a strong IgG antibody response to the conserved central repeat region of the CS protein and potent T-cell (CD4+) response.23,24 Antibody levels reach high concentration, often hundreds of micrograms per milliliter. The levels correlate with protection against infection or clinical malaria in several settings, but the absolute threshold level for protection has not been clearly defined yet.21,39 One study found an antibody titer of 121 EU/mL to be associated with the prevention of 50% malaria infections.40 CD4+ T cells show a vigorous response post-vaccination and are thought to confer protection.12 Most studies have reported an absence of substantial CS-specific CD8+ T cell response and, thus, is not an essential mediator of protection of RTS,S/AS01.41–43 Immunogenicity is short-lived without additional booster dosing. Antibody levels show an initial rapid decline with a mean half-life of 40 days, followed by a more gradual decline at about 20 months.39
Efficacy
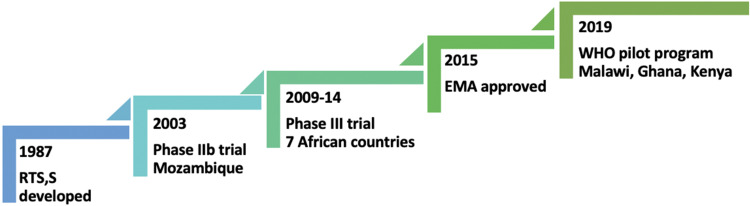
With the RTS,S/AS01 vaccine’s success in eliciting strong immunogenicity, a double-blind, phase IIb, randomized controlled trial was conducted in above two thousand children aged 1–4 years in southern Mozambique in 2003–2004 (Figure 1).44 It found that malaria’s incidence rate was 37% lower than in controls during the first six months after the third vaccine dose. Vaccine efficacy, calculated as one minus the incidence rate ratio, was 27% for all clinical episodes and 58% for severe disease. An additional single-blind follow-up for 12 months demonstrated the vaccine efficacy of 29% for all clinical episodes and 39% for severe malaria.45 Over the entire 18-month, the prevention was 35% and 49% for all episodes and severe episodes, respectively. Age at vaccine administration was not associated with vaccine efficacy. Subsequent double-blind phase IIb trials in Africa also showed significant protection in infants with an adjusted efficacy of about 65% during the first six months after vaccination.46–48
Figure 1.
Major events in the progress of RTS,S/AS01 vaccine.
With the encouraging results of early trials, a phase III randomized trial of RTS,S/AS01 vaccine (a collaboration between a private foundation, a vaccine manufacturer, and a public health agency) was conducted between 2009 to 2014 in seven sub-Saharan countries - Burkina Faso, Gabon, Ghana, Kenya, Malawi, Mozambique and the United Republic of Tanzania.25 The vaccination schedule consists of three baseline doses of 0.5 mL administered intramuscularly at 0, 1, and 2 months, followed by the fourth dose (booster) at 18 months after the 3rd dose. The three doses had an efficacy of about 28% in children (age 5–17 months) and about 18% in infants (age 6–12 weeks) against clinical malaria during a median follow-up after the first dose, 48 months for children and 38 months for infants. With a booster dose at 20 months after dose 1, efficacy increased to about 36% in children and about 26% in infants. Effectiveness waned over time, and without booster dosing, the risk of severe malaria increased by the trial end, notably in high-endemic areas.25
Further, a phase III extension study also demonstrated that the protection declined over time with a three-dose regimen with about 4% of overall estimated vaccine efficacy over seven years.49 The lower vaccine efficacy in the younger age (6–12 weeks) compared to older children (5–17 months) observed in the pivotal phase III trial might have resulted from interactions with other co-administered vaccine response, the presence of maternally acquired anti-CS antibodies, and immaturity of the immune system.25,50 The studies also showed substantial heterogeneity in efficacy in regions with varied transmission intensity (defined as the estimated local prevalence of asymptomatic parasitemia in children).49,51 The efficacy was lower among children with a higher exposure index.
Because malaria parasites are genetically different, the RTS,S/AS01 vaccine, based on genetic sequences derived from a single Plasmodium strain (3D7), has higher efficacy against parasites with a genotype that matches the CS protein allele in the vaccine than against the not matching strains. In a local African population, only <10% of the infections were vaccine-matched, and there was a modest loss of protection against divergent parasites.19 The allele-specific efficacy of the current vaccine has a profound implication for its wide deployment.
In conclusion, the protective efficacy of RTS,S/AS01 malaria vaccine is affected by multiple factors - more with a booster dose, more in older children than infants, short-term and waning with time, less in high transmission intensity populations, and low with genetically diverse parasites.
A next-generation RTS,S vaccine with additional CS antigens, called the R21 vaccine, is under investigation for the broader protective efficacy against genetically diverse parasites.38 Other prospects to improve vaccine efficacy include assessing alternate dosing schedules, booster doses at frequent intervals, enhanced immunogenicity by use of adjuvants, or as a part of a multicomponent product (in combination with vaccines that target other stages of the parasite’s life cycle).38–40
Safety
Safety and tolerability of RTS,S/AS01 vaccine match with other pediatric vaccines. There was an increased risk of febrile seizures within seven days after any vaccine doses among children in the older age group.25,50 Among the younger infants, this risk was only apparent after the fourth dose. There were no neurological sequelae following the febrile convulsions. In the pivotal phase III malaria vaccine trial, although the incidence of severe adverse events was similar across vaccine-group and control-group participants; however, meningitis was more likely in the former.25,50 The exact reason for meningitis remains unascertained and postulated due to diagnostic bias following frequent lumbar punctures or chemical reactivity due to AS01 adjuvant. In 2015, the European Medicines Agency found that the RTS,S/AS01 vaccine has an acceptable safety profile that would continue to be monitored (eg, in the pilot implementation program).52,53
Public Health Effects
After clinical trials showing modest efficacy of RTS,S/AS01 candidate against malaria, four mathematical models have quantified that addition of the vaccine to the existing malaria-control program could potentially prevent 6% to 30% of mortality in children aged <5 years in endemic regions, with significant public health benefit and cost-effectiveness in high parasite prevalence areas.4
In April 2019, the WHO has started a malaria vaccine pilot program to assess the effects on childhood mortality, its safety during routine use, and the feasibility of delivering four doses in children in three African countries - Malawi, Ghana, and Kenya.53 The three-dose intramuscular vaccination schedule for infants is at 6, 10, and 14 weeks of age with other routine childhood vaccination, and for older children, dosing to start between 5–17 months and at 1-month intervals, followed by a fourth booster dose 18 months after the 3rd dose in all age group.
Conclusion
The vaccine-based malaria-prevention strategy remains a high priority for sustained, substantial, and cost-effective control. Given the complexity of malaria immunity, the development of an effective vaccine remains a formidable challenge. The RTS,S/AS01 vaccine has shown low to modest efficacy in preventing clinical malaria by P. falciparum, and multiple factors further influence this efficacy. Because multiple dosing or dosing outside of the routine pediatric vaccination schedule requires considerable resources for wide vaccine deployment, it may endanger fund diversion from other malaria-control programs (mosquito eradication interventions and novel antimalarial drugs) to support the use of the vaccine. This vaccine should be taken as another addition to (rather than a replacement for) current measures. The policy recommendation and the future position of RTS,S/AS01 for childhood vaccination will depend on the ongoing pilot studies.
RTS,S/AS01 vaccination prevents clinical malaria; however, it might not be sufficient for global malaria eradication.
Disclosure
The authors report no conflicts of interest.
References
- 1.World Health Organization. World Malaria Report 2019. Geneva: WHO; 2019. [Google Scholar]
- 2.Pannu AK. Malaria today: advances in management and control. Trop Doct. 2019;49(3):160–164. doi: 10.1177/0049475519846382 [DOI] [PubMed] [Google Scholar]
- 3.Quispe AM, Pozo E, Guerrero E, et al. Plasmodium vivax hospitalizations in a monoendemic malaria region: severe vivax malaria? Am J Trop Med Hyg. 2014;91:11–17. doi: 10.4269/ajtmh.12-0610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penny MA, Verity R, Bever CA, et al. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet. 2016;387:367–375. doi: 10.1016/S0140-6736(15)00725-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy PE, Patrick Gorres J. Malaria vaccines since 2000: progress, priorities, products. NPJ Vaccines. 2020;5:48. doi: 10.1038/s41541-020-0196-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill AV. Vaccines against malaria. Philos Trans R Soc Lond B Biol Sci. 2011;366(1579):2806–2814. doi: 10.1098/rstb.2011.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stiepel RT, Batty CJ, MacRaild CA, Norton RS, Bachelder E, Ainslie KM. Merozoite surface protein 2 adsorbed onto acetalated dextran microparticles for malaria vaccination. Int J Pharm. 2021;593:120168. doi: 10.1016/j.ijpharm.2020.120168 [DOI] [PubMed] [Google Scholar]
- 8.Asali S, Raz A, Turki H, Mafakher L, Razmjou E, Solaymani-Mohammadi S. Restricted genetic heterogeneity of the Plasmodium vivax transmission-blocking vaccine (TBV) candidate Pvs48/45 in a low transmission setting: implications for the Plasmodium vivax malaria vaccine development. Infect Genet Evol. 2021;89:104710. doi: 10.1016/j.meegid.2021.104710 [DOI] [PubMed] [Google Scholar]
- 9.Kaslow DC. Malaria vaccine research & innovation: the intersection of IA2030 and zero malaria. NPJ Vaccines. 2020;5(1):109. doi: 10.1038/s41541-020-00259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matuschewski K. Vaccines against malaria-still a long way to go. FEBS J. 2017;284(16):2560–2568. doi: 10.1111/febs.14107 [DOI] [PubMed] [Google Scholar]
- 11.Hoffman SL, Oster CN, Plowe CV, et al. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science. 1987;237(4815):639–642. doi: 10.1126/science.3299709 [DOI] [PubMed] [Google Scholar]
- 12.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9(7):725–732. doi: 10.1038/ni.f.205 [DOI] [PubMed] [Google Scholar]
- 13.Girard MP, Reed ZH, Friede M, Kieny MP. A review of human vaccine research and development: malaria. Vaccine. 2007;25(9):1567–1580. doi: 10.1016/j.vaccine.2006.09.074 [DOI] [PubMed] [Google Scholar]
- 14.Daily JP. Malaria vaccine trials-beyond efficacy end points. N Engl J Med. 2012;367(24):2349–2351. doi: 10.1056/NEJMe1213392 [DOI] [PubMed] [Google Scholar]
- 15.Clemens J, Jodar L. Introducing new vaccines into developing countries: obstacles, opportunities and complexities. Nat Med. 2005;11(4 Suppl):S12–5. doi: 10.1038/nm1225 [DOI] [PubMed] [Google Scholar]
- 16.Bloom BR, Lambert PH, eds. The Vaccine Book. 15th ed. Amsterdam: Academic Press; 2003:345–370. [Google Scholar]
- 17.Sadoff JC, Ballou WR, Baron LS, et al. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science. 1988;240(4850):336–338. doi: 10.1126/science.3281260 [DOI] [PubMed] [Google Scholar]
- 18.RTS,S Malaria Vaccine Trial Team. Bojang KA, Milligan PJ, Pinder M, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358(9297):1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 19.Neafsey DE, Juraska M, Bedford T, et al. Genetic diversity and protective efficacy of the RTS,S/AS01 Malaria Vaccine. N Engl J Med. 2015;373(21):2025–2037. doi: 10.1056/NEJMoa1505819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nardin EH, Nussenzweig RS. T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu Rev Immunol. 1993;11:687–727. doi: 10.1146/annurev.iy.11.040193.003351 [DOI] [PubMed] [Google Scholar]
- 21.Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malar J. 2009;8:312. doi: 10.1186/1475-2875-8-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rts SCTP, Agnandji ST, Lell B, et al. First results of Phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365(20):1863–1875. [DOI] [PubMed] [Google Scholar]
- 23.Ballou WR, Cahill CP. Two decades of commitment to malaria vaccine development: glaxoSmithKline Biologicals. Am J Trop Med Hyg. 2007;77(6 Suppl):289–295. doi: 10.4269/ajtmh.2007.77.289 [DOI] [PubMed] [Google Scholar]
- 24.RTS,S Malaria Vaccine Evaluation Group. Stoute JA, Slaoui M, Heppner DG, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336(2):86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 25.RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–1164. doi: 10.1086/339409 [DOI] [PubMed] [Google Scholar]
- 27.Hoffman SL, Billingsley PF, James E, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin. 2010;6(1):97–106. doi: 10.4161/hv.6.1.10396 [DOI] [PubMed] [Google Scholar]
- 28.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433(7022):164–167. Erratum in: Nature. 2007;446(7131):102. doi: 10.1038/nature03188 [DOI] [PubMed] [Google Scholar]
- 29.Richie TL, Billingsley PF, Sim BK, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 2015;33(52):7452–7461. doi: 10.1016/j.vaccine.2015.09.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jongo SA, Urbano V, Church LWP, et al. Immunogenicity and protective efficacy of radiation-attenuated and chemo-attenuated PfSPZ vaccines in equatoguinean adults. Am J Trop Med Hyg. 2021;104(1):283–293. doi: 10.4269/ajtmh.20-0435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirima SB, Cousens S, Druilhe P. Protection against malaria by MSP3 candidate vaccine. N Engl J Med. 2011;365(11):1062–1064. doi: 10.1056/NEJMc1100670 [DOI] [PubMed] [Google Scholar]
- 32.Thera MA, Doumbo OK, Coulibaly D, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med. 2011;365(11):1004–1013. doi: 10.1056/NEJMoa1008115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payne RO, Milne KH, Elias SC, et al. Demonstration of the blood-stage plasmodium falciparum controlled human malaria infection model to assess efficacy of the P. falciparum Apical Membrane Antigen 1 Vaccine, FMP2.1/AS01. J Infect Dis. 2016;213(11):1743–1751. Erratum in: J Infect Dis. 2016;214(6):978. doi: 10.1093/infdis/jiw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grotendorst CA, Kumar N, Carter R, Kaushal DC. A surface protein expressed during the transformation of zygotes of Plasmodium gallinaceum is a target of transmission-blocking antibodies. Infect Immun. 1984;45(3):775–777. doi: 10.1128/IAI.45.3.775-777.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duffy PE, Pimenta P, Kaslow DC. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1993;177(2):505–510. doi: 10.1084/jem.177.2.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubler-Kielb J, Majadly F, Wu Y, et al. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc Natl Acad Sci U S A. 2007;104(1):293–298. doi: 10.1073/pnas.0609885104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manning JE, Oliveira F, Coutinho-Abreu IV, et al. Safety and immunogenicity of a mosquito saliva peptide-based vaccine: a randomised, placebo-controlled, double-blind, Phase 1 trial. Lancet. 2020;395(10242):1998–2007. doi: 10.1016/S0140-6736(20)31048-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin. 2010;6(1):90–96. doi: 10.4161/hv.6.1.9677 [DOI] [PubMed] [Google Scholar]
- 39.White MT, Verity R, Griffin JT, et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis. 2015;15(12):1450–1458. doi: 10.1016/S1473-3099(15)00239-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gosling R, von Seidlein L. The future of the RTS,S/AS01 Malaria vaccine: an alternative development plan. PLoS Med. 2016;13(4):e1001994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kebaier C, Voza T, Vanderberg J. Kinetics of mosquito-injected Plasmodium sporozoites in mice: fewer sporozoites are injected into sporozoite-immunized mice. PLoS Pathog. 2009;5(4):e1000399. doi: 10.1371/journal.ppat.1000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kester KE, Cummings JF, Ofori‐Anyinam O, et al. Randomized, double-blind, Phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200(3):337–346. doi: 10.1086/600120 [DOI] [PubMed] [Google Scholar]
- 43.Lalvani A, Moris P, Voss G, et al. Potent induction of focused Th1-type cellular and humoral immune responses by RTS,S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J Infect Dis. 1999;180(5):1656–1664. doi: 10.1086/315074 [DOI] [PubMed] [Google Scholar]
- 44.Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364(9443):1411–1420. doi: 10.1016/S0140-6736(04)17223-1 [DOI] [PubMed] [Google Scholar]
- 45.Alonso PL, Sacarlal J, Aponte JJ, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366(9502):2012–2018. doi: 10.1016/S0140-6736(05)67669-6 [DOI] [PubMed] [Google Scholar]
- 46.Aponte JJ, Aide P, Renom M, et al. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370(9598):1543–1551. doi: 10.1016/S0140-6736(07)61542-6 [DOI] [PubMed] [Google Scholar]
- 47.Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359(24):2521–2532. doi: 10.1056/NEJMoa0807381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdulla S, Oberholzer R, Juma O, et al. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359(24):2533–2544. doi: 10.1056/NEJMoa0807773 [DOI] [PubMed] [Google Scholar]
- 49.Olotu A, Fegan G, Wambua J, et al. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N Engl J Med. 2016;374(26):2519–2529. doi: 10.1056/NEJMoa1515257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.RTS,S Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367(24):2284–2295. doi: 10.1056/NEJMoa1208394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bejon P, White MT, Olotu A, et al. Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect Dis. 2013;13(4):319–327. Erratum in: Lancet Infect Dis. 2013;13(9):735. doi: 10.1016/S1473-3099(13)70005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.European Medicines Agency. First malaria vaccine receives positive scientific opinion from EMA EMA/CHMP/488348/2015. Press Office, editor. London: EMA; 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/07/WC500190447.pdf. Accessed November16, 2019. [Google Scholar]
- 53.World Health Organization. Malaria vaccine pilot launched in Malawi 2019. Geneva: WHO; 2019. Available from: https://www.who.int/news/item/23-04-2019-malaria-vaccine-pilot-launched-in-malawi. Accessed May3, 2020. [Google Scholar]